Abstract
Aim
To investigate the consequences of increased expression of caspase-9: 1) whether the caspase-9 overexpression resulted in cell death through apoptosis, 2) whether apoptosis could be triggered in normal and tumor cells, and 3) what is the role of caspase-9 in the process.
Methods
The caspase-9 fused to green fluorescent protein was expressed in primary cultures of anterior pituitary cells and of HeLa tumor cells. The expressed caspase-9 and the number of apoptotic and necrotic cells were determined using fluorescence microscopy.
Results
Overexpression of caspase-9 resulted in cell death of primary pituitary cells and HeLa cells. More than 94% of the cells died of apoptosis, which was triggered by the activation of caspase-9, since the cell deaths were prevented in the presence of caspase-9 specific inhibitor. HeLa cells were about 50% more resistant to apoptosis than pituitary cells.
Conclusions
Caspase-9 overexpression and its activation leads to apoptosis. It occurs both in normal and tumor cells. Since the majority of cancer therapy treatments initiate apoptosis through the caspase-9 activation, the modulation of caspase-9 expression may be exploited in designing new ways to control apoptosis in neurodegenerative or malignant diseases.
There are two types of cell death: necrosis and apoptosis. Apoptosis or programmed cell death is a process which controls the number and the quality of cells (1). Caspases are proteases, which have a central role in triggering and executing apoptosis (2,3). The two major pathways of triggering apoptosis are the intrinsic and extrinsic pathway (4). The main intrinsic pathway is characterized by mitochondrial dysfunction, with the release of cytochrome c, activation of caspase-9, and subsequently of caspase-3. The extrinsic pathway is activated at the cell surface through death receptor mediated activation of caspase-8 or caspase-10, followed by caspase-3 activation. This pathway may be amplified by caspase-9 activation (intrinsic pathway). Therefore, caspase-9 is one of the main initiator caspases (5). Like the other members of the caspase family, it is synthesized as an inactive zymogen and is activated through proteolytic processing. There are two main pathways for caspase-9 activation: within the apoptosome, a large protein complex, which consists of caspase-9, cytochrome c, and Apaf-1 (6-8), or by proteolytic cleavage by a previously activated caspase, which involves its dimerization (9-11).
Among other processes, caspase-9 triggers apoptosis early in the development of nervous system (12,13). Failure of caspase-9 activation is also associated with the resistance for apoptosis in testicular tumors (14). In addition, the gene for caspase-9 maps to the locus disrupted in human tumors, a hotspot for loss of heterozygosity in several cancers, including neuroblastomas (15). A number of initial studies supported the claim that cancer therapy triggered apoptosis by activating the extrinsic pathway of apoptosis (16). Subsequently, there is compelling evidence that the majority of cytotoxic drugs initiate cell death by triggering the cytochrome c/Apaf-1/Caspase-9-dependent pathway through the mitochondria (intrinsic pathway) (16). Embryonic stem cells with disrupted caspase-9 and thymocytes with disrupted Apaf-1 are resistant to cytotoxic drugs, but sensitive to death receptor triggering (extrinsic pathway) (13). In contrast, embryonic fibroblasts disrupted with caspase-8 are sensitive to cytotoxic drugs (17). The relative contributions of intrinsic and extrinsic pathways to apoptosis induced by cytotoxic drugs may also depend on the drug, dose, and kinetics (16). While there are data on the consequences of reduced amounts of caspase-9 in the cells, its overexpression has not been systematically investigated so far.
Here we report that caspase-9 overexpression triggers the activation of caspase-9 and apoptosis. It is likely that caspase-9 autoactivates itself under these conditions, since the caspase-9 specific inhibitor prevents the cell deaths. The apoptosis due to overexpression seems to be a ubiquitous process, since it occurs in normal and in tumor transformed cells. HeLa cells are only 50% less sensitive to cell death due to caspase-9 overexpression than the primary pituitary cells.
Material and methods
Expression of fluorescent fusion proteins
Mammalian expression plasmids encoding fusion proteins of green fluorescent protein (E), cytochrome c (Cytc), and caspase-9 (Casp9) were constructed as is described in the following publications: Casp9E (18), CytcE (19), and Casp9 (20). They were amplified using standard procedures and were introduced into the cells using LipofectAMINE PLUSTM reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. The amount of DNA introduced was 0.26 pmol and was kept constant in all experiments. Each type of DNA was introduced in at least 5 independent experiments; all types of fluorescent proteins were always expressed in parallel samples.
The optimal time between the introduction of the DNA for fluorescent fusion proteins and detection of their expression was determined for each type of cells separately and was equal in all experiments. At least five cultures were performed for each experiment. The number of cells was kept constant: about 100 000 pituitary and 70 000 HeLa cells for each sample. To decrease the variations between the samples due to the low number of fluorescent cells, the latter were counted throughout the sample.
Cell cultures
Primary cell cultures of pituitary cells from the anterior lobe were isolated from adult male rats (Wistar, body weight 200-300 g) as described by Ben-Tabou (21). Cells were placed on poly-L-lys-coated coverslips, incubated in a rich nutritive medium composed of 80% Dulbecco's Modified Eagle's Medium with Nutrient Mixture F-12 HAM (Invitrogen, Carlsbad, CA, USA), 9% Minimal essential medium with alpha modification (MEM Alpha Medium; Sigma, St. Louis, MO, USA), 0.4% D-glucose (Sigma), 25 mM N-hydroxyethylpiperazine-N’-2-ethanesulphonic acid (Hepes, Sigma), 2 mM L-glutamine, and 10% fetal bovine serum at 37°C, 5% CO2. HeLa cells were grown in MEM Alpha Medium (Sigma), using standard procedures. After 24 hours, DNA was introduced into primary or HeLa cells, as described above.
When the cells were grown in the presence of an inhibitor z-LEHD.fmk (2 μmol/L, BioVision, Mountain View, CA, USA), it was added immediately after the lipofection and was kept in the medium throughout the experiment. Apoptotic and necrotic cells were dyed by annexin conjugated with Alexa Fluor 568 (Invitrogen, Molecular Probes, Carlsbad, CA, USA) and Sytox Green (Invitrogen, Molecular Probes, Carlsbad, CA, USA), both according to the manufacturer’s instructions.
Image collection and statistical analyses
The images of fluorescent cells were captured using a Leica TCS SP confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany) with an oil immersion objective (40 × magnification and numerical aperture 1.25). Enhanced Green Fluorescence Protein (EGFP) was excited by argon laser at 488 nm and fluorescence was collected between 505 and 530 nm. The cells expressing the introduced DNA were identified by fluorescence microscopy (Olympus IX81, Olympus, Tokyo, Japan) through detection of EGFP.
The percentage of apoptotic cells in a sample was calculated by dividing the number of all apoptotic cells with the number of all dying cells in the sample. Apoptotic index (AI) was calculated as the ratio between the number of apoptotic cells and the number of all cells in the sample, ie, AI of fluorescent cells was the number of apoptotic fluorescent cells divided by the number of all fluorescent cells in a sample.
The primary data (or the calculated AI) from at least five independent experiments were plotted as box plot using Sigma Plot 10.0 (Systat Software, San Jose, CA, USA). Statistical analyses were performed using Statistical Package for the Social Sciences, version 13.0.1 (SPSS Inc., Chicago, IL, USA) with a module for exact tests. Unpaired exact Wilcoxon test was used to compare 2 groups, exact Kruskal-Wallis test to compare more than 2 groups, and Fisher exact test to compare the calculated ratios of cells. We considered values of samples as statistically significant when P<0.01.
The survival rates of cells expressing fluorescently labeled caspase-9 (Casp9E+Casp9) were compared with those expressing cytochrome c (CytcE) by calculating ratios between the medians of cell survival when CytcE was expressed and when Casp9E+Casp9 was expressed. The two ratios were plotted by Sigma plot 10.0.
Results
Overexpression of caspase-9 in culture cells
The cells expressing additional amounts of caspase-9 were identified using detection of fluorescent fusion proteins, either Casp9E or a fusion between cytochrome c and EGFP (CytcE, Figure 1A). When two different plasmids are introduced into the cells simultaneously, there is a high probability that both plasmids will enter the same cell (22). The fluorescent fusion protein CytcE expressed on its own was used as a control to monitor the cell death due to the overexpression of a fluorescent fusion protein itself.
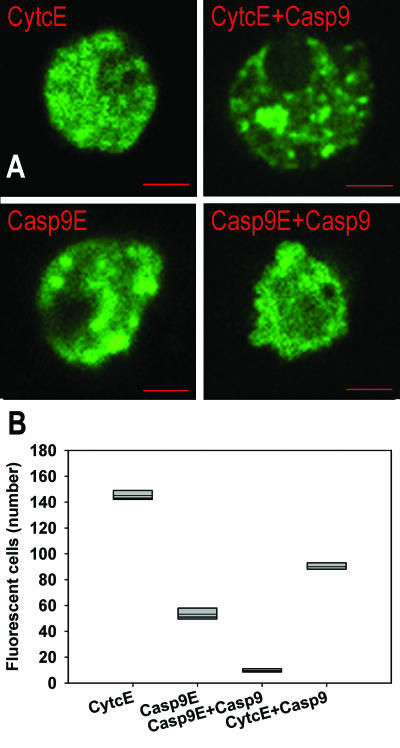
Figure 1.
Effects of overexpression of caspase-9 on the survival of cells. (A) Pituitary cells expressing fusion proteins cytochrome c-EGFP (CytcE), cytochrome c-EGFP and caspase-9 (CytcE+Casp9), caspase-9-EGFP (Casp9E) and caspase-9 in addition to caspase-9-EGFP (Casp9E+Casp9). A typical morphology associated with apoptosis was rarely observed in the cells overexpressing CytcE; apoptotic cells were observed frequently when Casp9E+Casp9 were overexpressed. Bar: 5 μm. (B) The survival of pituitary cells depends on the construct expressed; the differences in survival rates are statistically significant (P<0.001, Kruskal-Wallis test). Each box plot represents the results of 5 independent experiments; the thin line indicates the mean value.
The morphology of some primary pituitary cells expressing additional amounts of caspase-9 differed according to the construct introduced. Control cells, expressing the additional amounts of CytcE were morphologically indistinguishable from non-fluorescent cells (Figure 1A). The same was true for many cells expressing Casp9E. Some of these clearly showed apoptotic morphology. A typical vesicular structure of apoptotic cells was observed frequently in the samples where the part of the DNA for caspase-9 fusion protein was substituted by the plasmid expressing the full length caspase-9 only.
Overexpression of caspase-9 triggers cell death in primary pituitary cells
The relationship between the expression of fluorescent fusion proteins and cell survival is shown in Figure 1B. There were less fluorescent cells expressing Casp9E than the control CytcE (P<0.001, Kruskal-Wallis). Cells expressing the full length caspase-9 and Casp9E were more prone to death than those expressing the fluorescent fusion protein Casp9E. Also, significantly less cells survived when caspase-9 was overexpressed together with CytcE (CytcE+Casp9), compared with CytcE or with the equivalent amounts of CytcE expressed alone (data not shown). The survival of pituitary cells therefore seems to depend on the expression levels of caspase-9.
Overexpression of caspase-9 triggers apoptosis in the cells
The pituitary cells overexpressing caspase-9 could have died either in the process of apoptosis or necrosis. To distinguish between these possibilities, the cells were labeled with a fluorescent conjugate of annexin V (annexin V-568) (23) and the membrane impermeant dye Sytox Green, which detects necrotic cells (Figure 2A). Out of all dying cells, there were more than 94% of apoptotic cells in all samples: about 98% in cells overexpressing cytochrome c and their non-fluorescent controls and 94% in cells overexpressing caspase-9 in addition to cytochrome c (CytcE+Casp9) and their non-fluorescent controls. The difference between the two samples was statistically significant (P<0.001, Fisher exact test). However, we did not consider it practically significant, since the magnitude of the difference was very small. Therefore, almost all cell deaths due to caspase-9 overexpression occurred by apoptosis; necrosis did not contribute significantly to cell death.
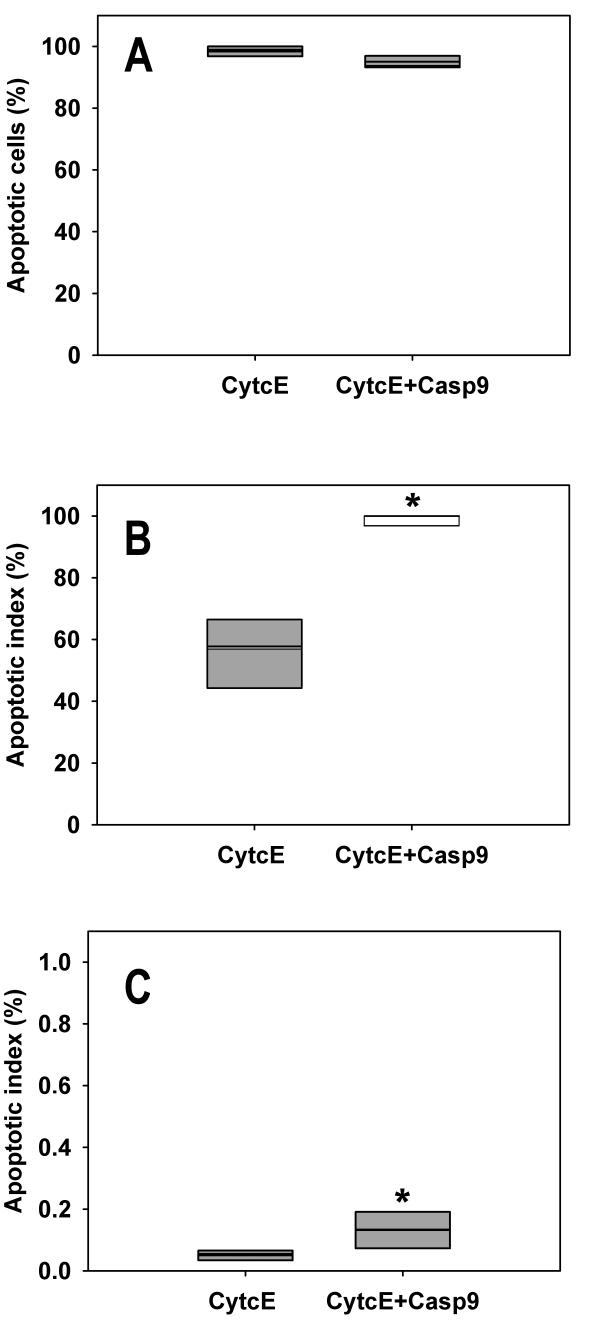
Figure 2.
Overexpression of caspase-9 triggers apoptosis in pituitary cells. (A) The percentage of apoptotic cells among all dead cells. There is a statistically significant difference between the cells overexpressing CytcE and CytcE+Casp9 from five independent experiments (P<0.001, Fisher exact test). Because the magnitude of the difference is very small, we do not consider it practically significant. (CytcE: cytochrome c-EGFP, CytcE+Casp9: cytochrome c-EGFP and caspase-9). The thin line indicates the mean value. (B) Apoptotic index (AI) of cells overexpressing the fluorescent fusion proteins. AI was calculated as the ratio of the numbers of annexin labeled fluorescent cells and all fluorescent cells. The addition of caspase-9 to CytcE significantly increases AI from 58% to 100% (medians; P<0.001 Fisher exact test). The thin line indicates the mean value. (C) Apoptotic index of control cells, compared with those overexpressing the fluorescent fusion proteins. The non-fluorescent cells were grown on the same coverslips as the fluorescent ones; however, they were not overexpressing the fluorescent proteins. Although nonfluorescent cells predominated, the apoptotic index was more than 100-fold lower than in the case of their fluorescent counterparts. There is a statistically significant difference between CytcE and CytcE+Casp9 (P<0.001, Fisher exact test). Because of the small size of the difference, we do not consider that the calculated statistical difference is practically significant. The thin line indicates the mean value.
To compare the incidence of apoptosis between samples expressing different amounts of caspase-9 we calculated the AI (Figure 2B). Median apoptotic index of cells expressing CytcE+Casp9 was 100% compared to 58% in cells overexpressing only CytcE (P<0.001, Fisher exact test).
The non-fluorescent cells were grown on the same coverslips as their fluorescent counterparts, but they did not express fluorescent fusion proteins. Apoptotic indices for both control groups of CytcE and CytcE+Casp9 were about 0.1% (Figure 2C); more than 100 times lower than in the case of fluorescent cells. Median AI of the two groups (CytcE and CytcE+Casp9) differed by only 0.05%; the statistical significance was calculated by Fisher exact test (P<0.001). Because the difference was very small, we did not consider the calculated statistical significance to be practically significant.
Overexpression of caspase-9 triggers its activation
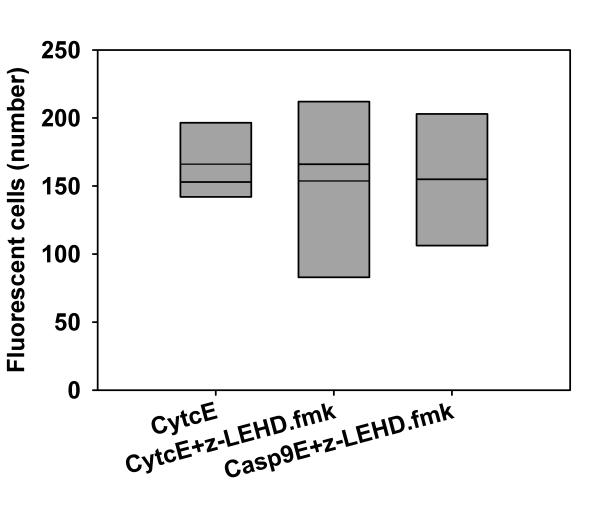
Overexpression of caspase-9 may result in apoptosis because of autoactivation of caspase-9 or as a consequence of activation of a different caspase. To distinguish between these possibilities, we investigated whether active caspase-9 is necessary for cell death. Cells overexpressing fluorescent caspase-9 (Casp9E) or cytochrome c (CytcE) were treated with caspase-9 specific inhibitor z-LEHD.fmk. No statistically significant difference was observed when CytcE was grown in the presence or absence of the inhibitor and when caspase-9 was expressed in the presence of its inhibitor (P = 0.926, Kruskal-Wallis test, Figure 3).
Figure 3.
Activation of caspase-9 is necessary for the apoptosis due to its overexpression. The pituitary cells were grown in the presence of 2 μM z-LEHD.fmk, where appropriate (+z-LEHD.fmk), after the introduction of DNA for fluorescent fusion proteins. There are no statistically significant differences between the samples (P = 0.926, Kruskal-Wallis test). CytcE: cytochrome c-EGFP, Casp9E: caspase-9-EGFP. The thin line indicates the mean value.
Caspase-9 overexpression causes apoptosis in tumor cells
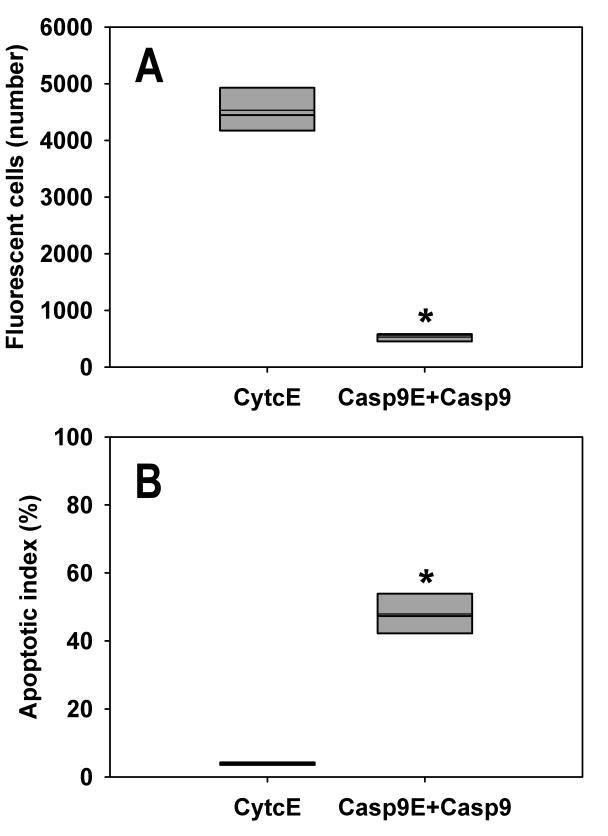
We introduced plasmids expressing both caspases Casp9E+Casp9 and a control protein CytcE into HeLa cells, resilient malignant cells derived from adenosquameous carcinoma of the cervix which are widely used in research (24). In the case of HeLa cells, the number of fluorescent cells that overexpressed Casp9E+Casp9 was significantly lower than the number of cells that expressed CytcE (P = 0.008, unpaired Wilcoxon test, Figure 4A).
Figure 4.
HeLa cells die of apoptosis due to caspase-9 overexpression. (A) The number of fluorescent cells significantly decreases when Casp9E+Casp9 is introduced into the cells (P = 0.008, Wilcoxon test). CytcE: cytochrome c-EGFP, Casp9E+Casp9: caspase-9 in addition to caspase-9-EGFP. The thin line indicates the mean value. (B) Apoptotic index was calculated from 5 independent experiments. In the case of the cells expressing Casp9E+Casp9, the apoptotic index increased significantly to 48%, from 4% when expressing CytcE (P<0.001, Fisher exact test). The thin line indicates the mean value.
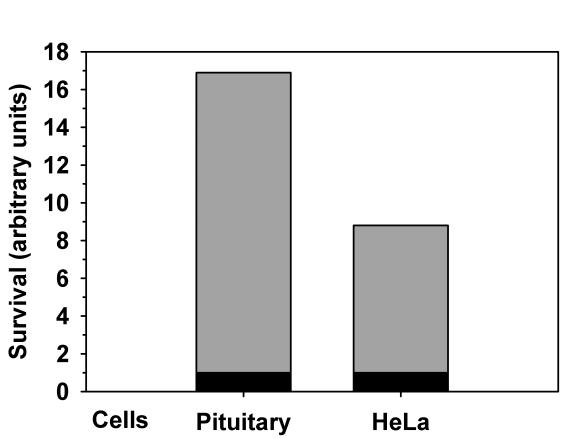
Similar to pituitary cells, overexpression of caspase-9 triggered apoptosis in HeLa cells (Figure 4B). Apoptotic index was significantly lower in cells with overexpressed CytcE than in cells with overexpressed Casp9E+Casp9 (P<0.001, Fisher exact test). To compare the resilience of primary and tumor transformed cells, we calculated the ratios between the surviving fluorescent cells overexpressing CytcE and Casp9E+Casp9 (Figure 5). Pituitary cells were 16 times more likely to die and HeLa cells were about 8 times more likely to die when overexpressing caspase-9 rather than cytochrome c.
Figure 5.
Survival rates of the pituitary and HeLa cells expressing Casp9E+Casp9 and CytcE. All expression levels were normalized to expression levels of Casp9E+Casp9. CytcE: cytochrome c-EGFP; Casp9E+Casp9: caspase-9 in addition to caspase-9-EGFP.
Discussion
We first studied the effects of overexpression of caspase-9 on the cell survival in rat anterior pituitary cells. Apoptosis has an important role in remodeling of anterior pituitary, since the pituitary has to respond continuously to transient but often repeated stimuli such as physiological and psychological stresses, throughout life (25). Therefore, pituitary cells from the anterior lobe are likely to be a good model for studying apoptosis. We showed that overexpression of caspase-9 caused cell death. Necrosis occurred in less than 6% of apoptotic dying cells, both in cells overexpressing caspase-9 and the controls. The apoptosis initiation, due to overexpression of caspase-9 was tested in pituitary cells and HeLa cells. Similar results were obtained from primary astrocytes, though there were on average less than 10 astrocytes expressing Casp9E (our unpublished data). Our results support the finding by Duan et al (20) that overexpression of caspase-9 increased the ratio of dying cancer cells MCF7, however in the case of MCF7, the cause of death was not investigated any further. We conclude that apoptosis initiation by overexpression of caspase-9 is ubiquitous, as it occurs in different types of cells, including tumor cells.
In this study, we overexpressed two components of the apoptosome, cytochrome c, and caspase-9. Only the overexpression of caspase-9 triggered apoptosis through the activation of caspase-9, since the rates of cell death were equal in the cells overexpressing cytochrome c in the presence or absence of the caspase-9 specific inhibitor. Cells that co-expressed caspase-9 and cytochrome c had a higher survival rate than cells that expressed only caspase-9. Cytochrome c, which is not conjugated with heme (apocytochrome c), can inhibit the apoptosome formation in vitro (26). While we cannot rule out that CytcE has an inhibitory effect, this inhibition is not complete, since apoptosis is increased when caspase-9 is co-expressed with CytcE. A possible explanation is that caspase-9 is activated directly by proteolytic processing of a caspase, without the formation of the apoptosome. Although there is a possibility that a caspase other than caspase-9 is activated first, the caspase-9 specific inhibitor z-LEHD.fmk completely prevented apoptosis in transfected cells. The same inhibitor was not able to prevent apoptosis triggered by rotenone (18). Therefore, caspase-9 itself may trigger apoptosis by its overexpression. One possibility is that because of the higher expression levels, the molecules of caspase-9 are brought closer together, which enables the dimerization necessary for their activation. This seems to be in contrast with the observation that it is difficult to achieve a dimerization of caspase-9 in solutions, at least in vitro (27,28). On the other hand, caspase-9 is an exception among the caspases since it can be active even in its procaspase (zymogen) form (27,29). If so, why is apoptosis not triggered by caspase-9 expressed in normal cells? There may be a balance between the expression levels of caspase-9 and its modulators. This is supported by the recent discovery of a dose-dependent inhibition of caspase-9 by HS-associated protein-1 (HAX-1) from cardiac myocytes (30).
The amounts of expressed caspase-9 differ among the tissues of the body. There is more caspase-9 mRNA in the ovaries and testes compared with the spleen, thymus, intestine, or colon (20). Therefore, different expression levels of caspase-9 are likely to have a physiological role. It was reported that too low levels of caspase-9 in tissues result in disturbances in the embryonic development and cause death in mice (12). A lower amount of caspase-9 was established also in the brain of patients with Alzheimer disease (31). Therefore, the amount of expressed caspase-9 must be highly regulated within the cells. A too low amount of this enzyme can result in neurodegenerative diseases or malignancies, while too high an amount can cause programmed cell death. Even mild overexpression of caspase-9 triggers the death of tumor transformed cells. The amount of expressed caspase-9 increases 3-fold to substitute the lack of caspase-2 in caspase-2 null mutants (32). Different expression levels of caspase-9 may be necessary for controlling cell quality and proliferation in the body.
Overexpression of caspases seems to be a common molecular mechanism for triggering apoptosis. Increased synthesis of caspase-2 and caspase-3 was found to precede apoptosis of some leukemic and colon carcinoma cell lines after the application of etoposide (33); in fact the ability of the tumor cells to up-regulate the caspase-2 and caspase-3 genes was related to the sensitivity of the cell lines to drug-induced apoptosis. Also, the treatment with both interferon-gamma and cytotoxic drugs increased caspase-8 expression and triggered apoptosis in the cell lines derived from the Ewing tumor (34). Since the majority of cancer therapy treatments initiate apoptosis through the activation of caspase-9 (16), it is important to study the ways of caspase-9 activation, which can be exploited to control apoptosis in neurodegenerative and malignant diseases.
Acknowledgments
We thank Dr Anna-Lisa Nieminen for the generous gift of plasmid encoding the fusion protein CytcE, Dr Donald Nicholson for a plasmid containing caspase-9, Alenka Frangež Štrukelj for technical assistance and Gaj Vidmar for statistical analysis. This work was supported by grant No. P3-0019 from the Ministry of Higher Education, Science, and Technology of the Republic of Slovenia.
References
- 1.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–6. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 4.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 5.Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–92. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 7.Saleh A, Srinivasula SM, Acharya S, Fishel R, Alnemri ES. Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation. J Biol Chem. 1999;274:17941–5. doi: 10.1074/jbc.274.25.17941. [DOI] [PubMed] [Google Scholar]
- 8.Cain K, Brown DG, Langlais C, Cohen GM. Caspase activation involves the formation of the aposome, a large (approximately 700 kDa) caspase-activating complex. J Biol Chem. 1999;274:22686–92. doi: 10.1074/jbc.274.32.22686. [DOI] [PubMed] [Google Scholar]
- 9.Ritter PM, Marti A, Blanc C, Baltzer A, Krajewski S, Reed JC, et al. Nuclear localization of procaspase-9 and processing by a caspase-3-like activity in mammary epithelial cells. Eur J Cell Biol. 2000;79:358–64. doi: 10.1078/S0171-9335(04)70040-0. [DOI] [PubMed] [Google Scholar]
- 10.Bitzer M, Armeanu S, Prinz F, Ungerechts G, Wybranietz W, Spiegel M, et al. Caspase-8 and Apaf-1-independent caspase-9 activation in Sendai virus-infected cells. J Biol Chem. 2002;277:29817–24. doi: 10.1074/jbc.M111898200. [DOI] [PubMed] [Google Scholar]
- 11.Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–94. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- 12.Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–37. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 13.Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–52. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 14.Mueller T, Voigt W, Simon H, Fruehauf A, Bulankin A, Grothey A, et al. Failure of activation of caspase-9 induces a higher threshold for apoptosis and cisplatin resistance in testicular cancer. Cancer Res. 2003;63:513–21. [PubMed] [Google Scholar]
- 15.Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, et al. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–9. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- 16.Debatin KM. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 2004;53:153–9. doi: 10.1007/s00262-003-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–76. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 18.Potokar M, Milisav I, Kreft M, Stenovec M, Zorec R. Apoptosis triggered redistribution of caspase-9 from cytoplasm to mitochondria. FEBS Lett. 2003;544:153–9. doi: 10.1016/s0014-5793(03)00494-0. [DOI] [PubMed] [Google Scholar]
- 19.Heiskanen KM, Bhat MB, Wang HW, Ma J, Nieminen AL. Mitochondrial depolarization accompanies cytochrome c release during apoptosis in PC6 cells. J Biol Chem. 1999;274:5654–8. doi: 10.1074/jbc.274.9.5654. [DOI] [PubMed] [Google Scholar]
- 20.Duan H, Orth K, Chinnaiyan AM, Poirier GG, Froelich CJ, He WW, et al. ICE-LAP6, a novel member of the ICE/Ced-3 gene family, is activated by the cytotoxic T cell protease granzyme B. J Biol Chem. 1996;271:16720–4. doi: 10.1074/jbc.271.28.16720. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Tabou S, Keller E, Nussinovitch I. Mechanosensitivity of voltage-gated calcium currents in rat anterior pituitary cells. J Physiol. 1994;476:29–39. [PMC free article] [PubMed] [Google Scholar]
- 22.Robins DM, Ripley S, Henderson AS, Axel R. Transforming DNA integrates into the host chromosome. Cell. 1981;23:29–39. doi: 10.1016/0092-8674(81)90267-1. [DOI] [PubMed] [Google Scholar]
- 23.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones HW, Jr, McKusick VA, Harper PS, Wuu KD. George Otto Gey. (1899-1970). The HeLa cell and a reappraisal of its origin. Obstet Gynec. 1971;38:945–9. [PubMed] [Google Scholar]
- 25.Nolan LA, Levy A. Temporally sensitive trophic responsiveness of the adrenalectomized rat anterior pituitary to dexamethasone challenge: relationship between mitotic activity and apoptotic sensitivity. Endocrinology. 2003;144:212–9. doi: 10.1210/en.2002-220241. [DOI] [PubMed] [Google Scholar]
- 26.Martin AG, Nguyen J, Wells JA, Fearnhead HO. Apo cytochrome c inhibits caspases by preventing apoptosome formation. Biochem Biophys Res Commun. 2004;319:944–50. doi: 10.1016/j.bbrc.2004.05.084. [DOI] [PubMed] [Google Scholar]
- 27.Renatus M, Stennicke HR, Scott FL, Liddington RC, Salvesen GS. Dimer formation drives the activation of the cell death protease caspase 9. Proc Natl Acad Sci U S A. 2001;98:14250–5. doi: 10.1073/pnas.231465798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao Y, Shiozaki EN, Srinivasula SM, Rigotti DJ, Fairman R, Shi Y. Engineering a dimeric caspase-9: a re-evaluation of the induced proximity model for caspase activation. PLoS Biol. 2005;3:e183. doi: 10.1371/journal.pbio.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stennicke HR, Deveraux QL, Humke EW, Reed JC, Dixit VM, Salvesen GS. Caspase-9 can be activated without proteolytic processing. J Biol Chem. 1999;274:8359–62. doi: 10.1074/jbc.274.13.8359. [DOI] [PubMed] [Google Scholar]
- 30.Han Y, Chen YS, Liu Z, Bodyak N, Rigor D, Bisping E, et al. Overexpression of HAX-1 protects cardiac myocytes from apoptosis through caspase-9 inhibition. Circ Res. 2006;99:415–23. doi: 10.1161/01.RES.0000237387.05259.a5. [DOI] [PubMed] [Google Scholar]
- 31.Engidawork E, Gulesserian T, Yoo BC, Cairns N, Lubec G. Alteration of caspases and apoptosis-related proteins in brains of patients with Alzheimer's disease. Biochem Biophys Res Commun. 2001;281:84–93. doi: 10.1006/bbrc.2001.4306. [DOI] [PubMed] [Google Scholar]
- 32.Troy CM, Rabacchi SA, Hohl JB, Angelastro JM, Greene LA, Shelanski ML. Death in the balance: alternative participation of the caspase-2 and -9 pathways in neuronal death induced by nerve growth factor deprivation. J Neurosci. 2001;21:5007–16. doi: 10.1523/JNEUROSCI.21-14-05007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Droin N, Dubrez L, Eymin B, Renvoize C, Breard J, Dimanche-Boitrel MT, et al. Upregulation of CASP genes in human tumor cells undergoing etoposide-induced apoptosis. Oncogene. 1998;16:2885–94. doi: 10.1038/sj.onc.1201821. [DOI] [PubMed] [Google Scholar]
- 34.Fulda S, Debatin KM. IFNgamma sensitizes for apoptosis by upregulating caspase-8 expression through the Stat1 pathway. Oncogene. 2002;21:2295–308. doi: 10.1038/sj.onc.1205255. [DOI] [PubMed] [Google Scholar]