Abstract
Aim
To assess the seroprevalence of human metapneumovirus (hMPV) in Croatia.
Methods
During 2005, a total of 137 serum specimens from Croatian patients aged from 6 days to 51 years, without respiratory symptoms, were collected at the Croatian National Institute of Public Health. The sera were examined using the indirect immunofluorescent assay.
Results
The overall hMPV seropositivity rate in the samples tested was 77.4% (106/137). The seropositivity rate increased from 18.7% in children aged between 6 months and 1 year to 100% in people older than 20 years of age. The highest proportion of titers ≥1:512 was found in children aged from 1 to 2 years.
Conclusion
Our results suggest that hMPV infection is present in Croatia, with primary infection occurring in early childhood. This is the first study that indicates the circulation of hMPV in Croatia.
Human metapneumovirus (hMPV) is a newly discovered respiratory virus assigned to the Paramyxoviridae family, Pneumovirinae subfamily, Metapneumovirus genus. It was first isolated in 2001 from nasopharyngeal aspirates obtained from young children in the Netherlands (1). Sequence analysis of several isolates identified two major genetic lineages (subtypes A and B) that can be further divided into subgroups A1, A2, B1, and B2 (2). HMPV causes acute respiratory tract infections in all age groups (3,4). In hospitalized young children, hMPV infection is commonly present as bronchiolitis with or without pneumonitis (5,6), whereas bronchitis, bronchospasm, and pneumonitis are most commonly seen in elderly patients (3). Since the initial report, hMPV has been studied all over the world and it has been reported on all continents (7). Seroprevalence surveys from the Netherlands (1), Japan (8), and Israel (9) indicated that virtually all children are infected by 5-10 years of age. The aim of this study was to demonstrate the presence of hMPV infection in Croatia, by examining sera from Croatian people for specific anti-hMPV antibodies by an indirect immunofluorescent assay (IFA).
Materials and methods
Serum samples
A total of 137 serum specimens from Croatian patients aged from 6 days to 51 years were examined at the Croatian National Institute of Public Health (CNIPH). The study was conducted as a part of the project “Disease Agents with Droplet Route of Spread,” approved by the Ethic Committees of Croatian National Institute of Public Health, University Children’s Hospital Zagreb, and University Hospital for Infectious Diseases. The sera from patients with a diagnosis of acute respiratory infection were excluded. The test samples were randomly selected from a bank of specimens collected from January 1 to December 31, 2005 from patients visiting the CNIPH for neurological (20%), lymphatic (35%), and skin disorders (15%), as well as various other diagnoses, such as hepatic lesions, myocarditis, and thrombocytopenia (30%). Serum samples were divided into six groups, according to the patient’s age: 0-6 months (n = 21), >6 months to 1 year (n = 16), >1-2 years (n = 11), >2-5 years (n = 20), >5-20 years (n = 45), and >20 years (n = 24).
Antigen preparation
LLC-MK2 cells (rhesus monkey kidney, European Collection of Cell Cultures, Feltham, UK) were grown in cell culture medium, consisting of minimal essential medium (MEM; Invitrogen, Paisley, UK), supplemented with 10% fetal bovine serum (Invitrogen, Paisley, UK). When the cell monolayer reached almost 100% confluency, cells were rinsed with phosphate-buffered saline (PBS) and infected with hMPV with addition of trypsin (Sigma-Aldrich, Saint Louis, MO, USA) in concentration of 1 μg/mL. HMPV isolate CAN97-83/A strain was kindly provided by Dr Guy Boivin, Centre de Recherche en Infectologie, Ste-Foy (Quebec), Canada. When cytopathic effect was evident, cells were dried and fixed on glass slides as previously described by Ebihara et al (8).
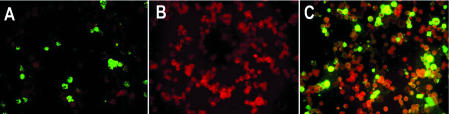
HMPV-infected cells were confirmed by IFA, using mouse monoclonal antibodies against nucleocapsid (N) and fusion (F) protein of hMPV, which reacts both against A and B hMPV genotypes prepared at Rochester General Hospital, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA (Figure 1A). Mock-infected cells were used as negative control (Figure 1B).
Figure 1.
Detection of human metapneumovirus in an indirect immunofluorescent assay. A) positive control (hMPV-infected cells recognized by monoclonal antibodies against N protein of hMPV, KE11-3); B) negative control (mock-infected cells); C) reaction with a positive serum sample.
Indirect immunofluorescence assay
Serum specimens were analyzed for the presence of anti-hMPV antibodies using IFA in the following way. Sera were diluted serially, beginning at 1:8. After incubation of sera with fixed cells at 37°C for 30 minutes, the slides were washed three times in PBS for 10 minutes. The slides were then incubated in the same conditions with commercial, mixed IFA conjugate consisting of goat antihuman IgA, IgG, and IgM antibodies conjugated with fluorescein isothiocyanate (Virion, Rüschlikon, Zürich, Switzerland). Green fluorescence was observed in the cytoplasm and on cell surface, but absent in the nucleus of the cells (Figure 1C). Sera that reacted to hMPV antigens at dilution equal or more than 1:8 were considered positive for hMPV antibodies.
Statistical analysis
Statistical analysis was performed by Fisher exact test using STATISTICA for Windows (StatSoft, Inc. Tulsa, OK, USA). P value <0.05 was considered to be significant.
Results
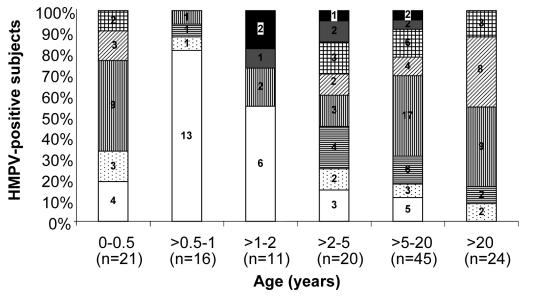
All 137 patients were Caucasians between 6 days to 51 years old. There were 74 men and 63 women. Overall 77.4% (106/137) of the samples tested were positive for hMPV antibodies. The seropositivity rate increased from 18.7% (3/16) in children aged between 6 months and 1 year to 45.5% (5/11) in children aged between 1 and 2 years, and 88.9% (40/45) in subjects aged 5-20 years. In this study, all subjects (24/24) older than 20 years had anti-hMPV antibodies (Figure 2). Statistical analysis by Fisher exact test showed that antibody prevalence was significantly lower in the age group from 6 months to 1 year, compared with antibody prevalence in all groups older than 2 years (P<0.001), but was not significantly different from the 1-2-year old age group (P = 0.206). Of the seropositive subjects, the proportion with titers ≥1:512 in the age groups 1-2 years, 2-5 years, and 5-20 years was 40% (2/5), 5.9% (1/17), and 5.0% (2/40), respectively (Figure 2). The difference between the 1-2-year group and the 5-20-year group was statistically significant (P = 0.024).
Figure 2.
Seroprevalence and antibody titers of human metapneumovirus in Croatia. Open bars – negative; dotted bars – 1:8; bars with horizontal lines – 1:16; bars with vertical lines – 1:32; bars diagonal lines – 1:64; grid bars – 1:128; gray bars – 1:256; closed bars – ≥1:512.
Discussion
The hMPV seropositivity rate in Croatian individuals of 77.4% found in our study is similar to the rate found in a Japanese serologic study performed in 2003 (8) on patients without respiratory symptoms and in Uruguayan study from 2005 (10) performed on healthy donor adults and children without any underlying pathology. In these studies, hMPV seropositivity rates were reported to be 72.5% and 80%, respectively. The seropositivity rate of 100% was found in the population of subjects older than 20 years, in contrast to Dutch and Japanese studies where hMPV seroprevalence reached 100% in the 5-10 age group (1,8). In addition, the results of serologic tests performed in the Netherlands showed that 25% of infants aged 6 months to 1 year were seropositive, increasing to 55% in children 1-2 years old and 70% in those 2-5 years old. Our study demonstrated slightly lower seroprevalence in the first two age groups (18.7%, 45.5%) and a higher seroprevalence in the 2-5 age group (85%).
In our study, 17 of 21 infants under 6 months of age (81%) had hMPV antibodies suggesting the presence of transplacentally derived maternal antibodies. In accordance with this is the lowest prevalence of 18.7%, found in the age group between 6 months and 1 year, when maternal antibodies would be expected to be at their nadir.
Seroprevalence increased with age consistent with increased exposure to hMPV. High titers of hMPV specific antibodies indicate recently acquired infection. Therefore, the highest proportion of titers ≥1:512 was found in the1-2 age group, when infants lack transplacentally derived maternal antibodies and are exposed to hMPV (this may be largely IgM). These findings indicate that the most of hMPV primary infections occur in early childhood (1-2 years of age).
Our results suggest that hMPV infection is present in Croatia, confirming the previous reports of hMPV worldwide distribution (7).
Acknowledgment
We thank Dr Guy Boivin of the Centre de Recherche en Infectologie, Ste-Foy (Quebec), Canada for providing a human metapneumovirus isolate (CAN97-83/A). We also thank Nevenka Mihaliček, Nataša Bauk, Mirela Josipović, Ljiljana Katičić, and Jelena Marić for technical assistance. This research was supported by the Ministry of Science, Education, and Sports of the Republic of Croatia, grant No. 0005002 (to G.M.G.).
References
- 1.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Hoogen BG, Herfst S, Sprong L, Cane PA, Forleo-Neto E, de Swart RL, et al. Antigenic and genetic variability of human metapneumoviruses. Emerg Infect Dis. 2004;10:658–66. doi: 10.3201/eid1004.030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boivin G, Abed Y, Pelletier G, Ruel L, Moisan D, Cote S, et al. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–4. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 4.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–90. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 5.Boivin G, De Serres G, Cote S, Gilca R, Abed Y, Rochette L, et al. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis. 2003;9:634–40. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freymouth F, Vabret A, Legrand L, Eterradossi N, Lafay-Delaire F, Brouard J, et al. Presence of the new human metapneumovirus in French children with bronchiolitis. Pediatr Infect Dis J. 2003;22:92–4. doi: 10.1097/00006454-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Hamelin ME, Abed Y, Boivin G. Human metapneumovirus: a new player among respiratory viruses. Clin Infect Dis. 2004;38:983–90. doi: 10.1086/382536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebihara T, Endo R, Kikuta H, Ishiguro N, Yoshioka M, Ma X, et al. Seroprevalence of human metapneumovirus in Japan. J Med Virol. 2003;70:281–3. doi: 10.1002/jmv.10391. [DOI] [PubMed] [Google Scholar]
- 9.Wolf DG, Zakay-Rones Z, Fadeela A, Greenberg D, Dagan R. High seroprevalence of human metapneumovirus among young children in Israel. J Infect Dis. 2003;188:1865–7. doi: 10.1086/380100. [DOI] [PubMed] [Google Scholar]
- 10.Mirazo S, Ruchansky D, Blanc A, Arbiza J. Serologic evidence of human metapneumovirus circulation in Uruguay. Mem Inst Oswaldo Cruz. 2005;100:715–8. doi: 10.1590/s0074-02762005000700005. [DOI] [PubMed] [Google Scholar]