Abstract
Management of chronic pain by intrathecal delivery is gaining increasing use. The aim of this article is to review the literature pertinent to implantable devices used for treatment of chronic pain, and to highlight what is known. Articles were obtained from Medline database and reviewed. Practical patient selection criteria, trial management, and surgical technique are described. Expert consensus guidelines for intrathecal medication use are also reviewed. Finally, an exhaustive description of known complications and future implications is discussed. We concluded that intrathecal pump seems to be overused, while there is still weak evidence to support its outcome. It is also recommended that future research focus on the outcome, measured by functional parameters rather than commonly used pain scores.
Pain is one of the most common reasons patients visit their doctors (1). If pain persists it not only can cause physical disability, but also place emotional, economic, psychological strain on patients and their families. Chronic pain is exceedingly prevalent. A variety of international studies have estimated that between 10.1%-55.2% of population are afflicted with pain (2). In the United States, it appears that 20 to 30% of the general population experience chronic or recurring pain (3). Approximately, 2/3 of these people have had pain for more than 5 years (4). These findings are consistent with the experience of other western European countries, such as Sweden and Denmark (3). In addition, pain is the most common symptom of patients presenting for palliative care and is present in 67% of patients with metastatic cancer (5).
Pain puts a considerable economic strain on the framework of society through lost productivity, disability, and health care utilization. The cost of chronic pain has been estimated to be as high as $100 billion a year in the United States. In a recent European study on individuals suffering from neuropathic pain, 76% visited their physician at least once in the past month. Employment status was affected in 43% of patients; those working missed an average (±standard deviation) of 5.5 ± 9.8 workdays during the past month (6). A study done in 2000 showed that 36 million workers missed work because of pain and that 83 million felt their pain interfered with participation in daily activities (4). These figures serve to clarify the need for effective long-term treatment modalities for chronic pain.
Intrathecal analgesia
Intrathecal analgesia has a long history marked by advancements in technique and discoveries of new indications. The beginnings of spinal analgesia are set in the late 19th century, soon after the discovery of cocaine as a local anesthetic. In 1898, August Bier produced the first documented spinal analgesia by injecting cocaine into the intrathecal space of himself, his assistant, and 6 patients undergoing operations on the lower part of the body (2,7). The potent anesthetic results were published in a hallmark paper that stirred immense interest and research in this treatment modality.
Among the first to use opioids for intrathecal analgesia was Rudolph Matas, who in 1900 discovered that mixing morphine with cocaine mitigated the adverse symptoms associated with intrathecal cocaine (8). His report was closely followed by that of the Japanese anesthesiologist Otojiro Kitagawa, who in 1901 published the use of intrathecal morphine in the treatment of vertebral inflammation (9).
The scientific study of spinal analgesia began soon after its discovery, but not until the discovery of opiate receptors in the spinal cord, beginning in 1973, was there a scientific rationale for intraspinal delivery of opioid drugs (10-12). Fields and Basbaum described the descending pain inhibition system in the substantia gelatinosa (13). This discovery laid the groundwork for the findings of J. Wang, who in 1979 reported the successful use of intrathecal morphine for treatment of intractable cancer pain (14). The report by Wang sparked increased utilization of spinal opioids.
The first uses of continuous spinal analgesia were demonstrated in the 1940s, providing the avenue for future long-term pain management with spinal opioids. It was introduced clinically in 1979 for obstetric analgesia (15).
The first clinical use of an implantable intrathecal opioid delivery device was demonstrated in 1981 for use in chronic pain of malignancy (8). The efficacy and safety of this modality was supported by a succession of studies, establishing it an alternative therapy for chronic pain states (16-20). The use of opioids in implantable drug delivery systems for chronic non-malignant pain is a more recent application. Although its efficacy has been repeatedly demonstrated, it remains controversial amidst fears of tolerance and drug addiction by many lay persons, government regulators, and even some health care professionals. While morphine, because of its history, duration of action, and ease of use, remains the gold standard of intraspinal analgesia, other agents were added to the repertoire of intraspinal therapy during the last twenty years. Alternative opioids such as hydromorphone, alpha adrenergic agents, and baclofen for neuropathic pain are currently being used.
Patient selection
Patients needing intrathecal drug delivery can be divided into 2 broad categories. The first category includes patients suffering from terminal illnesses such as cancer. These individuals generally respond well to intrathecal opioids if they have been successfully managed on oral opioids first. Invasive therapy should be considered if they have responded well to opioids, but have developed increasing pain and intractable side effects despite rotating oral opioids. However, if patients are terminal and their life expectancy is less than six months, one has to weigh the benefits vs the risks of such a venture knowing that it some times takes to stabilize patients on a given dosing pattern. This type of therapy is generally recommended for individuals with a life expectancy of greater than six months (21). It is important to rule out the presence of occult pathology in the spinal canal and any obstructive metastasis in the spinal column that could present a challenge during insertion of the catheter.
The second category of patients is those with chronic non-malignant pain, for example, failed low back surgery syndrome. The use of intrathecal drug delivery systems in chronic non-malignant pain is more controversial. One has to recognize that chronic non-malignant pain is complicated by physical, psychological, and behavioral factors. To be successful, a treatment must include a multidimensional approach that takes into account each of the elements of the biopsychosocial model. Clearly, treatment for chronic pain should consider conservative approaches before more invasive treatments are considered. These approaches include but are not limited to physical therapy and rehabilitation, psychological and behavioral intervention such as self-relaxation cognitive and behavioral therapies (eg, biofeedback), pharmacotherapy, minimally invasive interventions (such as epidural and transforaminal injections), and alternative therapies such as acupuncture.
Intrathecal drug delivery systems are implanted for chronic pain when conservative therapies have failed, surgery is ruled out, no active or untreated addiction exists, psychological testing indicates appropriateness for implantable therapy, medical contraindications have been eliminated (coagulopathies, infections), and a successful intrathecal drug trial has been completed (22).
Intrathecal pumps
Intrathecal pumps deliver small doses of medication directly to the spinal fluid. It consists of a small battery-powered, programmable pump (Figure 1) that is implanted under the subcutaneous tissue of the abdomen and connected to a small catheter tunneled to the site of spinal entry (Figure 2). Sophisticated drug dose regimens can be instituted. Implanted pumps need to be refilled every 1 to 3 months. There is no evidence showing whether it is more clinically effective to use bolus or continuous dosing.
Figure 1.

Intrathecal pump.
Figure 2.

Intrathecal catheter.
Trial
It is the recommendation of the authors that no intrathecal device should be implanted for pain management of chronic intractable pain without first performing a trial. The properly performed trial phase is of outmost importance. This phase determines whether a patient will benefit from an implant.
During the trial, the planned drug is infused through an indwelling catheter that could be placed intrathecally or epidurally. The advantage of intrathecal placement is that it is more physiologic and mimics what the patient will eventually receive. It is advised that an infusion is done and that the tip of the catheter be placed at or close to the dermatomal level transmitting the nociceptive impulse. The advantage of the epidural trial is avoiding intrathecal entry. It also allows a very specific response when the catheter tip is placed close to the dermatome and a small volume of infusate is used. However, one is to expect more systemic side-effect. Start with a dose of hydromorphone of 0.3mg/d slowly titrating to effect. Alternatively, morphine sulfate could be imitated at 0.2 mg/d with titration to approximately 2 mg/d. The trial is done for two to three days. Results are evaluated depending on patient’s and physician’s expectations. However, it must be said that a mild improvement in somebody’s symptoms should not be interpreted as a positive trial. Typically, one expects at least a 50% improvement in pain score and improvement in function for the trial to be considered successful. It is not known at what dose the trial should be considered a failure. It depends on the dose of opioid the patient was taking prior to the trial. But generally, the above dose range reflects the common practice. The lack of objective interpretation of trials has lead to unnecessary placement of these devices which in turn lead to a low success rate. Placebo trial use is controversial and due to ethical issues is not recommended by the authors.
Surgical technique
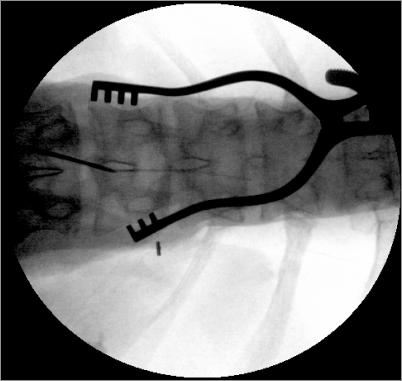
The placement of an implantable intrathecal pump consists of the catheter placement followed by implantation of the pump. The patient is placed in a lateral decubitus position, prepped, and draped in sterile fashion. Antibiotic prophylaxis is given 30 minutes prior to incision and anesthesia is induced. A posterior midline incision approximately 2-3 inches long is made from the skin to the supraspinous fascia at approximately L2-3 or 3-4. A 16g Touhy spinal needle is advanced through the incision into the epidural space. Meticulous attention to hemostasis is needed using electrocautery. The wound may be rinsed with antibiotic irrigation to reduce the risk of infection. A shallow angled paramedian approach with the Touhy needle allows easy rostral advancement of the catheter into the intrathecal space. To prevent catheter kinks, it is important that the needle enter the supraspinous fascia at the rostral end of the incision to ensure that the catheter has room caudally to gently exit and connect to the pump catheter. Some surgeons place a purse-string 2-0 silk suture around the Touhy needle before removing the needle to prevent cerebral spinal fluid (CSF) from entering the subcutaneous tissue creating a hygroma. Positioning of catheter is checked with fluoroscopy (Figure 3). Excessive CSF leaking may require a re-operation to correct the leak. After removing the needle, the catheter is anchored to the supraspinous fascia using a silastic anchor provided by the manufacturer. The anchor is placed around and over the catheter and sutured with interrupted 2-0 or 3-0 silk sutures. It is possible to puncture or occlude the catheter when suturing it. Therefore, before proceeding the end of the catheter should be checked for free flowing CSF and then clamped to prevent excessive leakage.
Figure 3.
Radiographic catheter placement.
Once the catheter is in place, the pump must be prepared for implantation. Careful review of the manufacturer’s recommendations for pump preparation is essential.
After the pump is prepared, an incision for the pump pocket is made in the right or left lower quadrant of the abdomen at or about the umbilical level. The pump should be placed below the belt line, but not too close to the anterior rib or iliac crest as it may lead to prolonged discomfort. Because of refilling requirements, it is important not to place the pump too deep. In an obese patient the pump should be placed at the mid-fat plane of the lower quadrant of the abdomen. If the patient is thin, then the pump may be placed at the rectus fascia. The incision for the pump pocket should be made just large enough to accommodate the pump. After creating the pump pocket, tunneling from the pump pocket to the back wound should be done with the tunneling device. It is vital that the tip of the tunneling device remains subcutaneous to prevent entering the peritoneal cavity or pleural cavity. Gentle subcutaneous guidance of the tip with a gently applied pressure on the skin overlying the tip will usually ensure safe tunneling. Once the tunnel has been created, the catheter can now be pulled through the tunnel to the pump site. The catheter should be measured, trimmed if needed, and connected to the pump. Prior to connecting the catheter, it is important to once again verify the free flow of CSF from the catheter. The pump is now placed into the pocket reservoir side up. Any excess catheter should be placed behind the pump to prevent damage to the catheter during refilling. The pump should be secured to the pocket by suturing to the abdominal fascia under the pump pocket. If there are no loops on the pump, then a Dacron pouch should be carefully placed over the pump and serve as an anchoring device. Over time, fibroblasts invade the Dacron pouch forming a tight fibrous capsule around the pump. Before closing the wounds, it is important to establish adequate hemostasis to prevent the risk of post-operative infections. The wounds should be closed in a two layer closure according to the preference of the surgeon.
After the wounds are closed and the patient is taken to the recovery area, the pump can be programmed to deliver the prescribed amount of medication. Because the pump tubing and catheter are not filled with medication, a bridge bolus must be performed in order to clear the dead space and deliver the prescribed medication dose.
Medications
Medications used in implantable drug delivery systems include opioids, local anesthetics, adrenergic agonists, N-methyl-D-aspartate receptor agonists, and other agents. Choosing among agents can be daunting for clinicians. Consensus guidelines have been developed by an expert panel to guide clinicians when there is a variation in practice and lack of firm evidence in support of either of these interventions. In this article, recommendations regarding medications are coherent with the consensus panel (23).
The first line of treatment includes morphine and hydromorphone, and has clear support from data and extensive clinical experience. Recent studies continue to support the fact that intrathecal morphine provides good analgesia in patients with chronic refractory pain (24,25). Another retrospective study examined the effects of hydromorphone after treatment with morphine found that nausea and drowsiness decreased. Initially lower extremity edema resolved but only temporary. Pain scores remained comparable after the switch from morphine to hydromorphone (26). Table 1 shows recommendations of dosing according to consensus guidelines.
Table 1.
Medication dosing with maximum concentration (27)
| Medication | Dose | Concentration | Comment |
|---|---|---|---|
| Morphine |
15 mg/d |
Max 30 mg/mL |
inflammatory masses |
| Hydromophone |
10 mg/d |
Max 30 mg/mL |
|
| Bupivacaine |
2-30 mg/d |
38 mg/mL |
elderly – blockade |
| Clonidine | 10-1000 μg/d | 2000 μg/mL | hypotension, sedation, peripheral edema, arrhythmia, rebound |
The second line of treatment may actually be chosen as first line in cases where an individual has prominently neuropathic symptoms. This consists of either hydromorphone or morphine with the addition of bupivacaine or clonidine. There is little data to confirm the safety of these mixed agents. Some of the expert panelists have concern regarding the hypotensive symptoms associated with clonidine. There is little evidence to support the efficacy of clonidine or bupivacaine as single agents.
Third line agents show clinical promise but both evidence and clinical experience is extremely limited. Third line drug combinations are chosen only after failure of first and second line drug combination treatments, either due to intolerable side effects or inadequate analgesia. Third line drug combinations include adding both bupivacaine and clonidine to either morphine or hydromorphone. Some case reports have suggested that bupivacaine may be more effective in cancer patients with intractable mechanical and visceral pain with small boluses in addition to basal rate (28). If this is unsatisfactory, another third line drug combination should be considered, for example, adding bupivacaine and clonidine to an alternative opioid, before progressing to fourth line agents.
Fourth line agents are not supported by clinical research evidence and experience, even by the most experienced pain practitioners. Fourth line agents include lipophilic opioid agents such as fentanyl and gamma-aminobutyric acid (GABA) agonists such as baclofen and midolazam. The reasoning behind using lipophilic opioids is that there would be less drug diffusion to the rostral brain centers. Baclofen is a safe and effective drug in the intrathecal space used extensively in the United States in the management of spasticity. Midazolam is used in preservative free solution in Europe, where they have experience with this drug in advanced cancer (29). The formulation in the United States has preservative, and it is not the recommendation of the Expert Consensus panel that it be used without further testing.
There has been advancement in the study of medication safety in the intrathecal space in the last ten years. A number of medications have been proven to be safe. Fentanyl, a potent anilinopiperidine analog for example, was evaluated in several retrospective studies and demonstrated good analgesia and no severe adverse effects (30-32). Methadone has also been studied in the intrathecal space. Three studies, two of which were prospective, demonstrated pain reduction and improvement in QOL scores, in 38 to 80% (33-35).
A double-blind, randomized, crossover, multicenter study was performed in 24 patients with intrathecal pumps with bupivacaine. For four consecutive months, their pumps were refilled with either the original opioid or its mixture with different concentrations of bupivacaine (4, 6, or 8 mg/d). Only one patient experienced mild side effects from intrathecal bupivacaine. It was found that the addition of bupivacaine to the intrathecal opioid failed to produce significant improvement in pain control, though individuals did have improved quality of life (QOL) scores (36). Conversely, in a retrospective study of a mixed population (cancer and failed back syndrome), individuals who failed opioids alone had bupivacaine added to the intrathecal solution. These patients were found to have significantly lower pain scores and also demonstrated a 23% dosage reduction in opioid dosage (37). Finally, adverse effects such as distal limb numbness, orthostatic hypotension, and urinary retention have been reported (36).
Ziconotide is a non-opioid, with several studies examining its efficacy in the intrathecal space. Most recently, a randomized, double-blind, placebo-controlled study of ziconotide in the intrathecal space was completed with 220 patients (38). In this study, a very slow titration was used to decrease side effect profile. Patients had significant pain relief when compared to placebo. It was found that a slower titration of ziconotide, a non-opioid analgesic, to a low maximum dose resulted in significant improvement in pain and was better tolerated than in two previous controlled trials (39) that used a faster titration to a higher mean dose.
Complications and management
Complications from the implantation of an intrathecal pump can be categorized into surgical, mechanical, pharmacological, and medical (endocrine, edema, infections).
Surgical complications
Surgical complications include: bleeding, neurological injury, infection, cerebral spinal leaks, shredded catheters, and malpositioned subcutaneous pockets.
Bleeding
Intra-operative bleeding can occur from ineffective local hemostasis during the procedure. It is important to identify and correct systemic factors prior to surgery by reviewing preoperative medications for prescribed anticoagulants, herbal, and over the counter medications that can increase the risk of bleeding. Patients who are anticoagulated are not candidates for this type of procedure until the coagulation returns to normal. Regional Anesthesia and Pain Medicine guidelines for anticoagulant use during intraspinal therapy should be followed to decrease the risk of bleeding (40).
Deep intraspinal bleeding in the epidural or intrathecal space, while extremely rare, is a much more serious problem and is associated with increased neurological morbidity. The use of fluoroscopy is essential in avoiding periosteum and spinal tumors which can be sources of bleeding during surgery. If bleeding persists, intraoperative neurosurgical consult for possible laminectomy would be necessary. Intraspinal bleeding can occur without the surgeon being aware of it. Significant persistent bleeding can lead to epidural hematoma, spinal cord compression, and paralysis. Post-operatively, it will present first with increasing back pain that rapidly progresses to neurological deficits, including motor weakness, sensory changes, and sphincter dysfunction. Later signs include fever and nuchal rigidity from blood in the subarachnoid space. Emergent magnetic resonance imaging (MRI) or computed tomography (CT) with contrast is warranted, along with a neurosurgical consult for possible epidural hematoma evacuation.
Superficial post-operative bleeding around the wound is another potential complication. A seroma or hematoma can appear at the pump site, presenting with swelling, pressure, and pain. Leakage of sanguinous or serosanguinous fluid may or may not be present from the wound itself. Often the site will have diffuse bruising. This is a self limiting problem that requires monitoring. Abdominal binders are sometimes helpful by applying direct pressure over the site to reduce the swelling and discomfort.
Catheter complications
Intrathecal pump catheter complications are the most common cause of failure in drug delivery (Table 3). Intra-abdominal positioning of the intrathecal pump may lead to the pump catheter neck to prematurely fracture and cause leakage (41).
Table 3.
The antimicrobial drugs of choice for prophylaxis during implantation of intrathecal pump
| Medication | Dose | Time of drug application |
|---|---|---|
| Cefazolin |
1-2 g IV |
30 min prior to incision |
| Clindamycin |
600 mg IV |
30 min prior to incision for B-lactam allergy |
| Vancomycin | 1 g IV | 30 min prior to incision; Methicillin Resistant Staph Aureus (MRSA) history |
Neurologic injury
Neurologic injury can result from the actual catheter placement, as well as from an inflammatory response that occurs at the catheter tip and is associated with drug delivery.
The implantation of spinal catheters for intrathecal drug delivery is done under fluoroscopy. The catheters are inserted through a spinal needle into the spinal canal. Damage to the nerve roots or the spinal cord itself during catheter insertion could occur, resulting in pain, sensory loss, and/or weakness. The deficits would present in the dermatomal distribution of the damaged nerve root. Damage to the spinal cord would lead to dysesthesias or myelopathies below the level of the damaged spinal cord. Also, intraparenchymal injury can occur (42,43), as well as cauda equina syndrome with pain, sensory loss, weakness, and bowel and bladder dysfunction. The deficits would present in multiple dermatomes in a saddle distribution. Neurologic injury can also develop later. One patient developed progressive necrotic myelopathy leading to paraplegia, a rare form of transverse myelitis (44). It is important for the surgeon to obtain pre-operative CT or MRI to check for canal stenosis, arachnoiditis, or other intraspinal abnormalities that would make insertion of the catheter more difficult.
Inflammatory mass
The development of neurologic symptoms can also be associated with the formation of an inflammatory mass at the tip of the spinal catheter. This inflammatory response leads to the development of an expanding sterile mass, known as a granuloma at the tip of the catheter. The practitioner should suspect the development of a granuloma if the pain presents as a new or increasing pain that worsens despite escalating doses of intrathecal opioids or if new neurologic symptoms arise. An MRI should be obtained to confirm the mass around the tip of the catheter. Catheter tip masses are visualized best by using intravenous contrast-enhanced T1-weighted images. The mass will appear as an enhancing lesion having the tip of the drug catheter embedded within it. For patients with contraindications to MRI, a high resolution CT-myelogram provides an alternative for detecting this type of mass (45). Failing to diagnose this condition could lead to permanent neurologic injury (46,47).
While the etiology is not clear, it appears that the use of high concentrations of opioid, morphine specifically, may be the culprit of this complication. Studies have shown that granuloma formation does not occur in patients receiving non-opioid intrathecal infusions, such as baclofen and therefore does not appear to be associated with catheter placement or infusion rates (48). Its onset appears to be within several months of the onset of the utilization of morphine in the intrathecal space. Typically, individuals present with an increase in pain that precedes signs and symptoms of neurologic deterioration (49). There is also a suggestion that clonidine used in combination with opioids may decrease the incidence of this complication. A broad consensus exists within the discipline of pain management that intrathecal opioids should be prescribed and maintained at the lowest effective dose for as long as possible (46). More specifically, it is recommended that the concentrations of the medications be kept as low as possible to decrease the incidence of granuloma formation. There is a causal relationship between intrathecal morphine sulfate infusion and the formation of catheter-tip inflammatory masses. The incidence of inflammatory masses increased with increasing dosage and concentration. In animal models, studies have not yet been able to distinguish between these variables (50-52). Once identified, the treatment and management of this complication is determined by the clinical condition of the patient. A neurosurgical evaluation should be obtained and the risk for neurologic injury assessed. If there are no neurologic deficits, then the pump medication with preservative free saline infused at the minimum pump rate should be replaced. Monthly serial MRIs to observe the regression of the mass is recommended. Once resolved, restart a non-offending opioid and monitor the patient closely for reoccurrence by obtaining an MRI every three months (53). If neurologic deficits are present, then spinal cord decompression, mass removal, and catheter removal are indicated. The pump itself could remain for future use if another catheter placement is determined to be appropriate. Consequently, masses identified early that do not significantly compress neural structures or compromise neurologic function are thought to be treated safely and effectively by removing the opioid from the pump and monitoring the patient until the mass has resolved.
Recent studies in animals by Allen et al (54) demonstrated granuloma formation is possible and predictably able to be elicited with almost all infusions. They infused the maximum concentration of each intrathecal drug used clinically. They demonstrated in dogs, that the agonists morphine, hydromorphone, methadone, and naloxone all could predictably induce granuloma. Fentanyl, interestingly, could not. For agonists that do produce masses, the concentration, rather than the total dose, appears to be an important determinant.
Infection
Preventing infections requires the use of strict sterile techniques, antibiotics, and monitoring. It is wise to use pre-operative and intra-operative antibiotics. Some practitioners advocate the use of intra-operative antibiotic irrigation as well (55). Since most operative infections are caused by staphylococcal infections, cephalosporin or vancomycin are used by most surgeons. The antimicrobial drugs of choice for prophylaxis during implantation are given in Table 2 (56).
Table 2.
Catheter complications from three Meditronic sponsored clinical studies (http://www.medtronic.com/)
| Catheter complication | Patients affected (%) |
|---|---|
| Dislodgement/migration |
6.1 |
| Fracture/break |
5.1 |
| Kink/occlusion |
4.0 |
| Cut/puncture |
3.0 |
| Disconnected from pump |
0.7 |
| Leak |
0.4 |
| Disconnect |
0.3 |
| Misplacement |
0.3 |
| Unknown |
0.1 |
| Tip fibrosis |
0.1 |
| Distal segment in cerebrospinal fluid | 0.1 |
If a patient is a known carrier of methicillin resistant Staphylococcus aureus (MRSA), it is wise to obtain a nasal swab pre-op and treat with vancomycin if found to be positive. Repeat nasal swabs should be done until clear before proceeding with the procedure. Cleansing the skin with hibiclens (chlorhexidine gluconate 4% topical liquid) prior to surgery may also offer some reduction in staphylococcal infections post-operatively.
Wound infections need to be identified early and treated aggressively to prevent serious complications. The practitioner should monitor the patient for increased pain at the surgical site, erythema, tenderness, swelling, drainage, fever, and leukocytosis. Consultants in infectious disease should be involved to determine the appropriate treatment course whenever infection is suspected.
Not all wound infections require the removal of the implanted hardware; superficial infections should be cultured and treated with appropriate antibiotics. More serious infections involving the catheter or pocket will require the removal of all the implanted hardware, followed by appropriate antibiotic therapy. Failure to remove the catheter and pump can lead to ongoing infection and potential progression of the infection. Once the material has been removed, the wound should be left open and wet to dry, with sterile normal saline dressings done until the wound closes on its own.
Infections involving the epidural or intrathecal space require immediate removal of all implanted devices and intravenous antibiotics. Epidural infections can lead to epidural abscess which can compress the thecal sac and cause neurologic injury. If an abscess is suspected, an MRI or CT should be obtained urgently. If positive, all implanted hardware should be removed, the spine decompressed, and appropriate antibiotics started.
Intrathecal infections are rare and present with fever, nuchal rigidity, changes in level of consciousness, leukocytosis, elevated erythrocyte sedimentation rate, and C reactive protein. The diagnosis is confirmed by positive bacterial CSF cultures. Removal of all implanted hardware and appropriate antibiotics treatment is necessary. Late infectious complications can also occur. One case was reported with transverse myelitis associated with an Acinetobacter Baumanii intrathecal pump catheter-related infection. The individual's clinical course improved with the co-administration of intravenous corticosteroids and antibiotics (57). Further, there is one retrospective study that suggests patients with neuropathic pain syndromes, particularly chronic regional pain syndrome had significantly more epidural space infections than patients with somatic pain (58).
Cerebral spinal fluid leaks
Cerebral spinal fluid leaks may occur in as much as 20% of the patients who have an intrathecal drug delivery system placed. The epidural space is accessed with a 17-gauge Tuohy needle followed by the placement of a smaller diameter catheter. When the needle is removed, a CSF leak is likely to occur. Persistent CSF leaks can lead to a post-dural puncture headache. In most instances these headaches will disappear over time. They can be managed conservatively with increased fluids, caffeine intake, and bed rest. However, some patients develop nausea, vomiting, photosensitivity, ringing in the ears, and too severe headache. An autologous epidural blood patch can be done to relieve the symptoms. The procedure should be done under fluoroscopy to avoid damaging the implanted catheter and strict attention to aseptic technique to avoid infection.
Hygroma
In a severe leak a hygroma may develop which is an accumulation of CSF subcutaneously near the dorsal incision. The complication will usually resolve spontaneously in 1-2 weeks. Aspiration of this fluid should be avoided due to the risk of contamination and subsequent infection. A large leak that is draining from the incision may require surgical revision.
Seroma
Formation of seromas is also common around the pump pocket. When a pocket is made, fluid accumulation at the pocket may develop. This can last for 1-2 months and is self-limiting. Abdominal binders are somewhat helpful in reducing the size and discomfort of the seroma and may promote healing. If infection is suspected, the fluid should be aspirated and a Gram stain, culture, and sensitivity obtained. All seromas will contain high levels of white blood cells; therefore bacteria must be present to confirm an infection. If infection is present, the pump reservoir and side port should not be accessed for fear of contamination. An appropriate antibiotic therapy should be initiated and hardware removed.
Medications
Medications errors are a common complication in intrathecal pump drug delivery. Drug refills must be done by trained individuals who are able to accurately assess pain, conduct physical examinations, and assess subtle changes in condition. It is also important that pain management centers must be extremely vigilant about their source of medications. An incident occurred when 8 of 13 patients experienced neurologic complications while receiving morphine in refills of their pumps during one 4-week period in a neurosurgical practice. Three persons underwent laminectomy for sterile abscesses and were left with new paralysis or leg weakness. After investigation, it was found that several bottles from the compounding pharmacy had contaminants (59).
Endocrine
In one retrospective study, it also has been found that patients receiving intrathecal opioids exhibited changes in their neuroendocrine function. In 73 patients with non-cancer pain, the majority of patients developed hypogonadotropic hypogonadism. Fifteen percent developed central hypocortisolism. Ninety-six per cent of men and 69% of women who received intrathecal opioids reported decreased libido. Hormone replacement ameliorated these effects (60).
Tolerance
Drug tolerance can best be described as the need for dose escalation for equivalent effect. There are multiple aspects to drug tolerance. It is thought that there are psychological or learned aspects to drug tolerance. There are also pharmacological or physiological aspects to drug tolerance. There appears to be no drug tolerance to gastrointestinal effects, as those are mediated by direct bowel receptors with less central nervous system control (61). There are multiple adaptations in the spine, including increased activities of sensory neuropeptides (calcitonin gene-related peptide and substance P) and their downstream messengers, prostaglandins, lipooxygenase metabolites, and endocannabinoid (62-64). Evidence is also accumulating that opioid tolerance is inhibited by N-methyl-D-aspartate receptor antagonists. In addition, intracellular cascades including those involving protein kinase C, can contribute to the development of neuroplastic changes that can be associated with degenerative neuronal changes in the spinal cord (65). There are suggestive parallels between these changes and those associated with peripheral nerve injury, leading some researchers to drawing a mechanistic similarity between opioid tolerance and neuropathic pain (66,67). Much research has elucidated the mechanisms of tolerance in animal studies, but there are difficulties in correlating their results with human subjects. Dose escalation in humans is moderated by a number of factors both in the subject and in the provider. Besides that, animal studies are unequivocal in the demonstration of tolerance via multiple mechanisms.
Future outlook
Just like any other technology that comes to replace an existing practice, intrathecal pump implantation has its advocates and its detractors. One concern is whether the elimination of systemic side-effects justifies a major procedure that has its own potential serious complications. In a fee-based medical system, there can be a financial incentive for the physician for placing intrathecal pumps. Conversely, there is a financial burden for the medical system for such an expensive device to be placed and then supported. A Canadian study from 2002 suggested that in patients who responded to this treatment, intrathecal drug treatment for failed low back syndrome is cost-effective in the long term, despite high initial costs of implantable devices (27).
Since there is no solid outcome evidence that supports their use, this article only attempts to highlight and update the achievement in the field so far and perhaps raise questions in the minds of the readers. Especially questions that the pain management community and the governing societies have failed to answer thus far. Randomized studies are still scarce. Funding prioritization from institutes such as the National Institute of Health (NIH) have not matched the need for this growing sector of health care. Therefore, adequate evidence is still missing. In fact, due to its invasive nature, we believe it needs to be spared for patients with advanced malignancies and perhaps select patients with chronic non-malignant pain where large doses of opioids are needed and side-effects limits additional dosing changes.
References
- 1.Kalb C. Taking a new look at pain. Newsweek. May 19, 2003. p. 43.
- 2.Miller RD. Miller’s anesthesia. 6th edition. Philadelphia, PA: Churchill Livingstone; 2004. [Google Scholar]
- 3.Weiner K. Pain issues: Pain is an epidemic. American Academy of Pain Management. Available from: http://www.aapainmanage.org Accessed: January 26, 2007.
- 4.Loeser JD, Butler SH, Chapman CR, Turk DC, editors. Bonica's management of pain. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 5.Smith TJ, Staats PS, Deer T, Stearns LJ, Rauck RL, Boortz-Marx RL, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040–9. doi: 10.1200/JCO.2002.02.118. [DOI] [PubMed] [Google Scholar]
- 6.McDermott AM, Toelle TR, Rowbotham DJ, Schaefer CP, Dukes EM. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain. 2006;10:127–35. doi: 10.1016/j.ejpain.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Bier A. Attempts over Cocainisirung of the Ruckenmarkers. Deutsche Zeitschrift für Chirurgie. 1899;51:361–9. [in German] [Google Scholar]
- 8.Onofrio BM, Yaksh TL, Arnold PG. Continuous low-dose intrathecal morphine administration in the treatment of chronic pain of malignant origin. Mayo Clin Proc. 1981;56:516–20. [PubMed] [Google Scholar]
- 9.Kitagawa O. On spinal anesthesia with cocaine. Japan Society of Surgery. 1901;3:185–91. [Google Scholar]
- 10.Pert CB, Snyder SH. Opiate receptor: demonstration in nervous tissue. Science. 1973;179:1011–4. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- 11.Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357–8. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- 12.Atweh SF, Kuhar MJ. Autoradiographic localization of opiate receptors in rat brain. I. Spinal cord and lower medulla. Brain Res. 1977;124:53–67. doi: 10.1016/0006-8993(77)90863-0. [DOI] [PubMed] [Google Scholar]
- 13.Basbaum AI, Clanton CH, Fields HL. Opiate and stimulus-produced analgesia: functional anatomy of a medullospinal pathway. Proc Natl Acad Sci U S A. 1976;73:4685–8. doi: 10.1073/pnas.73.12.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149–51. doi: 10.1097/00000542-197902000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Alper MH. Intrathecal morphine: a new method of obstetric analgesia? Anesthesiology. 1979;51:378–9. [PubMed] [Google Scholar]
- 16.Coombs DW, Maurer LH, Saunders RL, Gaylor M. Outcomes and complications of continuous intraspinal narcotic analgesia for cancer pain control. J Clin Oncol. 1984;2:1414–20. doi: 10.1200/JCO.1984.2.12.1414. [DOI] [PubMed] [Google Scholar]
- 17.Krames ES, Gershow J, Glassberg A, Kenefick T, Lyons A, Taylor P, et al. Continuous infusion of spinally administered narcotics for the relief of pain due to malignant disorders. Cancer. 1985;56:696–702. doi: 10.1002/1097-0142(19850801)56:3<696::aid-cncr2820560343>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Onofrio BM, Yaksh TL. Long-term pain relief produced by intrathecal morphine infusion in 53 patients. J Neurosurg. 1990;72:200–9. doi: 10.3171/jns.1990.72.2.0200. [DOI] [PubMed] [Google Scholar]
- 19.Follett KA, Hitchon PW, Piper J, Kumar V, Clamon G, Jones MP. Response of intractable pain to continuous intrathecal morphine: a retrospective study. Pain. 1992;49:21–5. doi: 10.1016/0304-3959(92)90183-C. [DOI] [PubMed] [Google Scholar]
- 20.Krames ES, Lanning RM. Intrathecal infusional analgesia for nonmalignant pain: analgesic efficacy of intrathecal opioid with or without bupivacaine. J Pain Symptom Manage. 1993;8:539–48. doi: 10.1016/0885-3924(93)90083-8. [DOI] [PubMed] [Google Scholar]
- 21.Hassenbusch SJ. Cost modeling for alternate routes of administration of opioids for cancer pain. Oncology. 1999;13(5) Suppl 2:63–7. . Williston Park. [PubMed] [Google Scholar]
- 22.Krames ES. Intraspinal opioid therapy for chronic nonmalignant pain: current practice and clinical guidelines. J Pain Symptom Manage. 1996;11:333–52. doi: 10.1016/0885-3924(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 23.Hassenbusch SJ, Portenoy RK, Cousins M, Buchser E, Deer TR, Du Pen SL, et al. Polyanalgesic Consensus Conference 2003: an update on the management of pain by intraspinal drug delivery–report of an expert panel. J Pain Symptom Manage. 2004;27:540–63. doi: 10.1016/j.jpainsymman.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Winkelmuller W, Burchiel K, Van Buyten J. Intrathecal opioid therapy for pain: efficacy and outcomes. Neuromodulation. 1999;2:67–76. doi: 10.1046/j.1525-1403.1999.00067.x. [DOI] [PubMed] [Google Scholar]
- 25.Kumar K, Kelly M, Pirlot T. Continuous intrathecal morphine treatment for chronic pain of nonmalignant etiology: long-term benefits and efficacy. Surg Neurol. 2001;55:79–86. doi: 10.1016/s0090-3019(01)00353-6. [DOI] [PubMed] [Google Scholar]
- 26.Anderson VC, Cooke B, Burchiel KJ. Intrathecal hydromorphone for chronic nonmalignant pain: a retrospective study. Pain Med. 2001;2:287–97. doi: 10.1046/j.1526-4637.2001.01052.x. [DOI] [PubMed] [Google Scholar]
- 27.Kumar K, Hunter G, Demeria DD. Treatment of chronic pain by using intrathecal drug therapy compared with conventional pain therapies: a cost-effectiveness analysis. J Neurosurg. 2002;97:803–10. doi: 10.3171/jns.2002.97.4.0803. [DOI] [PubMed] [Google Scholar]
- 28.Buchser E, Durrer A, Chedel D, Mustaki JP. Efficacy of intrathecal bupivacaine: how important is the flow rate? Pain Med. 2004;5:248–52. doi: 10.1111/j.1526-4637.2004.04039.x. [DOI] [PubMed] [Google Scholar]
- 29.Yaksh TL, Allen JW. The use of intrathecal midazolam in humans: a case study of process. Anesth Analg. 2004;98:1536–45. doi: 10.1213/01.ANE.0000122638.41130.BF. [DOI] [PubMed] [Google Scholar]
- 30.Willis KD, Doleys DM. The effects of long term intraspinal infusion therapy with noncancer pain patients: evaluation of patient, significant-other, and clinic staff appraisals. Neuromodulation. 1999;2:241–53. doi: 10.1046/j.1525-1403.1999.00241.x. [DOI] [PubMed] [Google Scholar]
- 31.Kamran S, Wright BD. Complications of intrathecal drug therapy. Neuromodulation. 2001;4:111–5. doi: 10.1046/j.1525-1403.2001.00111.x. [DOI] [PubMed] [Google Scholar]
- 32.Waara-Wolleat KL, Hildebrand KR, Stewart GR. A review of intrathecal fentanyl and sufentanil for the treatment of chronic pain. Pain Med. 2006;7:251–9. doi: 10.1111/j.1526-4637.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 33.Mironer YE, Tollison CD. Methadone in the intrathecal treatment of chronic nonmalignant pain resistant to other neruoaxial agents: The first experience. Neuromodulation. 2001;4:25–31. doi: 10.1046/j.1525-1403.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 34.Shir Y, Shapira SS, Shenkman Z, Kaufman B, Magora F. Continuous epidural methadone treatment for cancer pain. Clin J Pain. 1991;7:339–41. doi: 10.1097/00002508-199112000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Mironer YE, Haasis JC, Chapple ET. Successful use of methadone in neuropathic pain: A multicenter study by the National Forum of Independent Pain clinicians. Pain Digest. 1999;9:191–3. [Google Scholar]
- 36.Mironer YE, Haasis JC, Chapple I, Brown C, Satterthwaite JR. Efficacy and safety of intrathecal opioid/bupivacaine mixture in chronic nonmalignant pain: A double blind, randomized, crossover, multicenter study by the National Forum of Independent Pain Clinicians (NFIPC). Neuromodulation. 2002;5:208–13. doi: 10.1046/j.1525-1403.2002.02031.x. [DOI] [PubMed] [Google Scholar]
- 37.Deer TR, Caraway DL, Kim CK, Dempsey CD, Stewart CD, McNeil KF. Clinical experience with intrathecal bupivacaine in combination with opioid for the treatment of chronic pain related to failed back surgery syndrome and metastatic cancer pain of the spine. Spine J. 2002;2:274–8. doi: 10.1016/s1529-9430(02)00199-7. [DOI] [PubMed] [Google Scholar]
- 38.Rauck RL, Wallace MS, Leong MS, Minehart M, Webster LR, Charapata SG, et al. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31:393–406. doi: 10.1016/j.jpainsymman.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Staats PS, Yearwood T, Charapata SG, Presley RW, Wallace MS, Byas-Smith M, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63–70. doi: 10.1001/jama.291.1.63. [DOI] [PubMed] [Google Scholar]
- 40.Bergqvist D, Wu CL, Neal JM. Anticoagulation and neuraxial regional anesthesia: perspectives. Reg Anesth Pain Med. 2003;28:163–6. doi: 10.1053/rapm.2003.50133. [DOI] [PubMed] [Google Scholar]
- 41.Dickerman RD, Stevens QE, Schneider SJ. The role of surgical placement and pump orientation in intrathecal pump system failure: a technical report. Pediatr Neurosurg. 2003;38:107–9. doi: 10.1159/000068047. [DOI] [PubMed] [Google Scholar]
- 42.Harney D, Victor R. Traumatic syrinx after implantation of an intrathecal catheter. Reg Anesth Pain Med. 2004;29:606–9. doi: 10.1016/j.rapm.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 43.Huntoon MA, Hurdle MF, Marsh RW, Reeves RK. Intrinsic spinal cord catheter placement: implications of new intractable pain in a patient with a spinal cord injury. Anesth Analg. 2004;99:1763–5. doi: 10.1213/01.ANE.0000136421.69976.AE. [DOI] [PubMed] [Google Scholar]
- 44.Levin GZ, Tabor DR. Paraplegia secondary to progressive necrotic myelopathy in a patient with an implanted morphine pump. Am J Phys Med Rehabil. 2005;84:193–6. doi: 10.1097/01.phm.0000154897.26835.81. [DOI] [PubMed] [Google Scholar]
- 45.Hassenbusch S, Burchiel K, Coffey RJ, Cousins MJ, Deer T, Hahn MB, et al. Management of intrathecal catheter-tip inflammatory masses: a consensus statement. Pain Med. 2002;3:313–23. doi: 10.1046/j.1526-4637.2002.02055.x. [DOI] [PubMed] [Google Scholar]
- 46.Coffey RJ, Burchiel K. Inflammatory mass lesions associated with intrathecal drug infusion catheters: report and observations on 41 patients. Neurosurgery. 2002;50:78–86. doi: 10.1097/00006123-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 47.Langsam A. A case of spinal cord compression syndrome by a fibrotic mass presenting in a patient with an intrathecal pain management pump system. Pain. 1999;83:97–9. doi: 10.1016/s0304-3959(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 48.Yaksh TL. Coffey RJ. Spinal opiate toxicity. Proceedings of ASRA conference; 2004 Nov 18-21, Phoenix, AZ, USA. [Google Scholar]
- 49.Miele VJ, Price KO, Bloomfield S, Hogg J, Bailes JE. A review of intrathecal morphine therapy related granulomas. Eur J Pain. 2006;10:251–61. doi: 10.1016/j.ejpain.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Gradert TL, Baze WB, Satterfield WC, Hildebrand KR, Johansen MJ, Hassenbusch SJ. Safety of chronic intrathecal morphine infusion in a sheep model. Anesthesiology. 2003;99:188–98. doi: 10.1097/00000542-200307000-00029. [DOI] [PubMed] [Google Scholar]
- 51.Yaksh TL, Horais KA, Tozier NA, Allen JW, Rathbun M, Rossi SS, et al. Chronically infused intrathecal morphine in dogs. Anesthesiology. 2003;99:174–87. doi: 10.1097/00000542-200307000-00028. [DOI] [PubMed] [Google Scholar]
- 52.Yaksh TL, Hassenbusch S, Burchiel K, Hildebrand KR, Page LM, Coffey RJ. Inflammatory masses associated with intrathecal drug infusion: a review of preclinical evidence and human data. Pain Med. 2002;3:300–12. doi: 10.1046/j.1526-4637.2002.02048.x. [DOI] [PubMed] [Google Scholar]
- 53.Rauck R. Management of intrathecal pump complications. ASRA conference proceedings; 2005 Nov 17-20; Miami, FL; USA. [Google Scholar]
- 54.Allen JW, Horais KA, Tozier NA, Wegner K, Corbeil JA, Mattrey RF, et al. Time course and role of morphine dose and concentration in intrathecal granuloma formation in dogs: a combined magnetic resonance imaging and histopathology investigation. Anesthesiology. 2006;105:581–9. doi: 10.1097/00000542-200609000-00024. [DOI] [PubMed] [Google Scholar]
- 55.Paice JA, Penn RD, Shott S. Intraspinal morphine for chronic pain: a retrospective, multicenter study. J Pain Symptom Manage. 1996;11:71–80. doi: 10.1016/0885-3924(95)00099-2. [DOI] [PubMed] [Google Scholar]
- 56.Gyssens IC. Preventing postoperative infections: current treatment recommendations. Drugs. 1999;57:175–85. doi: 10.2165/00003495-199957020-00004. [DOI] [PubMed] [Google Scholar]
- 57.Ubogu EE, Lindenberg JR, Werz MA. Transverse myelitis associated with Acinetobacter baumanii intrathecal pump catheter-related infection. Reg Anesth Pain Med. 2003;28:470–4. doi: 10.1016/s1098-7339(03)00222-0. [DOI] [PubMed] [Google Scholar]
- 58.Hayek SM, Paige B, Girgis G, Kapural L, Fattouh M, Xu M, et al. Tunneled epidural catheter infections in noncancer pain: increased risk in patients with neuropathic pain/complex regional pain syndrome. Clin J Pain. 2006;22:82–9. doi: 10.1097/01.ajp.0000151872.97148.f6. [DOI] [PubMed] [Google Scholar]
- 59.Jones TF, Feler CA, Simmons BP, Melton K, Craig AS, Moore WL, et al. Neurologic complications including paralysis after a medication error involving implanted intrathecal catheters. Am J Med. 2002;112:31–6. doi: 10.1016/s0002-9343(01)01032-4. [DOI] [PubMed] [Google Scholar]
- 60.Abs R, Verhelst J, Maeyaert J, Van Buyten JP, Opsomer F, Adriaensen H, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215–22. doi: 10.1210/jcem.85.6.6615. [DOI] [PubMed] [Google Scholar]
- 61.Trang T, Quirion R, Jhamandas K. The spinal basis of opioid tolerance and physical dependence: Involvement of calcitonin gene-related peptide, substance P, and arachidonic acid-derived metabolites. Peptides. 2005;26:1346–55. doi: 10.1016/j.peptides.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 62.Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, et al. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 2000;20:7074–9. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Powell KJ, Ma W, Sutak M, Doods H, Quirion R, Jhamandas K. Blockade and reversal of spinal morphine tolerance by peptide and non-peptide calcitonin gene-related peptide receptor antagonists. Br J Pharmacol. 2000;131:875–84. doi: 10.1038/sj.bjp.0703655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong CS, Chang YC, Yeh CC, Huang GS, Cherng CH. Loss of intrathecal morphine analgesia in terminal cancer patients is associated with high levels of excitatory amino acids in the CSF. Can J Anaesth. 2002;49:561–5. doi: 10.1007/BF03017381. [DOI] [PubMed] [Google Scholar]
- 65.Vanderah TW, Suenaga NM, Ossipov MH, Malan TP, Jr, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001;21:279–86. doi: 10.1523/JNEUROSCI.21-01-00279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mao J, Mayer DJ. Spinal cord neuroplasticity following repeated opioid exposure and its relation to pathological pain. Ann N Y Acad Sci. 2001;933:175–84. doi: 10.1111/j.1749-6632.2001.tb05823.x. [DOI] [PubMed] [Google Scholar]
- 67.Melzack R, Coderre TJ, Katz J, Vaccarino AL. Central neuroplasticity and pathological pain. Ann N Y Acad Sci. 2001;933:157–74. doi: 10.1111/j.1749-6632.2001.tb05822.x. [DOI] [PubMed] [Google Scholar]