Abstract
Aim
To explain the variability in the behavioral response after spinal nerve ligation by investigating the relation between the development of neuropathic pain and the expression of inflammatory indicators, in dorsal root ganglia (DRG) and the spinal nerve.
Methods
Ninety-six male Sprague-Dawley rats were randomly assigned to the modified spinal nerve ligation, sham, and control group. Testing for pain-related behavior identified rats that successfully developed neuropathic pain (responders) and those which did not (non-responders). The extent of neuroinflammation in the two groups was assessed by immunohistochemical staining of dorsal root ganglions glial fibrillary acid protein (GFAP), and rat C3 complement receptor (OX-42).
Results
GFAP and OX-42 immunopositive cell density in the DRG and spinal nerve was significantly higher in hyperalgesic animals. DRG cell density was 3.96 ± 0.68 cells/2500 μm2 in GFAP responders’ group, compared with 2.76 ± 0.75 cells/2500 μm2 in non-responders’ group (Mann-Whitney U test, Z = -3.956, P<0.001). OX-42 density was 7.71 ± 1.03 cells/2500 μm2in responders and 4.75 ± 1.76 cells/2500 μm2 in non responders (Mann-Whitney U test, Z = -2.572, P = 0.01). Hyperalgesic behavior progressively increased during the testing period, although immunopositive cell density peaked on the fourth day post-injury and progressively decreased afterwards.
Conclusion
Our study suggests that inflammation has a decisive role in initiating neuropathic pain. Also, this study confirms that, for the sake of selecting appropriate subjects for mechanistic study, it is necessary to discriminate between experimental subjects that develop pain completely and those that do not.
In the last few decades, many different animal models of neuropathic pain have been developed in order to clarify the pathophysiological mechanisms of neuropathic pain (1). Most of these models are based on direct injury of sensory neurons, which in most cases leads to posttraumatic neuropathic pain, whose foremost important pathophysiological factor is immune activation (2). Therefore, when examining the potential role of immune activation in neuropathic pain, one has to take into consideration both classical immune cells such as macrophages, T lymphocytes, and immunocompetent cells (including endothelial cells, fibroblasts, and Schwann cells), which can release factors that are usually considered exclusively as immune-cell products, and immune-like spinal cord cells (astrocytes and microglia) (2).
To date, studies have generally focused on the dynamics of inflammatory cell migration into nerve tissue (3) and microglial activation (4), but very few have correlated these with the process of neuropathic pain development (5,6). The majority of authors report only the average behavioral response level of all treated animals (7,8), regardless of the fact that there was substantial inconsistency in behavioral response after nerve injury (9,10). Currently, there is no explanation for these behavioral differences in animals that received the same treatment (11).
The purpose of this study was to investigate the variability in the behavioral response after spinal nerve ligation. We focused on neuroinflammation in the dorsal root ganglion (DRG) and spinal nerve proximal to the injury site after spinal nerve ligation, which is characterized by activation of satellite glia cells as resident monocytic cells of the nervous system and the migration of blood circulating inflammatory cells (2,12). We compared the level of neuroinflammation between two groups of rats, one that displayed well-developed neuropathic signs (responders) and the other group that did not (non-responders). Our hypothesis was that local inflammation in the DRG and in the part of the spinal nerve proximal to the injury site differed significantly between responder and non-responder groups. If confirmed, this would suggest that inflammation has a decisive role in initiating neuropathic pain.
Material and methods
All experimental procedures and protocols have been approved by the Ethics Committee of the Split University Medical School. A total of 96 male Sprague-Dawley rats weighing 160-180 g were obtained from the Split University Animal Facility. Rats were randomly assigned to spinal nerve ligation (n = 46), sham (n = 22), and control (n = 28) surgery groups. For all surgical procedures, animals were anesthetized with 3% halothane in oxygen.
Spinal nerve ligation surgery was performed by the common modification of the previously described original method of Kim and Chung (13). A single surgeon performed all animal surgeries in order to avoid the possibility of inter-surgeon variability. Briefly, after exposure of the right paravertebral region, the sixth lumbar transverse process was removed, and the ventral rami of the right L5 and L6 spinal nerves were ligated with 6-0 silk thread and cut distal to the ligature. To minimize non-neural injury, muscles and intertransverse fascia were incised only at the site of the two ligatures, and articular processes were not removed. Sham surgery was performed identically, but without the passage of ligatures or transection of spinal nerves. Animals in the control group only received anesthesia and a lumbar skin incision to blind the observer during behavioral testing.
Animals were brought to animal care facility at least 24 hours before the first testing, which allowed them to get familiarized with the handling and environment. Behavioral testing sessions were performed on the day before surgery and on the fourth, eighth, and fifteenth day after surgery. Our strategy involved measuring changes in pain related behavioral responses over time in different surgery groups. By 22-gauge spinal anesthesia needle we stimulated the plantar skin of each hind paw of unrestrained rats 15 times for 1 second, with enough pressure to indent the skin but not to puncture it (11). As described previously (11), a hyperalgesia-type response (prolonged lifting of the paw, shaking, grooming, licking, and/or chewing of the paw) to noxious mechanical stimulation is the behavioral measure that best distinguishes between spinal nerve ligation and sham groups and, thereby, best identifies animals with successfully induced neuropathic pain. The degree of hyperalgesia was expressed as the difference between the probability of hyperalgesia response of the right and left paw for each animal.
At the end of sensory testing, rats were killed by anesthetic overdose. For the purposes of immunohistochemistry, L5 ganglia, along with a small contiguous part of the spinal nerve, were harvested after the second (4 days after the surgery – early spinal nerve ligation group, n = 7) or fourth (15 days after the surgery – late spinal nerve ligation group, n = 39) behavioral testing session. The control and the sham rat ganglia and spinal nerves were harvested only after the fourth behavioral testing. The tissue was fixed in 4% formaldehyde in 0.1 mmol/L phosphate buffer saline, and embedded in paraffin. Sections of 10 µm thickness were cut and mounted on silanized slides. After deparaffinization, pretreatment with 3% H2O2, and pre-incubation in 10% normal goat or swine serum, sections were then incubated overnight at 4°C with primary antibodies. The following antibodies were used: Glial Fibrillary Acid Protein (GFAP, dilution 1:1000), OX-42 (CD11b) (dilution 1:100), and Pan-T cells (dilution 1:1000) in PBS (Chemicon International Inc., Temecula, CA, USA). GFAP is a marker of activated DRG satellite cells, and OX-42 is another commonly used antibody that recognizes the complement receptor type 3 expression, which is greatly increased in hyperactive spinal microglia, monocyte/macrophages, and some neutrophil cells (3,14). Pan-T recognizes the rat T-cell receptor (TCR), which is present on the membrane of 97% of rat T-cells (15). For secondary detection, sections were incubated with biotinylated affinity purified secondary antibody for 1 hour in a humid box at room temperature, followed by incubation in avidin-biotin-peroxidase complex and 0.05% diaminobenzidine (Calbiochem, EMD Biosciences Inc., San Diego, CA, USA). After the final washes in PBS, sections were mounted and coverslipped. Positive and negative control staining was also performed.
For each section examined, we acquired 2-3 images by digital camera Olympus DP-11 (Olympus America, Mellville, NY, USA) using the same magnification, shutter speed, and digital gain for each image. The images were scrambled to blind the investigator to the source of the tissue. Each micrograph was divided into 50 × 50 μm squares. Mean number of immunopositively stained cells represented as stained areas per square was calculated by image-analysis software DP-Soft 3.1 (Olympus).
Statistical analysis
Behavioral test scores were analyzed with repeated-measurement ANOVA and one-way ANOVA. The GFAP and OX-42 immunopositive cell counts were analyzed using either Mann-Whitney U test or Kruskal-Wallis test followed by Dunn post hoc test. Significance levels were set at 0.05.
Results
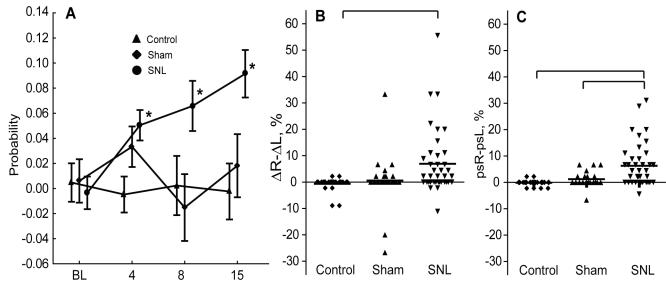
Spinal nerve ligation injury resulted in a significant increase in a hyperalgesia response probability. Before surgery, the mean baseline hyperalgesia response probability was similar in all groups of rats and there was no significant difference in the hyperalgesia response probability between right and left hind paws (Figure 1A). Following spinal nerve ligation surgery, the right hind paw hyperalgesia response probability increased progressively compared with that of the contralateral hind paw and the hind paw of sham operated and control rats, which remained at approximately pre-operative levels. Statistical analysis of the interaction between the type of surgery and time showed a significant difference between groups (repeated-measurement ANOVA; F = 2.18, P = 0.045). Post hoc comparisons revealed that spinal nerve ligation surgery induced a statistically significant increase in hyperalgesia response probability on all post-surgery testing days compared with the control group.
Figure 1.
(A) Postsurgical changes in probability of hyperalgesia response to stimulation by 22 gauge needle. Each value represents the mean difference in hyperalgesia response probability between the right and left paws. Data are presented as mean ± standard error of the mean. (B) ΔR-ΔL score represents the injury effect on the right side compared with the left as a percent averaged over the three postoperative testing sessions, and was calculated as 100 × [(average right postsurgical P)-(right baseline P)]-100 × [(average left postsurgical P)-(left baseline P)], where P is the probability of a hyperalgesia response. (C) psR–psL score represents postsurgical asymmetry and is calculated as 100 × [(average right postsurgical P)-(average left postsurgical P)]. On panels B and C means are reported with horizontal line. Asterisk denotes significant difference from baseline at a time point. The difference was analyzed by analysis of variance with Bonfferoni post hoc test; brackets denote post-hoc differences between groups. BL – baseline testing; SNL – Spinal nerve ligation
A subset of spinal nerve ligation operated animals did not show any signs of hyperalgesia. To identify these rats, we averaged each animal’s post-surgical responses over 3 separate testing sessions, and plotted them as behavioral changes referred to baseline values (ΔR-ΔL score) (Figure 1B) or simply as values of post-surgical behavioral asymmetry (psR-psL score) (Figure 1C). The score ΔR-ΔL on the right side represents the injury effect compared with the left as a percent, and is calculated as 100 × [(average right postsurgical P)-(right baseline P)]-100 × [(average left postsurgical P)-(left baseline P)], where P is the probability of a hyperalgesia response. The score psR–psL represents postsurgical asymmetry and is calculated as 100 × [(average right postsurgical P)-(average left postsurgical P)]. In both cases, the mean probability of a hyperalgesia response was markedly higher in the spinal nerve ligation than the control group, while the spinal nerve ligation group significantly differed from the sham group only when the hyperalgesia response was evaluated without referring it to the baseline values. Therefore, for the balance of the study, we considered only the hyperalgesia response probability asymmetry without comparing it to the baseline values.
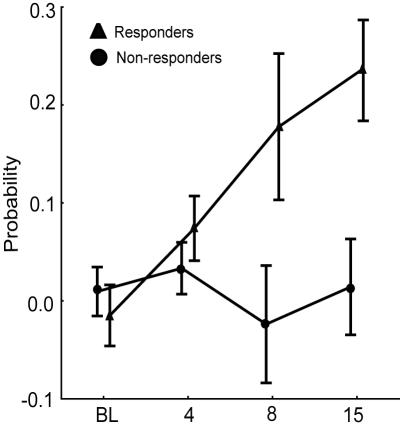
Hyperalgesia response probability values of the rats with late spinal nerve ligation showed a tendency to group into two clusters. Consequently, we divided the late spinal nerve ligation rats into two separate subgroups: those which had hyperalgesia probability value ≥5 and were considered to have developed hyperalgesia after spinal nerve ligation surgery (responders; n = 17, 43.6%) and those which had hyperalgesia probability value <5 and were considered not to have developed hyperalgesia after spinal nerve ligation surgery (non-responders; n = 22, 56.4%) (Figure 1C). The statistical analysis confirmed a significant difference in hyperalgesia response probability between responders and non-responders when grouped this way (Mann-Whitney U test, Z = -6.959, P<0.001). Figure 2 shows hyperalgesia probabilities in responders and non-responders on different testing sessions.
Figure 2.
The difference in probability of hyperalgesia response between responders and non-responders groups on different testing sessions. Data are presented as mean ± standard error of the mean.
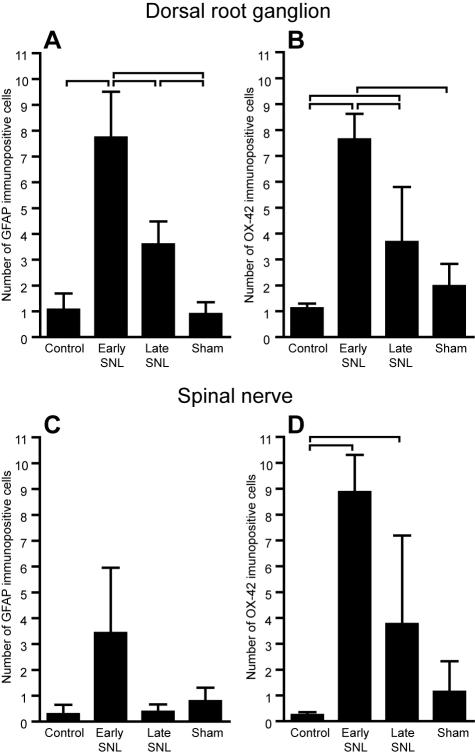
After immunohistochemical staining, we also examined if there was a significant difference in neuroinflammatory reaction between different surgery groups, as well as between responder and non-responder subgroups. Nerve injury led to an overall increase in GFAP and OX-42 immunopositive cell count in the L5 DRG after spinal nerve ligation, but not after sham surgery (Kruskal-Wallis test; for GFAP H = 82.42, P<0.001; for OX-42 H = 36.45, P<0.001) (Figure 3 A and B). This confirms that simple nerve manipulation, as was done in sham operated animals, is unable to provoke a significant neuroinflammatory response of ipsilateral DRG. For both GFAP and OX-42, the peak of immunopositivity was found on the fourth day (early spinal nerve ligation group), when the immunopositive cells were also most heavily stained (Figure 4). With Pan-T antibodies, only occasional lymphocytes were identified. Oval or irregularly shaped GFAP and OX-42 positive cells were also found among nerve fibers that pass among the islands of neuronal somata. The number of such cells differed significantly among different surgery groups (Kruskal-Wallis test; for GFAP H = 8.28, P = 0.0406; for OX-42 H = 17.99, P<0.001) (Figure 3 C and D). Peak values were again found in early spinal nerve ligation group.
Figure 3.
Mean number of glial fibrillary acid protein (GFAP) (A) and OX-42 (B) immunopositive cells in DRGs from different surgery groups. (C) and (D) show mean number of GFAP and OX-42 immunopositive cells in spinal nerve, respectively. All DRGs and spinal nerves were harvested 15 days after surgery, except early spinal nerve ligation (SNL) rats that were killed 4 days after the surgery, regardless of behavioral testing results (responders and non-responders). Data are reported as mean (±standard deviation) number of GFAP and OX-42 immunopositive cells counted in the ipsilateral L5 dorsal root ganglion and adjacent spinal nerve. Brackets denote Bonfferoni post-hoc differences between groups.
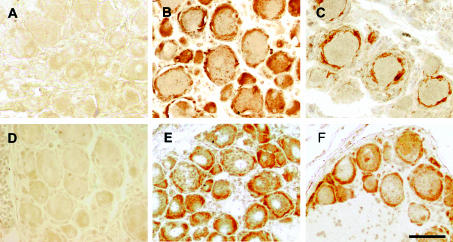
Figure 4.
Glial fibrillary acid protein (GFAP) and OX-42 immunoreactivity in dorsal root ganglion (DRG) after spinal nerve ligation. (A) Micrograph of the GFAP stained control group DRG section; GFAP stained DRG section harvested 4 (B), and 15 (C), days after spinal nerve ligation; (D) OX-42 stained control group DRG section; OX-42 stained DRG section harvested 4 days (E) and 12 days (F), after spinal nerve ligation. Scale bar, 50 µm.
Statistical analysis further indicated a significant difference in GFAP and OX-42 immunopositivity between responder and non-responder subgroups after spinal nerve ligation. Density of immunopositive DRG cells was 3.96 ± 0.68 cells/2500 μm2 in GFAP responders’ group, compared with 2.76 ± 0.75 cells/2500 μm2 in non-responders’ group (Mann-Whitney U test, Z = -3.956, P<0.001). The OX-42 positive cell density was 7.71 ± 1.03 cells/2500 μm2 in responders and 4.75 ± 1.76 cells/2500 μm2 in non responders (Mann-Whitney U test, Z = -2.572, P = 0.01). These results do not show a clear distinction between activated satellite glia and circulating activated macrophages in the DRG and the nerve proximal to the nerve injury after the spinal nerve ligation surgery.
Discussion
Our study showed that spinal nerve ligation was a valid neuropathic pain trigger, but not all rats subjected to spinal nerve ligation surgery necessarily developed neuropathic pain. The sensory testing showed that the spinal nerve ligation pain model induced significant hyperalgesia, while sham surgery did not. These findings support our earlier report (11). Although most studies report only the average response level, we showed the inconsistency in behavior after peripheral nerve injury, which was also demonstrated by other authors (9,10). This observation has been confirmed in clinical setting (16). To our knowledge, the present study is the first to demonstrate an injury-induced difference in DRG and spinal nerve neuroinflammation between animals that developed neuropathic pain after nerve injury (responders) and those that did not (non-responders). In a similar study, Kim et al (17) showed that an increase in spinal levels of vasoactive instestinal polypeptide and neuropeptide Y after peripheral nerve injury was not sufficient for the development of neuropathic pain. In their study, only 25 (22.32%) out of 112 operated animals developed robust signs of neuropathic pain, which supports our current findings. Although our results showed that there was a significant difference in the number of activated neuroinflammatory cells between responders and non-responders, the unpredictable effect of peripheral nerve injury on pain behavior may be the result of many additional contributing factors such as anatomic variability in neural pathways and peripheral nerve distribution, difference in the extent of adjacent tissue damage and inflammation due to uncontrolled differences in the degree of local injury, variable responsiveness of different subject cohorts to injury, and different environmental factors (11).
We examined the correlation of behavior with neuroinflammation caused by spinal nerve ligation. We used GFAP as a marker of activated satellite cells and OX-42 as a marker of activated spinal microglia, monocyte/macrophages, and some neutrophil cells (3,14). Antibodies to the rat T-cell receptor, which is present on the membrane of almost all of rat T-cells, were also used (15). However, only occasional immunopositive T-cells were seen. Similar results have been reported by Lu et al (18), although other authors described more consistent lymphocyte infiltration of DRGs (19).
Our findings, together with previous observations (14,20-22), indicate that the increase in the GFAP and OX-42 immunopositve cell counts in DRGs, spinal nerves or the dorsal horn of the spinal cord correlates with the onset of hyperalgesia after nerve injury, and that the peak immunopositive cell count may be found between the third and tenth day after the nerve injury, with a later significant count decrease. Although the majority of studies have focused on spinal cord inflammatory infiltration, a lack of blood-tissue barrier in the DRG (12) leads us to focus on inflammation of the DGR and spinal nerve, where resident immunologic response cells may interact with circulating ones. We suspect that the increased number of OX-42 cells in the DRG and spinal nerve represents an activation or phenotypic shift of satellite glial cells, but we cannot determine from the present observations whether their increased number may be in part the result of recruitment of hematogenous monocytes (23). A substantially lower effect of injury on GFAP expression among nerve fibers probably occurs because activated neuroinflammatory cells are mostly localized in the proximity of neuronal somata, while OX-42 positive cells are uniformly distributed between the nerve fiber and somata regions (Figure 3 C and D). Our results also show that, even though the peak inflammation cells count of the DRG and spinal nerve was registered on the fourth day post-injury, with a decrease thereafter, behavioral testing showed a continuous linear increase in hyperalgesic response. This suggests that inflammation may be a trigger for neuropathic pain development, which afterwards has a self-maintaining mechanism.
In conclusion, we observed a significantly higher overall neuroinflammatory cell density in rats with spinal nerve ligation surgery that successfully developed pain than in those that did not develop pain. A progressive increase of pain behavior is registered after injury, although the neuroinflammatory infiltrate starts to decrease 4 days after injury. These results suggest that neuroinflammation may be an important pain initiating trigger, and that the failure to fully develop an inflammatory response limits the expression of neuropathic pain behavior. Further studies, especially mechanistic ones, should include behavioral testing as an inclusion criterion to assure the selection of appropriate subjects that have successfully developed pain after nerve injury.
Acknowledgments
We are grateful to Mrs Asja Miletić for her skilful technical assistance. This work was supported by the Ministry of Science, Education, and Sports of the Republic of Croatia (grants No. 0216001 and 216-2160528-0522).
References
- 1.Hogan Q. Animal pain models. Reg Anesth Pain Med. 2002;27:385–401. doi: 10.1053/rapm.2002.33630. [DOI] [PubMed] [Google Scholar]
- 2.Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 3.George R, Griffin JW. Delayed macrophage responses and myelin clearance during Wallerian degeneration in the central nervous system: the dorsal radiculotomy model. Exp Neurol. 1994;129:225–36. doi: 10.1006/exnr.1994.1164. [DOI] [PubMed] [Google Scholar]
- 4.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–2. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 5.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Zhang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, et al. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain. 2005;118:125–36. doi: 10.1016/j.pain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–94. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Eschenfelder S, Blenk KH, Janig W, Habler H. Spontaneous activity of axotomized afferent neurons after L5 spinal nerve injury in rats. Pain. 2000;84:309–18. doi: 10.1016/s0304-3959(99)00211-0. [DOI] [PubMed] [Google Scholar]
- 9.Cui JG, Holmin S, Mathiesen T, Meyerson BA, Linderoth B. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain. 2000;88:239–48. doi: 10.1016/S0304-3959(00)00331-6. [DOI] [PubMed] [Google Scholar]
- 10.Kontinen VK, Paananen S, Kalso E. The effects of the alpha2-adrenergic agonist, dexmedetomidine, in the spinal nerve ligation model of neuropathic pain in rats. Anesth Analg. 1998;86:355–60. doi: 10.1097/00000539-199802000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101:476–87. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber RC, Shadiack AM, Bennett TA, Sedwick CE, Zigmond RE. Changes in the macrophage population of the rat superior cervical ganglion after postganglionic nerve injury. J Neurobiol. 1995;27:141–53. doi: 10.1002/neu.480270203. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 14.Hu P, McLachlan EM. Inflammation in sympathetic ganglia proximal to sciatic nerve transection in rats. Neurosci Lett. 2004;365:39–42. doi: 10.1016/j.neulet.2004.04.077. [DOI] [PubMed] [Google Scholar]
- 15.Hunig T, Wallny HJ, Hartley JK, Lawetzky A, Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. Differential reactivity with subsets of immature and mature T lymphocytes. J Exp Med. 1989;169:73–86. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali Z, Meyer RA, Belzberg AJ. Neuropathic pain after C7 spinal nerve transection in man. Pain. 2002;96:41–7. doi: 10.1016/s0304-3959(01)00413-4. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Back SK, Kim J, Sung B, Hong SK, Na HS. Increases in spinal vasoactive intestinal polypeptide and neuropeptide Y are not sufficient for the genesis of neuropathic pain in rats. Neurosci Lett. 2003;342:109–13. doi: 10.1016/s0304-3940(03)00254-4. [DOI] [PubMed] [Google Scholar]
- 18.Lu X, Richardson PM. Responses of macrophages in rat dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1993;22:334–41. doi: 10.1007/BF01195557. [DOI] [PubMed] [Google Scholar]
- 19.Hu P, McLachlan EM. Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience. 2002;112:23–38. doi: 10.1016/s0306-4522(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 20.Inoue K, Tsuda M, Koizumi S. ATP- and adenosine-mediated signaling in the central nervous system: chronic pain and microglia: involvement of the ATP receptor P2X4. J Pharmacol Sci. 2004;94:112–4. doi: 10.1254/jphs.94.112. [DOI] [PubMed] [Google Scholar]
- 21.Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol. 2001;439:127–39. [PubMed] [Google Scholar]
- 22.Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–75. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 23.Smith ML, Jr, Adrian EK., Jr On the presence of mononuclear leucocytes in dorsal root ganglia following transection of the sciatic nerve. Anat Rec. 1972;172:581–7. doi: 10.1002/ar.1091720311. [DOI] [PubMed] [Google Scholar]