Abstract
Aim
To evaluate risk factors related to total mortality in an unselected population of patients implanted with a cardioverter defibrillator.
Methods
Survival analysis was performed retrospectively investigating the records of 77 consecutive patients implanted with defibrillators (median 67 years, range 38-83 years; 63 men). All patients were followed regularly in 3-month intervals. The cause of mortality was assessed clinically, including post-mortem examination of device to assess possible arrhythmogenic death. Predictors were evaluated by Kaplan-Meier analysis with log-rank tests and by Cox regression analysis (proportional hazards).
Results
Defibrillator recipients had a mean (±SD) ejection fraction of 34 ± 13%, left ventricular end-diastolic dimension (LVEDD) of 6.24 ± 0.8 cm, QRS duration of 129 ± 34 ms, and body mass index (BMI) of 26.4 ± 4.3 kg/m2. Atrial fibrillation was present in 32 patients, paroxysmal fibrillation in 23, and permanent fibrillation in 9 patients. The estimate of mean survival time for all patients was 51.5 (95% confidence interval 46.6-56.5) months. During the study period 11/77 (14%) patients died. Mean follow-up time was 24.5 months (range 0.2-60.7) for survivors and 7.6 months (range 1.5-42) for non-survivors. Independent predictors of mortality were the NYHA class (P = 0.004), BMI≤26 kg/m2 (P = 0.024), presence of paroxysmal or permanent atrial fibrillation (P = 0.014), and absence of arterial hypertension (P = 0.010). LVEDD showed a weak significant effect on survival (P = 0.049).
Conclusion
Patients with implantable cardioverter defibrillator and a normal to lower BMI or atrial fibrillation had a significantly higher overall mortality. These factors may be indicative of end stage heart failure or diseases associated with high sympathetic activation.
Patients with implantable cardioverter defibrillator, in addition to an intrinsic residual risk of sudden cardiac death due to a reduced effectiveness of defibrillators, have a high incidence of co-morbidities, which increase mortality in this population. Several studies have demonstrated that the implantation of a cardioverter defibrillator for both secondary and primary prevention significantly reduces total mortality by preventing sudden cardiac death (1-3). However, the death rate from non-arrhythmic causes among patients with implantable cardioverter defibrillator is substantial (4) and deserves further investigation. The purpose of this study was to investigate various risk factors with respect to total mortality in an unselected population of patients with implanted cardioverter defibrillator devices.
Subjects and methods
Subjects
We followed-up clinical characteristics of 77 patients (median age 67 years, range 38-83 years; 63 men and 14 women) who received an implantable cardioverter defibrillator at our institution between 2001 and 2005. All patients gave written informed consent for implantation of the device. The survival analysis was carried out retrospectively investigating the records of the patients implanted with defibrillators. Treatment with an implantable cardioverter defibrillator was indicated for secondary prevention of sudden death in 72 patients. Ventricular tachycardia was documented in 51 (66%) and ventricular fibrillation in 21 (27%) patients. An implantable cardioverter defibrillator was inserted for prophylactic indication in 5 patients based on the MADIT-II criteria (2). Single-chamber implantable cardioverter defibrillator was implanted in 43 patients, dual-chamber implantable cardioverter defibrillator in 29 patients, and cardiac-resynchronization defibrillator in 5 patients with advanced heart failure. Patients with no indications for antibradycardia pacing received a single-chamber defibrillator (n = 41), which was programmed to back up pacing at a lower rate limit of 40 beats per minute in order to avoid right ventricular pacing. In 13 patients, a dual-chamber defibrillator was implanted, mainly for enhanced discrimination of atrial from ventricular arrhythmias, but programmed to dual chamber pacing/dual chamber sensing/inhibited response to sensing (DDI) back-up mode. In patients with indications for dual chamber pacing, defibrillators were programmed either to dual chamber pacing/dual chamber sensing/dual response to sensing/rate modulation (DDDR) mode at a lower rate limit of 60 beats per minute in 9 patients or to a functional atrial pacing/atrial sensing/inhibited response to sensing/rate modulation (AAIR) mode in 7 patients favoring intrinsic ventricular conduction. Only 2 patients with permanent atrial fibrillation were set to a lower rate limit of 60 beats per minute in ventricular pacing/ventricular sensing/inhibited response to sensing/rate modulation (VVIR) mode.
Methods
Clinical assessment of co-morbidities and weight and height measurement were performed at the time of device implantation. At this stage, all ICD recipients were without any signs of overt heart failure. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Hypertension was defined by systolic and/or diastolic blood pressure ≥130 and/or ≥85 mm Hg or if the patient was receiving antihypertensive medications. Resting heart rate was derived from surface electrocardiogram (ECG), recorded before device implantation. Atrial fibrillation was defined as a history of electrocardiographically documented paroxysmal or permanent atrial fibrillation. Renal insufficiency was defined by baseline creatinine concentration of ≥132 µmol/L. Diabetes mellitus was defined by a fasting plasma glucose level of ≥7 mmol/L or if the patient was undergoing antidiabetic treatment. Diagnosis of chronic obstructive pulmonary disease was based on abnormal spirometry results. A history of myocardial infarction was defined as a previous cardiac event with elevated peak creatine kinase-MB fraction and/or new electrocardiographic changes suggestive of acute myocardial infarction. After cardioverter defibrillator implantation, all patients were followed on a regular 3-month basis at our outpatient clinic. The primary cause of death refers to the event that led to death. Mode of death was assessed clinically. In addition, defibrillator devices were examined to establish whether the cause of death was arrhythmogenic or non-arrhythmogenic. Overall, the deaths were classified as cardiac or non-cardiac.
Statistical analysis
Analyses were performed using the Statistical Package for the Social Sciences, version 12.0 (SPSS Inc., Chicago, IL, USA) and PEPI 4.0 software (Salt Lake City, UT, USA). Characteristics of subjects were presented as medians and range or as means and standard deviations. Kolmogorov-Smirnov test was used to check the normal distribution of data. Unpaired t tests or Mann-Whitney test were used for comparison of continuous variables and Fisher exact test was used for nominal parameters. Survival curves were generated by the Kaplan-Meier procedure and compared using the log-rank test. Significant variables affecting the outcome were incorporated into a multivariate Cox proportional-hazards regression model. P-value ≤0.05 was considered statistically significant.
Results
Study population
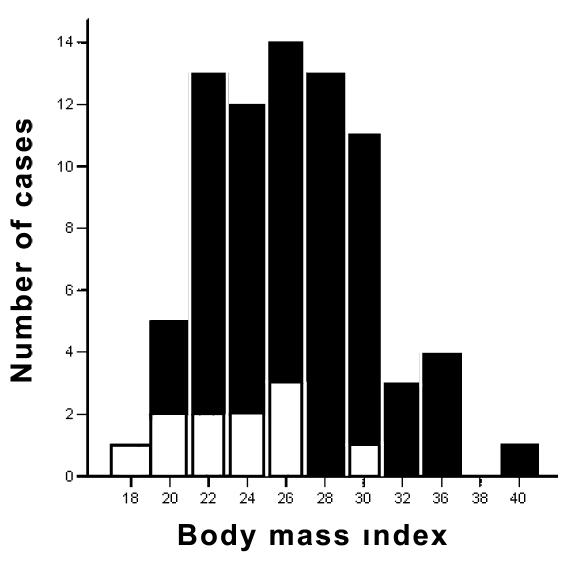
The baseline clinical characteristics of the patient cohort are summarized in Table 1 (5,6). Mean ejection fraction was 34 ± 13%, mean QRS duration 129 ± 34, and median BMI 26.1 kg/m2 (range 17.3–40.1). For survivors median BMI was 26.6 kg/m2 (range 19.5-40.1) and 24.0 kg/m2 (range 17.3-30.1) for non-survivors (Figure 1). Non-survivors had significantly lower BMI (P = 0.015), higher NYHA functional class (P = 0.014), greater LVEDD (P = 0.008), and higher prevalence of atrial fibrillation (P = 0.007). Notably, only 2 out of 11 (18%) patients in the non-survivor group had a history of arterial hypertension, compared with 40 out of 66 (61%) in the survivor group (P = 0.014) There were no significant differences in the use of amiodarone, beta blocker, or lipid lowering drug therapy. Device examination revealed appropriate antitachycardia pacing and/or shock therapy in 43 (56%) patients, but there were no differences between survivors and non-survivors. Patients receiving appropriate discharges had lower BMI than patients without defibrillator therapies (25.1 kg/m2, range 19.5-36.3 vs 27.4 kg/m2, range 17.3-40.1, respectively; P = 0.096). Inappropriate shocks were delivered to 9 patients (8 survivors and 1 non-survivor) because of rapidly conducted supraventricular tachycardias and lead fracture in 1 patient. Inadequate defibrillator therapies were more frequent in patients with single-chamber than dual-chamber devices(6/43 vs 3/29 patients, respectively; P = 0.262).
Table 1.
Clinical baseline characteristics of the patients with implantable cardioverter defibrillator according to survival
| Patients |
P† |
|||
|---|---|---|---|---|
| Variable* | total (n = 77) | survivors (n = 66) | non-survivors (n = 11) | |
| Age, years (range) |
66 (38-83) |
66 (38-83) |
67 (46-80) |
0.839 |
| Male/female, No. (%) |
63/14 (82/18) |
54/12 |
9/2 |
1.000 |
| Body mass index, kg/m2 |
26.4 ± 4.3 |
26.9 ± 4.3 |
23.5 ± 3.5 |
0.015 |
| Ejection fraction, % |
34 ± 13 |
35 ± 14 |
30 ± 7 |
0.127 |
| Left ventricular end-diastolic dimension, cm |
6.24 ± 0.8 |
6.14 ± 0.8 |
6.86 ± 0.7 |
0.008 |
| Mitral regurgitation, No. (%): |
0.136 |
|||
| none |
9 (12) |
8 (10) |
1 (1) |
|
| mild |
43 (56) |
38 (49) |
5 (6) |
|
| moderate |
22 (28) |
19 (25) |
3 (4) |
|
| severe |
3 (4) |
1 (1) |
2 (3) |
|
| Heart rate, beats per minute |
69.9 ± 11.1 |
69.7 ± 10.7 |
70.6 ± 13.9 |
0.804 |
| QRS duration, ms |
129 ± 34 |
127 ± 34 |
137 ± 27 |
0.351 |
| NYHA functional class, No. (%):‡ |
0.014 |
|||
| I |
15 (20) |
15 (20) |
0 |
|
| II |
35 (45) |
31 (40) |
4 (5) |
|
| III |
22 (29) |
18 (23) |
4 (5) |
|
| IV |
5 (6) |
2 (3) |
3 (4) |
|
| Etiology, No. (%): |
0.281 |
|||
| ischemic cardiomyopathy |
50 (65) |
43 (56) |
7 (9) |
|
| non-ischemic cardiomyopathy |
17 (22) |
13 (17) |
4 (5) |
|
| idiopathic dilated cardiomyopathy |
15 (19) |
15 (19) |
0 |
|
| valvular cardiomyopathy |
2 (3) |
1 (1) |
1 (1) |
|
| cardiac arrhythmias |
10 (13) |
10 (13) |
0 |
|
| Indication, No. (%): |
0.275 |
|||
| ventricular fibrillation |
21 (27) |
20 (26) |
1 (1) |
|
| ventricular tachycardia |
51 (66) |
41 (53) |
10 (13) |
|
| primary prevention |
5 (6) |
5 (6) |
0 |
|
| Device type, No. (%):§ |
0.303 |
|||
| single-chamber |
43 (56) |
39 (51) |
4 (5) |
|
| dual-chamber |
29 (38) |
23 (30) |
6 (8) |
|
| cardiac resynchronization device |
5 (6) |
4 (5) |
1 (1) |
|
| Pacing mode, No. (%):§ |
0.016 |
|||
| VVI or DDI – back-up pacing mode |
54 (70) |
50 (65) |
4 (5) |
|
| DDDR |
9 (12) |
5 (6) |
4 (5) |
|
| AAIR |
7 (9) |
6 (8) |
1 (1) |
|
| VVIR |
2 (3) |
1 (1) |
1 (1) |
|
| Biventricular pacing |
5 (6) |
4 (5) |
1 (1) |
|
| Atrial fibrillation, No. (%): |
0.007 |
|||
| permanent |
9 (12) |
6 (8) |
3 (4) |
|
| paroxysmal |
23 (30) |
17 (22) |
6 (8) |
|
| none |
45 (58) |
43 (56) |
2 (3) |
|
| History of myocardial infarction, No. (%) |
43 (56) |
36 (47) |
7 (9) |
0.634 |
| Coronary revascularization (CABG/PCI), n (%) |
31 (40) |
28 (36) |
3 (4) |
0.422 |
| Diabetes, n (%) |
11 (14) |
10 (13) |
1 (1) |
0.826 |
| Renal dysfunction, No. (%) |
10 (13) |
8 (10) |
2 (3) |
0.485 |
| Obstructive lung disease, No. (%) |
13 (17) |
11 (14) |
2 (3) |
0.839 |
| Arterial hypertension, No. (%) |
42 (55) |
40 (52) |
2 (3) |
0.014 |
| Antiarrhythmic medication, No. (%): |
0.489 |
|||
| beta blocker |
24 (31) |
21 (27) |
3 (4) |
|
| amiodarone |
10 (13) |
7 (9) |
3 (4) |
|
| beta blocker + amiodarone |
34 (44) |
30 (39) |
4 (5) |
|
| no antiarrhythmic drug |
9 (12) |
8 (10) |
1 (1) |
|
| Lipid lowering drug |
31 (40) |
28 (36) |
3 (4) |
0.422 |
| Defibrillator therapy (ATP and/or shock), No. (%) | 43 (56) | 34 (44) | 9 (12) | 0.758 |
*Abbreviations: NYHA – New York Heart Association; CABG – coronary artery bypass graft; PCI – percutaneous coronary intervention; ATP – antitachycardia pacing; VVIR – ventricular pacing-ventricular sensing-inhibited response to sensing-rate modulation; AAIR – atrial pacing-atrial sensing-inhibited response to sensing-rate modulation; DDDR – dual chamber pacing-dual chamber sensing-dual response to sensing-rate modulation; DDI – dual chamber pacing-dual chamber sensing-inhibited response to sensing.
†χ2 test or Fisher exact test for nominal characteristics, t-test or Mann-Whitney test for continuous characteristics.
‡According to the New York Heart Association Classification (5).
§According to the NASPE/BPEG generic pacemaker code for antibradyarrhythmia and adaptive-rate pacing and antitachyarrhythmia devices (6).
Figure 1.
Distribution of the body mass index (BMI) among the survivors (n = 66) and non-survivors (n = 11). Mean BMI ± standard deviation was 26.9 ± 4.3 kg/m2 for survivors (closed bars), and 23.5 ± 3.5 kg/m2 for non-survivors (open bars).
Follow-up data
Overall, more than a half of patients were re-hospitalized during the follow-up, mostly due to progression of heart failure. During the study period, 11 out of 77 patients (14%) died. Median follow up time was 24.5 months (range 0.2-60.7) for survivors and 7.6 months (range 1.5-42) for non-survivors. Kaplan-Meier estimates of the 1, 2, 3, and 4-year cumulative probability of survival were 90%, 86%, 83%, and 77%, respectively. The estimate of mean survival time for all patients was 51.5 (95% confidence intervals [CI] 46.6-56.5) months.
Risk factors for mortality
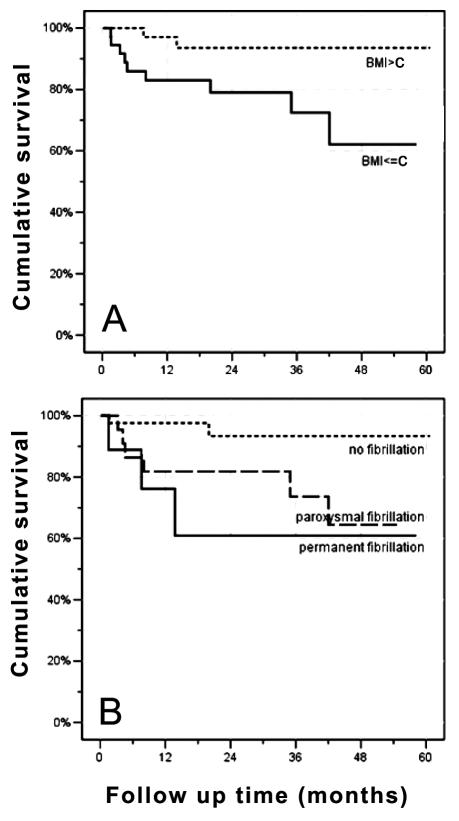
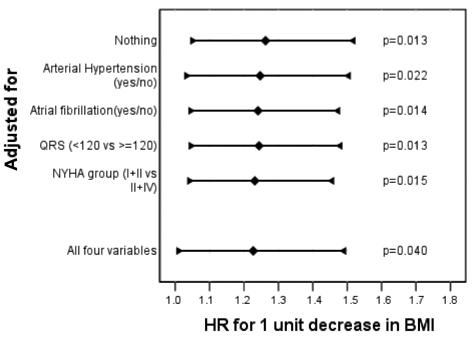
Using Kaplan-Meier estimates of survival, the following variables showed a significant impact on survival (Table 2): BMI (P = 0.024), atrial fibrillation (P = 0.014), NYHA class (P = 0.004), LVEDD (P = 0.049), and a negative history of arterial hypertension (P = 0.010). The Kaplan-Meier survival curves for BMI below and above the median (26.1 kg/m2) and for the presence of atrial fibrillation – either in the paroxysmal or the permanent form – are shown in Figure 2. QRS duration ≥120 ms at the time of cardioverter defibrillator implantation did not reach statistical significance (P = 0.072). No significant difference was found for the pacing mode according to the Kaplan-Meier method. Under the assumption of proportional hazard ratios (HR), the Cox regression model showed that the following variables had a significant impact on survival: BMI as a continuous variable (HR for one unit decrease in BMI: 1.26; 95% CI, 1.05-1.52; P = 0.013), the presence of atrial fibrillation (HR 5.58; 95% CI, 1.20-25.99; P = 0.029), LVEDD (HR for one cm increase 2.41, 95% CI, 1.18-4.92; P = 0.016), and the absence of arterial hypertension (HR 0.17; 95% CI, 0.037-0.789; P = 0.024). The resting heart rate at baseline had no significant impact on survival (P = 0.996). When the effect of BMI as a continuous variable was assessed in a multivariate Cox regression analysis, which included all the variables with a relevant effect on survival, the HR resulting from one unit decrease in BMI remained significant (P = 0.040) (Figure 3).
Table 2.
Association between various risk factors and Kaplan-Meier survival curves calculated by the log-rank test
| Variable | P |
|---|---|
| Age (≤median = 67 y vs>median) | 0.742 |
| Body mass index (≤median = 26.1 kg/m2 vs>median) | 0.024 |
| Ejection fraction (EF): | |
| EF≤35% vs >35% | 0.332 |
| EF≤25% vs >25% | 0.686 |
| Mitral regurgitation | 0.272 |
| Left ventricular end-diastolic dimension (LVEDD), cm | |
| Four groups by quartiles at 5.7 cm, 6.2 cm, 6.9 cm | 0.049 |
| QRS duration (<120 ms vs ≥120 ms) | 0.072 |
| NYHA* functional class: | |
| All four NYHA classes | 0.004 |
| NYHA class<III vs≥III | 0.057 |
| Pacing mode | 0.129 |
| Indication: | |
| Ventricular fibrillation; ventricular tachycardia; primary prevention | 0.212 |
| Ventricular fibrillation; ventricular tachycardia | 0.119 |
| Atrial fibrillation: | 0.030 |
| Permanent or paroxysmal vs none | 0.014 |
| Permanent vs none | 0.005 |
| Paroxysmal vs none | 0.041 |
| Coronary intervention | 0.397 |
| History of myocardial infarction | 0.642 |
| Defibrillator therapy | 0.114 |
| Diabetes | 0.578 |
| Renal dysfunction | 0.408 |
| Obstructive lung disease | 0.961 |
| Arterial hypertension | 0.010 |
| Antiarrhythmic medication: | 0.464 |
| Beta blocker vs none | 0.927 |
| Amiodarone vs none | 0.466 |
| Beta blocker and amiodarone vs none | 0.969 |
| Beta blocker vs amiodarone | 0.222 |
| Lipid lowering drug | 0.223 |
*NYHA – New York Heart Association.
Figure 2.
(A) Kaplan-Meier Estimates of the cumulative probability of survival in patients with a body mass index (BMI) below and above the median (26.1 kg/m2). The difference in survival between the two groups was significant (P = 0.024). (B) Kaplan-Meier cumulative probability of survival categorized by absence or presence of atrial fibrillation. The three groups differ significantly with respect to survival (P = 0.030). The presence of permanent or paroxysmal atrial fibrillation was significantly associated with an adverse outcome (P = 0.014). C – median.
Figure 3.
Crude (top line) and adjusted hazard ratios (HR; 95% confidence intervals marked by whiskers) for one unit decrease in body mass index (BMI) with respect to survival. The effect on survival remained significant (P = 0.040) after adjustment for other variables with an impact on outcome.
Cause of death
Overall, a cardiac cause of death was recorded in 7 out of 11 patients. Pump failure was the most common cause of cardiac death (6 out of 11 patients). Sudden cardiac death due to incessant ventricular tachycardia was noticed in a single patient (Table 3). Device examination of the single chamber defibrillator revealed that all programmed therapies were delivered ineffectively in this patient, including 4 shocks at maximal output. Re-hospitalization and consecutive in-hospital death were observed in 8 patients, whereas out-of-hospital death occurred in 3 patients. According to clinical circumstances, progressive heart failure was the cause of death in 6 patients – 4 patients died in the setting of an intensive care unit, 1 patient died in a retirement home, and 1 at home, with progressive dyspnea. Non-cardiac deaths occurred in a total of 4 patients. One patient with diabetes died due to lower limb infection and 1 due to a femur fracture followed by several re-operations. One patient, with respiratory distress syndrome, developed irreversible multiorgan failure, and 1 patient died due to the major intracerebral hemorrhage on oral anticoagulation.
Table 3.
Cause of death among the total study population (n = 77)
| Cause of death | Non-survivors, n (%) |
|---|---|
| Number of deaths | 11 (100) |
| Cardiac deaths: | 7 (64) |
| pump failure | 6 (55) |
| incessant ventricular tachycardia | 1 (9) |
| Non-cardiac deaths: | 4 (36) |
| sepsis (not device related) | 2 (18) |
| multiorgan failure | 1 (9) |
| intracerebral hemorrhage | 1 (9) |
Discussion
The major findings of this study were that lower BMI, presence of atrial fibrillation, NYHA class, and absence of arterial hypertension were predictors of mortality after implantation of cardioverter defibrillator. Low BMI in progressive diseases, such as congestive heart failure, was shown to be a powerful prognostic marker of poor outcome and increased mortality (7). To the best of our knowledge, it has not been previously studied whether BMI is associated with the outcome of patients implanted with a cardioverter defibrillator. In general, obesity, arterial hypertension, and other components of the metabolic syndrome are well known risk factors for the development of cardiovascular diseases and associated with increased mortality over the long-term (8,9) However, in large population-based cohort studies, a “J” or “U” curve effect was described, in which increased mortality was shown not only in obese individuals but also in individuals with a low BMI (10,11). Horwich et al (12) found significantly better survival curves for overweight and obese than for underweight patients with heart failure. The association between traditional cardiovascular risk factors and an adverse clinical outcome in heart failure patients is referred to as “reverse epidemiology” or the “obesity paradox” (13). Kalantar-Zadeh et al (14) identified different possible causes of adverse clinical outcome in heart failure, such as the “malnutrition-inflammation complex syndrome,” endotoxin-lipoprotein hypothesis, and time discrepancies among competitive risk factors. Obese patients may have protective alterations in neurohormonal status, including sympathetic and renin-angiotensin pathways or circulation cytokine levels. Fat stores may indicate preserved metabolic efficiency and/or energetic reserves. Lommi et al (15) found higher blood ketone bodies as an indicator of malnutrition in patients with congestive heart failure than in the control group. Depletion of liver glycogen favors fatty acid oxidation and ketone body production. Sympathetic stimulation and noradrenaline activation, increased secretion of tumor necrosis factor and other cytokines, as well as an increased cortisol/dehydroepiandrosterone balance are shown to contribute to the malnutrition status (16,17). The findings of the present study emphasize that continuous weight control of patients treated with defibrillator devices is required. Given the potential morbidity and mortality of heart failure in normal to underweight BMI patients, special cardiac training programs and nutrition plans may help to prevent progressive weight loss (18).
There is evidence that the presence of lower systemic arterial pressure may be an indicator of a poor prognosis in congestive heart failure (19). In support of this hypothesis, we found arterial hypertension in only a minority of non-survivors, whereas about a half of survivors were hypertensive. From a clinical perspective, the outcome might have been influenced by the fact that obese or normal weight patients with higher blood pressure tolerate optimal medical treatment, including angiotensin-converting enzyme inhibitors and beta-blockers in adequate doses, better than lower weight individuals with pre-existing arterial hypotension.
Recently, a subgroup analysis performed by MADIT II investigators demonstrated that BMI≥30 kg/m2 was associated with an increased risk of ventricular tachycardia or ventricular fibrillation (20). However, there was no relationship between increased BMI and the combined end point, including death. In the present study, the delivery of appropriate device therapy for ventricular tachyarrhythmias was observed in more than a half of the defibrillator patients, but there was no difference in the frequency of therapies between the group of survivors and non-survivors. In order to answer the question whether differences in the outcome were based on the type of the implanted device, we performed further analysis not only with respect to the device type but also to the programmed pacing mode of the defibrillator. Significant differences found between the group of survivors and non-survivors according to the pacing mode but not according to the device type, a clinically relevant finding. However, Kaplan-Meier analysis detected no significant impact of the pacing mode on survival. It is well known that dual-chamber and especially single-chamber cardioverter defibrillators are ineffective in the treatment of progressive heart failure, but very effective in treatment of serious, life threatening arrhythmias. In this context, it is noteworthy that we avoided right ventricular pacing for the conventional device types as much as possible, unless there was a clear indication for antibradycardia pacing. Nowadays, an increasing rate in implantation of cardiac resynchronization defibrillators may improve clinical outcome, particularly in the subset of patients with severe heart failure (21).
In the present study, Kaplan-Meier survival curves comparing all four NYHA classes showed a highly significant difference. LVEDD categorized by quartiles was weakly significant. The difference in survival stratified by ejection fraction, however, did not reach statistical significance. Advanced NYHA functional class and prolonged QRS duration seem to be more accurate predictors of mortality, as has been shown previously (22,23). According to our analysis, the majority of patients died due to progressive heart failure. There is evidence that pump failure is more likely the cause of death than malignant arrhythmia in end-stage heart failure (4).
Presence of atrial fibrillation – either permanent or paroxysmal – was associated with higher mortality in patients with implantable cardioverter defibrillator. In this respect, results of the present study are consistent with previous data showing that chronic atrial fibrillation is independently associated with an increase in mortality among patients with impaired left ventricular function but also in patients without structural heart disease (24-26). Deneke et al (27) demonstrated that the occurrence of paroxysmal atrial fibrillation early after cardioverter defibrillator implantation was associated with a higher risk of death. Parkash et al (28) identified a history of atrial fibrillation as one of the valid independent predictors of one-year mortality in patients with implantable cardioverter defibrillators. Consequently, a clinician has to pay attention to the development of atrial fibrillation, which might be detected by the electrogram storage capabilities of the device during the follow-up. Strategies for adequate rate and/or rhythm control of atrial fibrillation, together with oral anticoagulation, may help to reduce the overall mortality (29).
Statin therapy has been shown to improve the outcome not only in heart failure patients but also in the population with implantable cardioverter defibrillators, because of its anti-inflammatory effect (30,31). Although, more survivors than non-survivors were treated with statins, we were not able to show significant differences in terms of survival because of the limited sample size. As a shortcoming, only half of the patients with ischemic cardiomyopathy were actually treated with statins. In the light of the recent data, clinicians should prescribe statin-type hypolipidemic drugs more widely to patients with coronary artery disease and implanted defibrillator.
Some limitations of our study must be taken into account in the interpretation of study results. First, the total number of patients in this study was smaller than in previously published defibrillator trials comparing different treatment arms. However, our study included non-selected consecutive patients who were representative of the heterogeneous population followed in an outpatient clinic. Second, the limited number of non-survivors encountered during follow-up may have reduced the power to detect statistically significant differences for other variables. Although the present data suggested that lower BMI was associated with a poor outcome in patients with implantable cardioverter defibrillator, further studies are needed to confirm this observation on larger populations.
In conclusion, the present study demonstrated that normal to lower BMI, presence of atrial fibrillation, absence of arterial hypertension, advanced NYHA class, and LVEDD were unfavorable predictors for survival in an unselected population implanted with cardioverter defibrillators. Apart from regular device interrogations, high-risk individuals may be identified by the above-mentioned characteristics. Continuous medical care, particularly with respect to the multidimensional problems in heart failure, is necessary to improve the prognosis and prevention of sudden death.
Acknowledgment
The authors thank Mynda Schreuer, PhD, who performed all statistical analyses. There is no form of financial support or grant associated with this study.
References
- 1.A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337:1576–83. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–8. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 5.Fisher JD. New York Heart Association Classification. Arch Intern Med. 1972;129:836. [PubMed] [Google Scholar]
- 6.Bernstein AD, Camm AJ, Fletcher RD, Gold RD, Rickards AF, Smyth NP, et al. The NASPE/BPEG generic pacemaker code for antibradyarrhythmia and adaptive-rate pacing and antitachyarrhythmia devices. Pacing Clin Electrophysiol. 1987;10:794–9. doi: 10.1111/j.1540-8159.1987.tb06035.x. [DOI] [PubMed] [Google Scholar]
- 7.Perna ER, Cimbaro Canella JP, Macin SM, Bayol PA, Kriskovich JO, Vargas Morales W, et al. Body mass index obtained before discharge is a powerful long-term prognostic marker in patients with decompensated heart failure. J Card Fail. 2004;10(Suppl 4):S124. [Google Scholar]
- 8.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 9.Wilhelmsen L, Rosengren A, Eriksson H, Lappas G. Heart failure in the general population of men–morbidity, risk factors and prognosis. J Intern Med. 2001;249:253–61. doi: 10.1046/j.1365-2796.2001.00801.x. [DOI] [PubMed] [Google Scholar]
- 10.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 11.Mosterd A, Cost B, Hoes AW, de Bruijne MC, Deckers JW, Hofman A, et al. The prognosis of heart failure in the general population: The Rotterdam Study. Eur Heart J. 2001;22:1318–27. doi: 10.1053/euhj.2000.2533. [DOI] [PubMed] [Google Scholar]
- 12.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–95. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 13.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91:891–4. doi: 10.1016/s0002-9149(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–44. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 15.Lommi J, Koskinen P, Naveri H, Harkonen M, Kupari M. Heart failure ketosis. J Intern Med. 1997;242:231–8. doi: 10.1046/j.1365-2796.1997.00187.x. [DOI] [PubMed] [Google Scholar]
- 16.Anker SD, Swan JW, Volterrani M, Chua TP, Clark AL, Poole-Wilson PA, et al. The influence of muscle mass, strength, fatigability and blood flow on exercise capacity in cachectic and non-cachectic patients with chronic heart failure. Eur Heart J. 1997;18:259–69. doi: 10.1093/oxfordjournals.eurheartj.a015229. [DOI] [PubMed] [Google Scholar]
- 17.Anker SD, Clark AL, Kemp M, Salsbury C, Teixeira MM, Hellewell PG, et al. Tumor necrosis factor and steroid metabolism in chronic heart failure: possible relation to muscle wasting. J Am Coll Cardiol. 1997;30:997–1001. doi: 10.1016/s0735-1097(97)00262-3. [DOI] [PubMed] [Google Scholar]
- 18.Lissin LW, Gauri AJ, Froelicher VF, Ghayoumi A, Myers J, Giacommini J. The prognostic value of body mass index and standard exercise testing in male veterans with congestive heart failure. J Card Fail. 2002;8:206–15. doi: 10.1054/jcaf.2002.126812. [DOI] [PubMed] [Google Scholar]
- 19.Cowburn PJ, Cleland JG, Coats AJ, Komajda M. Risk stratification in chronic heart failure. Eur Heart J. 1998;19:696–710. doi: 10.1053/euhj.1997.0820. [DOI] [PubMed] [Google Scholar]
- 20.Singh JP, Hall WJ, McNitt S, Wang H, Daubert JP, Zareba W, et al. Factors influencing appropriate firing of the implanted defibrillator for ventricular tachycardia/fibrillation: findings from the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II). J Am Coll Cardiol. 2005;46:1712–20. doi: 10.1016/j.jacc.2005.05.088. [DOI] [PubMed] [Google Scholar]
- 21.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco TN, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 22.Pulignano G, Del Sindaco D, Tavazzi L, Lucci D, Gorini M, Leggio F, et al. Clinical features and outcomes of elderly outpatients with heart failure followed up in hospital cardiology units: data from a large nationwide cardiology database (IN-CHF Registry). Am Heart J. 2002;143:45–55. doi: 10.1067/mhj.2002.119608. [DOI] [PubMed] [Google Scholar]
- 23.Bode-Schnurbus L, Bocker D, Block M, Gradaus R, Heinecke A, Breithardt G, et al. QRS duration: a simple marker for predicting cardiac mortality in ICD patients with heart failure. Heart. 2003;89:1157–62. doi: 10.1136/heart.89.10.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 25.Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients. Circulation. 1991;84:40–8. doi: 10.1161/01.cir.84.1.40. [DOI] [PubMed] [Google Scholar]
- 26.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 27.Deneke T, Lawo T, Gerritse B, Lemke B. The European GEM DR Trade Mark Investigators. Mortality of patients with implanted cardioverter/defibrillators in relation to episodes of atrial fibrillation. Europace. 2004;6:151–8. doi: 10.1016/j.eupc.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Parkash R, Stevenson WG, Epstein LM, Maisel WH. Predicting early mortality after implantable defibrillator implantation: a clinical risk score for optimal patient selection. Am Heart J. 2006;151:397–403. doi: 10.1016/j.ahj.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Van den Berg MP, Tuinenburg AE, Crijns HJ, Van Gelder IC, Gosselink AT, Lie KI. Heart failure and atrial fibrillation: current concepts and controversies. Heart. 1997;77:309–13. doi: 10.1136/hrt.77.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwich TB, MacLellan WR, Fonarow GC. Statin therapy is associated with improved survival in ischemic and non-ischemic heart failure. J Am Coll Cardiol. 2004;43:642–8. doi: 10.1016/j.jacc.2003.07.049. [DOI] [PubMed] [Google Scholar]
- 31.Vyas AK, Guo H, Moss AJ, Olshansky B, McNitt SA, Hall WJ. Reduction in ventricular tachyarrhythmias with statins in the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol. 2006;47:769–73. doi: 10.1016/j.jacc.2005.09.053. [DOI] [PubMed] [Google Scholar]