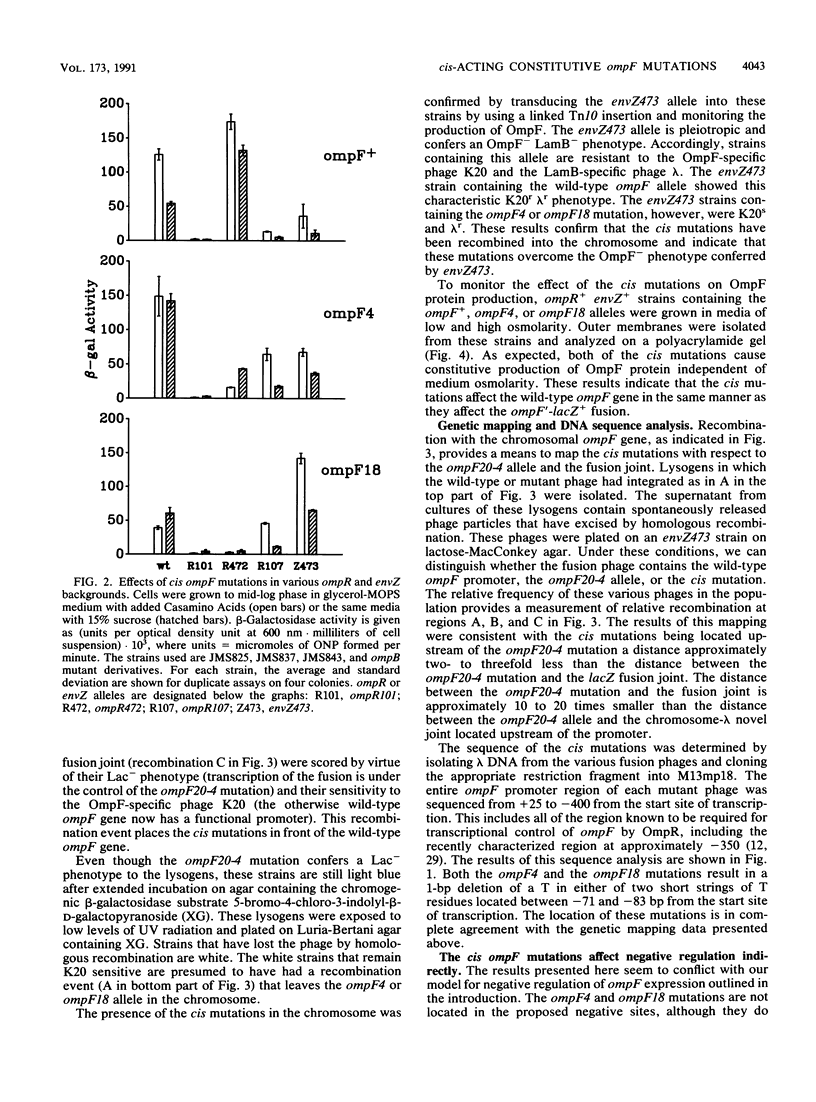
Abstract
OmpR and EnvZ differentially control the transcription of the major outer membrane porin genes, ompF and ompC, in Escherichia coli in response to the osmolarity of the medium. We have previously provided evidence that OmpR works both positively and negatively at the ompF promoter to give the characteristic switch from OmpF to OmpC production with increasing osmolarity. Here, we describe the isolation of cis-acting ompF mutations that affect negative regulation by OmpR by affecting the three-dimensional structure of the promoter region as measured by agarose gel mobility. These results further clarify the mechanism by which OmpR negatively regulates ompF expression, suggesting a model in which OmpR forms a repressive loop in the ompF promoter region.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Nakasai F., Mizushima S., Mizuno T. Phosphorylation of a bacterial activator protein, OmpR, by a protein kinase, EnvZ, results in stimulation of its DNA-binding ability. J Biochem. 1989 Jul;106(1):5–7. doi: 10.1093/oxfordjournals.jbchem.a122817. [DOI] [PubMed] [Google Scholar]
- Albright L. M., Huala E., Ausubel F. M. Prokaryotic signal transduction mediated by sensor and regulator protein pairs. Annu Rev Genet. 1989;23:311–336. doi: 10.1146/annurev.ge.23.120189.001523. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chisholm D. A convenient moderate-scale procedure for obtaining DNA from bacteriophage lambda. Biotechniques. 1989 Jan;7(1):21–23. [PubMed] [Google Scholar]
- Forst S. A., Delgado J., Inouye M. DNA-binding properties of the transcription activator (OmpR) for the upstream sequences of ompF in Escherichia coli are altered by envZ mutations and medium osmolarity. J Bacteriol. 1989 Jun;171(6):2949–2955. doi: 10.1128/jb.171.6.2949-2955.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler R. G., Degnen G. E., Cox E. C. Mutational specificity of a conditional Escherichia coli mutator, mutD5. Mol Gen Genet. 1974;133(3):179–191. doi: 10.1007/BF00267667. [DOI] [PubMed] [Google Scholar]
- Friedman D. I. Integration host factor: a protein for all reasons. Cell. 1988 Nov 18;55(4):545–554. doi: 10.1016/0092-8674(88)90213-9. [DOI] [PubMed] [Google Scholar]
- Garrett S., Silhavy T. J. Isolation of mutations in the alpha operon of Escherichia coli that suppress the transcriptional defect conferred by a mutation in the porin regulatory gene envZ. J Bacteriol. 1987 Apr;169(4):1379–1385. doi: 10.1128/jb.169.4.1379-1385.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Bacteriophage resistance in Escherichia coli K-12: general pattern of resistance. J Bacteriol. 1975 Mar;121(3):983–993. doi: 10.1128/jb.121.3.983-993.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo M. M., Slauch J. M., Silhavy T. J. Signal transduction in bacteria: kinases that control gene expression. New Biol. 1990 Jan;2(1):5–9. [PubMed] [Google Scholar]
- Inokuchi K., Furukawa H., Nakamura K., Mizushima S. Characterization by deletion mutagenesis in vitro of the promoter region of ompF, a positively regulated gene of Escherichia coli. J Mol Biol. 1984 Sep 25;178(3):653–668. doi: 10.1016/0022-2836(84)90243-2. [DOI] [PubMed] [Google Scholar]
- Kato M., Aiba H., Mizuno T. Molecular analysis by deletion and site-directed mutagenesis of the cis-acting upstream sequence involved in activation of the ompF promoter in Escherichia coli. J Biochem. 1989 Mar;105(3):341–347. doi: 10.1093/oxfordjournals.jbchem.a122665. [DOI] [PubMed] [Google Scholar]
- Kawaji H., Mizuno T., Mizushima S. Influence of molecular size and osmolarity of sugars and dextrans on the synthesis of outer membrane proteins O-8 and O-9 of Escherichia coli K-12. J Bacteriol. 1979 Dec;140(3):843–847. doi: 10.1128/jb.140.3.843-847.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiino D. R., Phillips G. J., Silhavy T. J. Increased expression of the bifunctional protein PrlF suppresses overproduction lethality associated with exported beta-galactosidase hybrid proteins in Escherichia coli. J Bacteriol. 1990 Jan;172(1):185–192. doi: 10.1128/jb.172.1.185-192.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Maeda S., Mizuno T. Evidence for multiple OmpR-binding sites in the upstream activation sequence of the ompC promoter in Escherichia coli: a single OmpR-binding site is capable of activating the promoter. J Bacteriol. 1990 Jan;172(1):501–503. doi: 10.1128/jb.172.1.501-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Mizushima S. Novel rpoA mutation that interferes with the function of OmpR and EnvZ, positive regulators of the ompF and ompC genes that code for outer-membrane proteins in Escherichia coli K12. J Mol Biol. 1987 Jun 20;195(4):847–853. doi: 10.1016/0022-2836(87)90489-x. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kato M., Jo Y. L., Mizushima S. Interaction of OmpR, a positive regulator, with the osmoregulated ompC and ompF genes of Escherichia coli. Studies with wild-type and mutant OmpR proteins. J Biol Chem. 1988 Jan 15;263(2):1008–1012. [PubMed] [Google Scholar]
- Mizuno T., Mizushima S. Characterization by deletion and localized mutagenesis in vitro of the promoter region of the Escherichia coli ompC gene and importance of the upstream DNA domain in positive regulation by the OmpR protein. J Bacteriol. 1986 Oct;168(1):86–95. doi: 10.1128/jb.168.1.86-95.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T. Static bend of DNA helix at the activator recognition site of the ompF promoter in Escherichia coli. Gene. 1987;54(1):57–64. doi: 10.1016/0378-1119(87)90347-7. [DOI] [PubMed] [Google Scholar]
- Morona R., Reeves P. The tolC locus of Escherichia coli affects the expression of three major outer membrane proteins. J Bacteriol. 1982 Jun;150(3):1016–1023. doi: 10.1128/jb.150.3.1016-1023.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon B. T., Ronson C. W., Ausubel F. M. Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7850–7854. doi: 10.1073/pnas.83.20.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow K. S., Silhavy T. J., Garrett S. cis-acting sites required for osmoregulation of ompF expression in Escherichia coli K-12. J Bacteriol. 1986 Dec;168(3):1165–1171. doi: 10.1128/jb.168.3.1165-1171.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersaud A., Norioka S., Inouye M. Characterization of OmpR binding sequences in the upstream region of the ompF promoter essential for transcriptional activation. J Biol Chem. 1989 Nov 5;264(31):18693–18700. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. DNA looping. Science. 1988 Apr 8;240(4849):127–128. doi: 10.1126/science.3353710. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A., McDonald G. A. Regulation of outer membrane protein synthesis in Escherichia coli K-12: deletion of ompC affects expression of the OmpF protein. J Bacteriol. 1984 Aug;159(2):555–563. doi: 10.1128/jb.159.2.555-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauch J. M., Garrett S., Jackson D. E., Silhavy T. J. EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J Bacteriol. 1988 Jan;170(1):439–441. doi: 10.1128/jb.170.1.439-441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauch J. M., Silhavy T. J. Genetic analysis of the switch that controls porin gene expression in Escherichia coli K-12. J Mol Biol. 1989 Nov 20;210(2):281–292. doi: 10.1016/0022-2836(89)90330-6. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Owen J. Mechanisms of spontaneous and induced frameshift mutation in bacteriophage T4. Genetics. 1985 Apr;109(4):633–659. doi: 10.1093/genetics/109.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Garrett S., Sodergren E., Silhavy T. J. Mutations that define the promoter of ompF, a gene specifying a major outer membrane porin protein. J Bacteriol. 1985 Jun;162(3):1054–1060. doi: 10.1128/jb.162.3.1054-1060.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Hall M. N., Silhavy T. J. Isolation and characterization of mutations altering expression of the major outer membrane porin proteins using the local anaesthetic procaine. J Mol Biol. 1983 May 25;166(3):273–282. doi: 10.1016/s0022-2836(83)80085-0. [DOI] [PubMed] [Google Scholar]
- Tsui P., Helu V., Freundlich M. Altered osmoregulation of ompF in integration host factor mutants of Escherichia coli. J Bacteriol. 1988 Oct;170(10):4950–4953. doi: 10.1128/jb.170.10.4950-4953.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]





