Abstract
Aim
To determine peripheral blood lymphocyte subsets – T cells, helper T cells, cytotoxic T cells, B cells, and natural killer cells, natural killer cell cytotoxicity, serum cortisol concentration, and lymphocyte glucocorticoid receptor expression in Croatian combat veterans diagnosed with chronic posttraumatic stress disorder (PTSD); and to examine the relationship between the assessed parameters and the time passed since the traumatic experience.
Methods
Well-characterized group of 38 PTSD patients was compared to a group of 24 healthy civilians. Simultaneous determination of lymphocyte subsets and the expression of intracellular glucocorticoid receptor was performed using three-color flow cytometry. Natural killer cell cytotoxicity was measured by 51Cr-release assay and the serum cortisol concentration was determined by radioimmunoassay.
Results
We found higher lymphocyte counts in PTSD patients than in healthy controls (2294.7 ± 678.0/μL vs 1817.2 ± 637.0/μL, P = 0.007) and a positive correlation between lymphocyte glucocorticoid receptor expression and the number of years that passed from the traumatic experience (rs = 0.43, P = 0.008). Lymphocyte glucocorticoid receptor expression positively correlated with serum cortisol concentration both in PTSD patients (r = 0.46, P = 0.006) and healthy controls (r = 0.46, P = 0.035).
Conclusion
This study confirmed that the immune system was affected in the course of chronic PTSD. Our findings also indicated that the hypothalamic-pituitary-adrenal axis profile in PTSD was associated with the duration of the disorder. Due to the lack of power, greater sample sizes are needed to confirm the results of this study.
Prolonged or frequently repeated stress response during symptomatic episodes in chronic posttraumatic stress disorder (PTSD) can result in neuroendocrine and immune alterations, posing serious threat to mental and physical health (1,2). Evidence suggests that PTSD is related to increased medical morbidity, particularly from cardiovascular and autoimmune diseases (3). With controversial findings when neurobiology of PTSD is concerned, the patophysiological mechanisms underlying increased susceptibility to disease are not clear (4,5). However, it has been implicated that the sympathetic-adrenal-medullary (SAM) and the hypothalamic-pituitary-adrenal axes are the key mediators in this process (6,7).
The immune system interacts with the hypothalamic-pituitary-adrenal axis in a bidirectional fashion to maintain homeostasis. Being the primary effector of the stress response, cortisol modifies the complex cytokine network and, consequently, leukocyte function and recirculation (8). These effects are achieved through its interaction with the specific intracellular glucocorticoid receptors (9).
Studies of the leukocyte recirculation (10,11), immune cells function (12), and hypothalamic-pituitary-adrenal axis activity (5) in PTSD yielded controversial results. Overall findings support the hypothesis that immune activation in PTSD may be associated with Th2 cytokine shift and alterations in the proinflammatory cytokine system (4). Besides, it is believed that PTSD is linked with low plasma cortisol levels and higher glucocorticoid receptor expression, suggesting enhanced feedback sensitivity to cortisol (13). In contrast to these findings, Gotovac et al (14) showed that Croatian combat veterans with PTSD, approximately 6 years after traumatic event, had lower expression of glucocorticoid receptor in lymphocyte subsets, with higher serum cortisol concentration than healthy subjects. Majority of other studies did not take into account the time passed since the trauma and their samples mainly included Vietnam veterans (15) or Holocaust survivors (16), who had greater time gap since the traumatic experience than Croatian war veterans.
Considering the strong discrepancies in the results published to date, we performed a cross-sectional study to evaluate the correlation between PTSD in Croatian combat war veterans and the percentages of circulating lymphocyte subsets, natural killer cell cytotoxicity as a measure of immune function, and the serum cortisol concentration with lymphocyte glucocorticoid receptor expression as components of hypothalamic-pituitary-adrenal axis. The emphasis was put on the relationship between the assessed parameters and the time passed since the traumatic experience.
Subjects and methods
Subjects
PTSD patients were Croatian combat veterans, all men (Table 1), recruited from the outpatient PTSD program at Vrapče Psychiatric Hospital during the year 2004. Before entering the study, all patients met the International Classification of Disorders (ICD)-10 (17) PTSD criteria, the official classification in Croatian psychiatric practice. For the purposes of this study, the diagnosis of PTSD, as well as the specificities of clinical picture (frequency and intensity of all and selected PTSD symptoms during the period of one month prior to rating), were determined by using the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV (18) based Clinician-Administered PTSD Scale (CAPS) (19) and all 38 patients met the criteria for chronic PTSD. Only the patients without psychiatric premorbidity or comorbidity, including major depression, were selected for the purpose of this study. Each patient had at least one “reexperiencing symptom” within the criterion B, at least 3 “avoidance and numbing symptoms” within the criterion C, and at least 2 “hyperarousal symptoms” within the criterion D. No patients had subsyndromal form of PTSD. In relation to DSM-IV A (traumatic event) criterion, each patient witnessed another person’s death and experienced a threat to their physical integrity and/or wounding. On the basis of intrusive thoughts content, the focal traumatic experience was identified and the time passed until assessment was recorded. The PTSD-group was relatively homogenous related to the severity of the illness. The average severity of PTSD was qualified as moderate (median = 4; interquartile range = 4 to 5), according to the Clinical Global Impression Severity (CGI-S) scale (20). Control subjects were sex-matched healthy civilians, hospital and laboratory personnel with no combat experience.
Table 1.
Participants’ demographic and clinical characteristics
| Participants |
|||
|---|---|---|---|
| Variable* | PTSD patients (n = 38) | healthy volunteers (n = 24) | P† |
| Age (mean±SD) | 44.3 ± 9.4 | 39.0 ± 8.4 | 0.028 |
| Tobacco use | 24 | 13 | 0.330 |
| Marital status: | 0.443 | ||
| married | 21 | 12 | |
| unmarried/divorced/widower | 17 | 12 | |
| Lives with the family‡ | 32 | 22 | 0.329 |
| Education: | 0.006 | ||
| elementary school | 5 | 0 | |
| high school | 28 | 13 | |
| university education | 5 | 11 | |
| Work status: | 0.012 | ||
| employed | 22 | 21 | |
| retired | 11 | 0 | |
| unemployed | 5 | 3 | |
| CAPS (mean±SD): | |||
| reexperiencing | 16.5 ± 2.2 | 0 | |
| avoidance/numbing | 26.1 ± 3.1 | 0 | |
| hyperarousal | 15.7 ± 3.3 | 0.5 ± 1.1 | |
| total | 58.3 ± 7.0 | 0.5 ± 1.1 | <0.001 |
| Delayed§ | 20/38 | ||
| Years from trauma (median, range) | 12 (8-13) | ||
*Abbreviations: PTSD – posttraumatic stress disorder; SD – standard deviation; CAPS – Clinician-Administered PTSD Scale.
†Two-sided values obtained using Fisher exact tests for 2×2 tables, Pearson χ2 tests for 3×2 tables, and t tests; statistically significant if P<0.05.
‡Lives with the family.
§The onset of symptoms after the first six months of traumatic incident.
Prior to blood drawing, healthy civilians were examined by an experienced physician and their histories were recorded. All controls had negative history of psychiatric disorders.
None of the participants used any psychotropic medication, drug, or reported alcohol abuse for at least one month, and did not suffer from any infectious, allergic, or endocrine disorder. They had no symptoms or signs of acute or chronic physical illness.
The study was approved by the Ethic Committee of the hospital and written informed consent was obtained from all subjects.
Samples
Heparinized and unheparinized blood samples (10 mL each) were collected by venipuncture between 8 and 9 am in Vacutainer tubes (Becton Dickinson Vacutainer System Europe, Grenoble, France). A part of the whole heparinized blood (50 μL per tube) was used for the lymphocyte immunophenotyping and glucocorticoid receptor expression determination by flow cytometry. Peripheral blood mononuclear cells (PBMC) were separated by centrifugation on Ficoll-Hypaque (Pharmacia AB, Uppsala, Sweden) density gradient and used for natural killer cell cytotoxicity determination. The sera for determination of cortisol concentration were stored at -80°C.
Immunophenotyping and intracellular glucocorticoid receptor determination
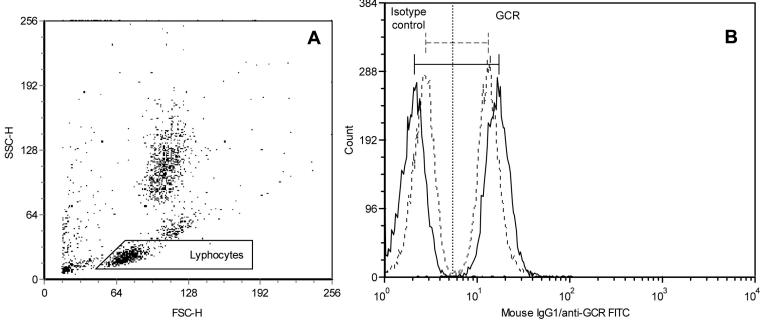
Lymphocyte counts were determined by flow cytometry, using Flow-CountTM Fluorospheres (Coulter Corporation, Miami, USA) according to the manufacturer’s protocol. Flow cytometric, three-color staining method was used for the simultaneous labeling of surface lymphocyte markers and intracellular lymphocyte glucocorticoid receptor expression. The method was previously described in detail (14). Briefly, surface staining was achieved by phycoerytrin (PE) conjugated anti-CD3, anti-CD16,56, anti-IgG2a, and peridinin chlorophyll protein (PerCP) conjugated anti-CD4, anti-CD8, anti-CD20, anti-IgG1 monoclonal antibodies (MoAb) (Becton Dickinson, Heidelberg, Germany), 5 μL per 50 μL of whole blood each. After incubation in the dark for 15 minutes, cells were washed and fixed, followed by erythrocytes lysis. The cells were then washed in the permeabilization buffer, containing predetermined optimal concentration of fluorescein isothiocyanate (FITC) conjugated anti-glucocorticoid receptor MoAb (21) or 5 μL of isotype control, left for 20 minutes in the dark, washed with permeabilization buffer again, and resuspended in 500 μL of fixation buffer. Prepared samples were run on FACSCalibur flow cytometer and analyzed by CELLQuest software (Becton Dickinson, Heidelberg, Germany). At least 5000 events in the light-scatter lymphocyte gate were acquired and the percentages of surface marker positive cells were determined in PE vs PerCP dot plots. The relative quantity of lymphocyte glucocorticoid receptor (mean glucocorticoid receptor fluorescence), expressed as mean fluorescence intensity, was calculated as the difference between mean values of glucocorticoid receptor and isotype control (Figure 1). The instrument calibration was performed on a daily basis.
Natural killer cell cytotoxicity
The in vitro natural killer cell cytotoxicity toward 51Cr-labeled K562 target cells (185 MBq/mL, Amersham, Little Chalfont, UK) was performed according to the previously described technique (22). We represented natural killer cell activity as the mean percentage of lysis across three effector-target cell ratios (25:1, 50:1, 100:1) (Table 2). Lytic units were not calculated because not all of our data fitted the assumptions required for lytic unit transformation. To avoid missing data and minimize the number of comparisons and consequent type I error, only the mean percentage of lysis was included in the overall analyses.
Table 2.
Comparison of the immune and endocrine parameters between PTSD patients and healthy volunteers*
| Findings in participants |
Statistics |
||||
|---|---|---|---|---|---|
| Variable† | PTSD patients (n = 38) | healthy volunteers (n = 24) | t | P | d‡ |
| Lymphocytes (count/μL) | 2294.7 (2077.8-2511.5) | 1817.2 (1544.3-2090.1) | 2.763 | 0.007 | 0.006 |
| T cells (%) | 70.7 (67.5-73.8) | 67.9 (63.6-71.9) | 0.812 | 0.419 | 0.028 |
| Helper T cells (%) | 44.8 (42.0-47.5) | 42.2 (38.7-45.6) | 1.023 | 0.310 | 0.017 |
| Cytotoxic T cells (%) | 22.8 (20.3-25.6) | 20.8 (17.9-24.1) | 0.768 | 0.444 | 0.039 |
| B cells (%) | 11.2 (9.8-12.8) | 11.5 (9.7-13.5) | -0.237 | 0.813 | 0.050 |
| Natural killer cells (%) | 16.2 (13.7-18.8) | 17.93 (14.7-21.5) | -0.276 | 0.783 | 0.044 |
| Natural killer cell cytotoxicity (mean):§ | 45.5 (39.2-51.7) | 47.4 (39.4-55.4) | -0.778 | 0.439 | 0.033 |
| 25:1 | 31.6 (25.5-37.8) | 35.0 (27.5-42.5) | -0.711 | 0.480 | |
| 50:1 | 46.5 (40.1-52.4) | 49.5 (41.7-51.4) | -0.629 | 0.532 | |
| 100:1 | 56.5 (50.8-62.2) | 59.6 (52.2-67.1) | -0.684 | 0.497 | |
| Lymphocyte glucocorticoid receptor (m.f.i.) | 42.5 (38.5-46.6) | 39.21 (34.5-44.3) | 0.856 | 0.394 | 0.022 |
| Cortisol (μg/100 mL) | 13.7 (11.1-16.5) | 12.0(9.1-15.4) | 1.140 | 0.258 | 0.011 |
*Abbreviations: PTSD – posttraumatic stress disorder; m.f.i. – mean fluorescence intensity.
†Values are means or backtransformed means with 95% confidence intervals in the parentheses. To meet t test assumptions variables were transformed as follows: square for T cells (CD3+), logarithm for cytotoxic T cells (CD3+CD8+), square root for B cells (CD20+), natural killer cells (CD56+CD16+), glucocorticoid receptor, and cortisol.
‡Threshold value calculated using the false discovery rate method. If the P value exceeds corresponding d, performed test is not statistically significant at 0.05 level.
§Percentage of lysis represented as the mean of three effector:target cell ratios (25:1, 50:1, 100:1), separate effector:target cell ratios were not included in the calculation of d values.
Serum cortisol determination
Serum cortisol concentration was determined by the radioimmunoassay kit CORT-CT2 (Schering S.A. Cis bio international, Gif-sur-Yvette, France). All samples were analyzed in duplicates following manufacturer’s protocol. The sensitivity of the assay was 0.17 μg/100mL, and the intra-assay and inter-assay coefficients of variation were less than 6% and 8%, respectively.
Statistical analysis
Categorical data were analyzed using Fisher exact or Pearson χ2 tests. Since not all continuous variables were normally distributed, as confirmed with Shapiro-Wilk W test, transformations were applied to meet parametric tests assumptions of normality and homogeneity of variance (assessed by Levene test). The group differences were tested with analysis of covariance (ANCOVA), using age as covariate, and two-tailed t tests. Data could not be modeled to meet multivariate test assumptions, leaving the greater possibility of type I error because of the multiple comparisons performed. Therefore, we used the false discovery rate method (23) for 9 group comparisons and 15 planned correlations. Partial correlations controlling for age and smoking in both groups, including “delayed” factor only in PTSD group, were performed. Correlations with the number of years since the trauma were examined using nonparametric Spearman rank order correlations because this variable could not be normalized. All statistical analyses were performed with Statistica, version 6 (StatSoft, Inc., Tulsa, OK, USA).
Results
Group comparisons
Groups were not matched by age, education, and work status. PTSD patients were slightly older, less educated, and substantial proportion of them was retired (Table 1). The age difference was considered as possible confounding factor, but ANCOVA, using age as a covariate, showed no significant effect of age on the investigated variables (Table 2). Results based on raw P value from t test indicated higher lymphocyte count in PTSD patients and the calculated power yielded 80% for group sample sizes of 38 and 24. Calculation for two-sided two-sample t test was based on the observed difference of 477.4/μL, with group standard deviations of 678.0 and 637.0, and with significance level (α) set to 0.05. However, with α set to 0.006, as indicated by false discovery rate threshold value (d), calculated power yielded only 56%, with a sample size of 49 participants per group needed to achieve the power of 80%. No other measured parameter significantly different between the PTSD patients and healthy volunteers (Table 2).
Correlational analyses
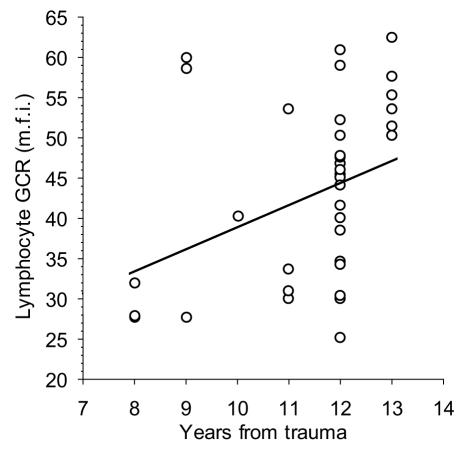
No biological parameter significantly correlated with total CAPS scores, but the number of years after trauma positively correlated with lymphocyte glucocorticoid receptor expression, as indicated by the raw P value (Figure 2). Positive correlation was also found between lymphocyte glucocorticoid receptor expression and serum cortisol concentration in the PTSD patients (r = 0.46, P = 0.006, d = 0.003) and healthy volunteers (r = 0.46, P = 0.035, d = 0.010). However, false discovery rate analysis indicated type I error since all P values exceeded corresponding d values (Table 2). The other planned correlations of cortisol concentration, lymphocyte count, and natural killer cell cytotoxicity with the number of years from trauma, were not statistically significant.
Figure 2.
Correlation between the glucocorticoid receptor (GCR) expression in lymphocytes and number of years passed since the trauma. Spearman rank order correlation controlled by the false discovery rate at α = 0.05 for total number of 15 correlations performed in this study: rs = 0.43, P = 0.008, d = 0.007; m.f.i. – mean fluorescence intensity.
Discussion
Our study showed increased lymphocyte count in PTSD patients and positive correlation of the lymphocyte glucocorticoid receptor expression with the time passed since traumatic experience. The effect of PTSD on the peripheral blood lymphocyte subsets, the immune cell function measured by natural killer cell cytotoxicity, and the components of hypothalamic-pituitary-adrenal axis was not observed.
Generally, few studies on the immunity in PTSD have been published to date and even fewer addressed well-characterized fully developed PTSD according to the DSM-IV criteria. Our study could not confirm either the previous findings of higher peripheral blood percentages of helper T cells (CD4) and natural killer cells (CD16,56) in Croatian PTSD patients (24), or higher percentages of T cells (CD3), CD4, and cytotoxic T cells (CD8) in Vietnam veterans (11). However, other studies also reported the lack of differences in the peripheral blood lymphocyte phenotype in combat-related (12), or childhood sexual abuse-related (10) chronic PTSD. Recently, Boscarino et al (3) showed that Vietnam veterans with PTSD had higher lymphocyte counts, consistent with the higher prevalence of autoimmune diseases. We noticed that PTSD patients had higher lymphocyte counts in this group, as well as in other examined PTSD groups (our unpublished data), although this rise could not be contributed to any particular lymphocyte subpopulation, as seen from the lack of difference between percentages of subpopulations. However, observed difference in lymphocyte count must be interpreted with caution because of the possibility of type I error, as indicated by false discovery rate analysis. Our future study will address the same question by examining a larger group of patients (minimally 49 per group, as shown by the sample size analysis).
Research on the immune function in PTSD showed generally an increased delayed-type hypersensitivity (3,25), higher percentage of perforin positive natural killer cells (24), and inconclusive natural killer cell cytotoxicity measurements (12,26,27). According to the results from chronic stress studies of decreased natural killer cell cytotoxicity (28), the same would be expected in chronic PTSD. Nevertheless, it was shown that Croatian professional soldiers released from concentration camp had higher natural killer cell cytotoxicity (29). Moreover, our preliminary results of a six years follow-up indicate that natural killer cell cytotoxicity declines over time in chronic PTSD, after the initial increase within eight years from the trauma, compared with healthy individuals (30). In this study, we were not able to show the group difference in natural killer cell cytotoxicity, as well as expected negative correlation with the time passed since the trauma. Prospective studies are needed to elucidate the dynamics of the immune function in chronic PTSD.
In contrast to immunological studies, extensive research has been done regarding the components of hypothalamic-pituitary-adrenal axis in PTSD (5). In fact, low cortisol level with enhanced negative feedback sensitivity to cortisol, ie, higher number of glucocorticoid receptors, is considered to be a distinctive biological feature of PTSD that separates this disorder from other psychiatric conditions such as depression (31). Simultaneous determination of cortisol and its receptor enables better characterization of the hypothalamic-pituitary-adrenal axis than cortisol alone (32). Using this approach, we found that Croatian combat veterans had higher cortisol concentration with lower lymphocyte glucocorticoid receptor expression (14). This finding was attributed to the limited duration of PTSD compared with other studies, and thus shorter duration of the stress response with preserved negative feedback by higher cortisol levels. During the repeated activation of stress response, as seen in PTSD, the stress system re-establishes stability at a higher set point by the process called allostasis that, over time, becomes a burden for the living system (allostatic load) (33). Thus, we expect that PTSD patients will adopt distinctive hypothalamic-pituitary-adrenal axis profile if the disorder persists. Present study investigated a group of PTSD patients with longer duration of the disorder (8 to 13 in this study vs 6 to 8 years in the previous study), so the lack of difference between the groups was not surprising. Moreover, Yehuda et al (34) showed a positive correlation between the amount of cortisol suppression after a low dose of dexamethasone (indirect glucocorticoid receptor sensitivity measure) and the number of years passed since the traumatic event. The hypothesis that the duration of allostatic load is the key factor in the modulation of hypothalamic-pituitary-adrenal axis activity is further supported by the positive correlation between lymphocyte glucocorticoid receptor expression and the number of years from the trauma found in this study (Figure 1). Preliminary results from our ongoing follow-up study, which show significant decrease of the glucocorticoid receptor expression in PTSD patients after six years follow up period, also support this hypothesis (30).
Another positive correlation between cortisol concentration and lymphocyte glucocorticoid receptor observed in this study suggests that factors other than endogenous glucocorticoids are involved in the mutual modulation of glucocorticoid receptor expression and cortisol secretion. A possible candidate is interleukin (IL)-6, which directly stimulates production of glucocorticoids (35) and also up-regulates glucocorticoid receptor and expression of glucocorticoid receptor mRNA in peripheral blood mononuclear cells (36). Although some studies reported increased IL-6 in cerebrospinal fluid (37) and sera (38) of PTSD patients, our future studies will further investigate the proposed relationship.
We should also point out the limitations of this study. The age difference could have confounded the results because aging is associated with the immunological changes that resemble those following chronic stress (39). Since this difference was very small and statistically controlled for by ANCOVA, it should have not interfered with the results. Another possible limitation is that 9 healthy controls were chosen from psychiatry hospital personnel (psychiatrists and technicians) who are believed to experience higher amount of stress than laboratory personnel or other healthy civilians not working in the hospital. Careful examination of the biological data from these individuals did not show any deviations from their counterparts in the control group. Although the examined group of patients was well-defined, relatively homogeneous, and without psychiatric comorobidities, depressive symptoms were not controlled for, neither in the PTSD nor in the control group.
Cross-sectional research of PTSD is connected with many obstacles, and inconsistency of the results in literature may be due to differences in gender, age, stressor type, diagnostic instrument chosen, and timing of the assessment in relation to the trauma onset. Moreover, biological changes in PTSD are very small and large sample sizes are needed to obtain enough statistical power to confirm them. Because of the lack of power, results from this study are more indicative than conclusive. More controlled laboratory challenges could reveal group differences in biological parameters in relation to acute individualized traumatic stimuli (40) and long term prospective studies (41) could confirm the dynamics of endocrine and immune parameters in PTSD implicated in this study.
sFigure 1. Representative flow cytometric analysis of glucocorticoid receptor expression in lymphocytes of the patient with posttraumatic stress disorder (PTSD). Lymphocytes were gated on light scatter dot plot (A), and the relative quantity of glucocorticoid receptor was calculated as the difference between mean fluorescence intensity of glucocorticoid receptor and isotype control (B). Overlaying histograms show that the glucocorticoid receptor expression is higher in PTSD patient. SSC-H – side scatter-hight; FSC-H – forward scatter-hight; GCR – glucocorticoid receptor; FITC – fluorescein isothiocyanate; full line – patient with posttraumatic stress disorder, dashed line – healthy volunteer.
Acknowledgments
This work was supported by grants from the Ministry of Science, Education and Sports of the Republic of Croatia (No. 0021003 to Dragan Dekaris). We gratefully acknowledge the assistance of Timea Berki (Department of Immunology and Biotechnology, University of Pécs, Faculty of Medicine, Pécs, Hungary) who provided us with anti-glucocorticoid receptor monoclonal antibody.
References
- 1.McEwen BS. The neurobiology and neuroendocrinology of stress. Implications for post-traumatic stress disorder from a basic science perspective. Psychiatr Clin North Am. 2002;25:469–94. doi: 10.1016/s0193-953x(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 2.Sabioncello A, Gotovac K, Vidović A, Gagro A, Markotić A, Rabatić S, et al. The immune system under stress. Period Biol. 2004;106:317–23. [Google Scholar]
- 3.Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann N Y Acad Sci. 2004;1032:141–53. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- 4.Wong CM. Post-traumatic stress disorder: advances in psychoneuroimmunology. Psychiatr Clin North Am. 2002;25:369–83. doi: 10.1016/s0193-953x(01)00006-5. [DOI] [PubMed] [Google Scholar]
- 5.de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–67. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Kiecolt-Glaser JK, Glaser R. Psychoneuroimmunology and health consequences: data and shared mechanisms. Psychosom Med. 1995;57:269–74. doi: 10.1097/00006842-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Bornstein SR, Rutkowski H. The adrenal hormone metabolism in the immune/inflammatory reaction. Endocr Res. 2002;28:719–28. doi: 10.1081/erc-120016992. [DOI] [PubMed] [Google Scholar]
- 8.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 9.Tuckermann JP, Kleiman A, McPherson KG, Reichardt HM. Molecular mechanisms of glucocorticoids in the control of inflammation and lymphocyte apoptosis. Crit Rev Clin Lab Sci. 2005;42:71–104. doi: 10.1080/10408360590888983. [DOI] [PubMed] [Google Scholar]
- 10.Wilson SN, van der Kolk B, Burbridge J, Fisler R, Kradin R. Phenotype of blood lymphocytes in PTSD suggests chronic immune activation. Psychosomatics. 1999;40:222–5. doi: 10.1016/S0033-3182(99)71238-7. [DOI] [PubMed] [Google Scholar]
- 11.Boscarino JA, Chang J. Higher abnormal leukocyte and lymphocyte counts 20 years after exposure to severe stress: research and clinical implications. Psychosom Med. 1999;61:378–86. doi: 10.1097/00006842-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Laudenslager ML, Aasal R, Adler L, Berger CL, Montgomery PT, Sandberg E, et al. Elevated cytotoxicity in combat veterans with long-term post-traumatic stress disorder: preliminary observations. Brain Behav Immun. 1998;12:74–9. doi: 10.1006/brbi.1997.0513. [DOI] [PubMed] [Google Scholar]
- 13.Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am. 2002;25:341–68. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]
- 14.Gotovac K, Sabioncello A, Rabatic S, Berki T, Dekaris D. Flow cytometric determination of glucocorticoid receptor (GCR) expression in lymphocyte subpopulations: lower quantity of GCR in patients with post-traumatic stress disorder (PTSD). Clin Exp Immunol. 2003;131:335–9. doi: 10.1046/j.1365-2249.2003.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanter ED, Wilkinson CW, Radant AD, Petrie EC, Dobie DJ, McFall ME, et al. Glucocorticoid feedback sensitivity and adrenocortical responsiveness in posttraumatic stress disorder. Biol Psychiatry. 2001;50:238–45. doi: 10.1016/s0006-3223(01)01158-1. [DOI] [PubMed] [Google Scholar]
- 16.Yehuda R, Golier JA, Kaufman S. Circadian rhythm of salivary cortisol in Holocaust survivors with and without PTSD. Am J Psychiatry. 2005;162:998–1000. doi: 10.1176/appi.ajp.162.5.998. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: WHO; 1992. [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington (DC): APA; 1994. [Google Scholar]
- 19.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 20.Guy W. ECDEU assessment manual for psychopharmacology. Revised. Washington (DC): US Government Printing Office; 1976. [Google Scholar]
- 21.Berki T, Kumanovics G, Kumanovics A, Falus A, Ujhelyi E, Nemeth P. Production and flow cytometric application of a monoclonal anti-glucocorticoid receptor antibody. J Immunol Methods. 1998;214:19–27. doi: 10.1016/s0022-1759(98)00037-4. [DOI] [PubMed] [Google Scholar]
- 22.Timonen T, Saksela E, Ranki A, Hayry P. Fractionation, morphological and functional characterization of effector cells responsible for human natural killer activity against cell-line targets. Cell Immunol. 1979;48:133–48. doi: 10.1016/0008-8749(79)90106-0. [DOI] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B Methodological. 1995;57:289–300. [Google Scholar]
- 24.Skarpa I, Rubesa G, Moro L, Manestar D, Petrovecki M, Rukavina D. Changes of cytolytic cells and perforin expression in patients with posttraumatic stress disorder. Croat Med J. 2001;42:551–5. [PubMed] [Google Scholar]
- 25.Watson IP, Muller HK, Jones IH, Bradley AJ. Cell-mediated immunity in combat veterans with post-traumatic stress disorder. Med J Aust. 1993;159:513–6. doi: 10.5694/j.1326-5377.1993.tb138003.x. [DOI] [PubMed] [Google Scholar]
- 26.Mosnaim AD, Wolf ME, Maturana P, Mosnaim G, Puente J, Kucuk O, et al. In vitro studies of natural killer cell activity in post traumatic stress disorder patients. Response to methionine-enkephalin challenge. Immunopharmacology. 1993;25:107–16. doi: 10.1016/0162-3109(93)90014-h. [DOI] [PubMed] [Google Scholar]
- 27.Ironson G, Wynings C, Schneiderman N, Baum A, Rodriguez M, Greenwood D. Posttraumatic stress symptoms, intrusive thoughts, loss, and immune function after Hurricane Andrew. Psychosom Med. 1997;59:128–41. doi: 10.1097/00006842-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauc G. Dabelic, Dumic J, Flogel M. Stressin and natural killer cell activity in professional soldiers. Ann N Y Acad Sci. 1998;851:526–30. doi: 10.1111/j.1749-6632.1998.tb09031.x. [DOI] [PubMed] [Google Scholar]
- 30.Vidović A, Gotovac K, Vilibić M, Sabioncello A, Rabatić S, Folnegović-Šmalc V, et al. Changes of immune and endocrine parameters in PTSD over time. In: Reine CS, editor. Journal of neuroimmunology. Special issue: abstracts of the Seventh international congress of neuroimmunology; 2004 Sep 28-Oct 2; Venice, Italy. Amsterdam: Elsevier; 2004. p. 139. [Google Scholar]
- 31.Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–14. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- 32.Yehuda R, Lowy MT, Southwick SM, Shaffer D, Giller EL., Jr Lymphocyte glucocorticoid receptor number in posttraumatic stress disorder. Am J Psychiatry. 1991;148:499–504. doi: 10.1176/ajp.148.4.499. [DOI] [PubMed] [Google Scholar]
- 33.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 34.Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM. Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology. 2004;29:389–404. doi: 10.1016/s0306-4530(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 35.Barkhudaryan N, Dunn AJ. Molecular mechanisms of actions of interleukin-6 on the brain, with special reference to serotonin and the hypothalamo-pituitary-adrenocortical axis. Neurochem Res. 1999;24:1169–80. doi: 10.1023/a:1020720722209. [DOI] [PubMed] [Google Scholar]
- 36.Angeli A, Masera RG, Sartori ML, Fortunati N, Racca S, Dovio A, et al. Modulation by cytokines of glucocorticoid action. Ann N Y Acad Sci. 1999;876:210–20. doi: 10.1111/j.1749-6632.1999.tb07641.x. [DOI] [PubMed] [Google Scholar]
- 37.Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, et al. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 2001;9:209–17. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- 38.Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R, et al. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry. 1999;45:833–9. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 39.Bauer ME. Stress, glucocorticoids and ageing of the immune system. Stress. 2005;8:69–83. doi: 10.1080/10253890500100240. [DOI] [PubMed] [Google Scholar]
- 40.Glover DA, Steele AC, Stuber ML, Fahey JL. Preliminary evidence for lymphocyte distribution differences at rest and after acute psychological stress in PTSD-symptomatic women. Brain Behav Immun. 2005;19:243–51. doi: 10.1016/j.bbi.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kellner M, Yehuda R, Arlt J, Wiedemann K. Longitudinal course of salivary cortisol in post-traumatic stress disorder. Acta Psychiatr Scand. 2002;105:153–5. doi: 10.1034/j.1600-0447.2002.01012.x. [DOI] [PubMed] [Google Scholar]