Abstract
We evaluated the role of exposure analysis in assessing whether ochratoxin A aristolochic acid are the agents responsible for causing Balkan endemic nephropathy. We constructed a framework for exposure analysis using the lessons learned from the study of endemic goiter within the context of an accepted general model. We used this framework to develop an exposure analysis model for Balkan endemic nephropathy, evaluated previous findings from the literature on ochratoxin A and aristolochic acid in the context of this model, discussed the strength of evidence for each, and proposed approaches to address critical outstanding questions. The pathway for exposure to ochratoxin A is well defined and there is evidence that humans have ingested ochratoxin A. Factors causing differential exposure to ochratoxin A and how ochratoxin A is implicated in Balkan endemic nephropathy are not defined. Although there is evidence of human exposure to aristolochic acid and that its effects are consistent with Balkan endemic nephropathy, a pathway for exposure to aristolochic acid has been suggested but not demonstrated. Factors causing differential exposure to aristolochic acid are not known. Exposure analysis results suggest that neither ochratoxin A nor aristolochic acid can be firmly linked to Balkan endemic nephropathy. However, this approach suggests future research directions that could provide critical evidence on exposure, which when linked with findings from the health sciences, may be able to demonstrate the cause of this disease and provide a basis for effective public health intervention strategies. One of the key unknowns for both agents is how differential exposure can occur.
In the approximately 60 years since Balkan endemic nephropathy was first reported, much has been learned about the clinical features and epidemiology of the disease. While it is generally agreed that at least one environmental toxin must be involved, and many have been proposed, the responsible agent has not been established. New cases continue to be reported today. The evaluation of suspected agents has largely focused on toxicological aspects, although a limited number of studies have attempted to evaluate the extent to which exposure might be consistent with the epidemiology of the disease. Several of these studies have suggested that certain agents are unlikely to cause the disease, while consistent but inconclusive evidence was found for others (1). These preliminary exposure analysis studies, combined with what is currently known about the toxicology and epidemiology, provide a basis for researchers to focus their attention on the most likely possibilities. At this point in time, the two potential agents for which reasonable cases can be made on the basis of both toxicity and exposure evidence are materials derived from plants of the Aristolochia species and mycotoxins, with particular emphasis on ochratoxin A (2). These two agents were the subject of the recent symposium from which this paper originates (3). We note, however, that other agents or combinations of agents that may not be proposed at this time remain a possibility.
The underpinning of exposure analysis is a model strategy that includes identification of the agent(s), the source(s) for the agent, the pathway(s) by which the agent gets to humans; exposure to the agent; and the disease attributed to the agent. This basic model is well established and described in the environmental health literature (4-8). It has been applied extensively in setting regulatory standards for environmental pollutants as a part of the risk assessment process.
Building on this general approach, Voice et al (1) recently proposed six criteria that can be used to evaluate the evidence for proposed agents. Two criteria relate to the nephrotoxic and carcinogenetic evidence, which are the subject of an extensive literature, and are not the subject of this paper. From the remaining criteria, we suggest that a convincing case for a proposed agent from an exposure analysis perspective should include the following evidentiary elements:
1. Exposure levels – evidence that exposure to this agent occurs in the endemic areas at levels capable of causing both nephrotoxic and carcinogenic effects based on the current toxicological evidence.
2. Exposure pathway – evidence on how the agent moves from an identified point of origin or source to the human receptors that is consistent with the known physical, chemical, and biological properties of the agent and environmental conditions and processes in endemic areas.
3. Exposure follows spatial pattern – evidence that significantly higher exposure occurs in endemic areas than in non-endemic areas and potentially in Balkan endemic nephropathy than in non-Balkan endemic nephropathy households.
4. Explanation for absence of Balkan endemic nephropathy in other exposure situations – evidence that either exposure does not occur in other locations where there is a source of the agent, or other factors that substantially mitigate the extent to which exposure results in disease.
5. Absence of other arguments – absence of strong alternative explanations for evidence offered as supportive.
In this article, we first discussed and built a framework for exposure analysis in the context of environmentally-linked endemic diseases that considered both environmental science and health science issues. The case of iodine deficiency and goiter provided a basis for this discussion as the underlying environmental and health science issues are well understood and the historical issues associated with the disease have similarities to those of Balkan endemic nephropathy. We then applied this approach to evaluating the available exposure evidence for ochratoxin A and aristolochic acid in the context of the evidentiary elements outlined above. We concluded discussions of ochratoxin A and aristolochic acid by identifying critical questions that future exposure analysis studies should addressed for these two proposed agents.
Exposure analysis (general model and endemic goiter case study)
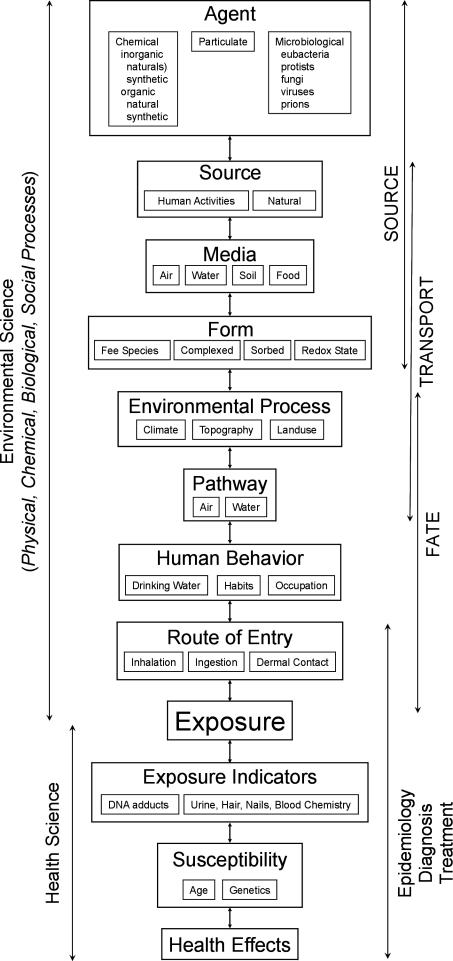
We have taken the formulations of the various exposure models of (4-8) and recast them in light of the environmental and health sciences (Figure 1) with exposure as the interface between the two sciences. In this context, we define environmental science as the application of the principles of physical, chemical, biological, and social processes to understand the source(s), transport, and fate of environmental agents. Dimensions of the health sciences include toxicology, epidemiology, diagnosis, pathology, and treatment of the disease. We suggest that this model could be useful in understanding Balkan endemic nephropathy, as well as any disease related to exposure to a specific environmental agent. Approaching the diagram deterministically, the arrows in the environmental science component follow the flow of knowledge from the source to exposure, while the arrows in the health science imply the flow of knowledge from exposure to establish the role of the agent in causing disease. However, the diagram also suggests the value of taking an inferential approach, in which observations are used as the basis for formulating testable hypotheses for those elements that lead to exposure and disease. In practice, we often work with fragmentary evidence at multiple points along the diagram and must use the available knowledge to postulate upstream processes and assess downstream consequences. Using a combination of forward (deterministic) and reverse (inferential) approaches, the goal is to ultimately complete the full source-to-disease picture.
Figure 1.
Proposed exposure analysis model for environmentally-linked endemic diseases. Modified from Ott (3,4) and Lioy (5).
To illustrate the various aspects of the exposure analysis model shown in Figure 1, we used what is known from many years of study of endemic goiter. Simple goiter is an enlargement of the thyroid gland when it is unable to meet metabolic demands. The original questions surrounding the etiology of endemic goiter in the United State and elsewhere in the world were similar to those related to Balkan endemic nephropathy. For example, the disease is spatially located, related to a naturally occurring agent, many hypotheses have been proposed for its etiology, and cases occur today. The development of a complete understanding of the etiology of endemic goiter is one example of how knowledge and approaches from both the environmental and the health research communities must be integrated to produce the full picture. With this knowledge, effective public health intervention strategies have been developed.
Endemic goiter is a very old, global disease (9) that was identified in the USA as early as 1797 (10). Early surveys of the incidence of goiter in Native Americans (1908 and 1923) began to reveal higher incident rates in the northern portion of the USA, as well as in western mountainous areas (11). The spatial distribution of endemic goiter in the USA was better defined using health records of draftees of World War I and showed that the highest incidence rates occurred in the Great Lakes and Pacific Northwest regions of the country, and that there was a gradient toward lower rates in the south (12).
Although over 40 hypotheses have been proposed to cause goiter, five have received the greatest attention; 1) iodine deficiency; 2) hard drinking water; 3) goitrogenic substances (goitrogenic factors) in food, water, soils/rocks; 4) pathogens; and 5) vitamin A deficiency (12). When iodine was discovered in 1811, it was suggested that it might cure goiter (13), and by 1820 it was being successfully used (14).
By 1927, studies of the spatial relationship between the lack of iodine in the environment and occurrence of goiter were being reported (15). The lack of environmental iodine is caused by two factors: 1) certain soil and rock types (16) are naturally deficient in iodine and 2) iodine has been removed from the environment due to leaching by water. Conditions that enhance removal of iodine include those associated with glaciation or climate conditions that allow for high rainfall (17).
We now put this background information on endemic goiter in the context of the framework for exposure analysis illustrated in Figure 1. We note that environmental agents are diverse and include natural and synthetic organic and inorganic chemical, particulate matter, and microbes. Some of the toxic organics, such as ochratoxin A, can be generated by microbial process. Sources for toxic agents can be natural (ultimately soils, rocks) or products of human activities such as industrial wastes. Human activities such as mining can also disrupt the environmental and enhance the availability of agents. The ultimate source for iodine is mineral weathering which adds its concentrations to soil and seawater and subsequently to food and marine biota, respectively. Except for releases of radioactive iodine, there is little disruption of its environmental behavior due to human activities (18).
Typically, there are four media in which an agent can occur: air, water, soil, and food. For iodine, water, soil, and food are important. One of the more challenging aspects is often to determine the form of the agent in the media. The form of the chemical can significantly change its toxicity, mobility, and bioavailability. Methyl mercury, for example, is significantly more toxic than elemental mercury. The geochemistry of iodine is fairly well established (18). Some of the iodine in the environmental exists as the free ionic species (iodide, I-), some complexed to oxygen (iodate, IO3-) and some sorbed to soils material as an organic complex. The particular form of iodine in the environment is in part of function of its redox state. Iodide is easily solubilized and transported in the environment, the dominate form in drinking water and the form necessary for health.
It must also be considered that the Earth is a dynamic system driving various physical, chemical, and biological processes. These environmental processes play a role in the environmental behavior of an agent. In terms of environmental health issues, knowledge of these processes can help to identify areas of risk and therefore understand the spatial patterns of the disease (16). For example, it is now recognized that the formation of methyl mercury relies upon the interaction of dissolved mercury, organic carbon, and sulfate. With this knowledge, preliminary maps have been created where environmental conditions and processes are conducive for this interaction and thus identify potential areas at risk for methyl mercury exposure in the USA (19). Similarly, knowledge of the factors influencing the availability of iodine, as discussed, has made it possible to predict areas of risk for goiter around the globe (20).
The three basic transport pathways for transportation are air, water, food. Water (typically groundwater) and food are major pathways for iodine.
Human behavioral patterns are a significant influence on the fate of environmental contaminants leading to exposure. Eating habits and occupation are examples, but frequently human behavior is not well defined. Knowledge of the location of soils and rocks that are naturally depleted in iodine or depleted by some environmental process such as running water allows prediction of the potential for exposure. Most endemic areas are inland. Populations in coastal areas who are at risk because of iodine depleted soils and water may not contract the disease because of higher rates of marine fish consumption (11,15,21) This serves to illustrate how understanding the human behavioral aspects, like environmental processes, can help explain the spatial distribution of diseases.
The final aspect is the route of entry of the agent. Inhalation, ingestion, and dermal contact are the major routes and as in the preceding discussion, ingestion is the route for iodine.
Defining these eight environmental science issues establishes that exposure (or lack of exposure) to iodine happens, the conditions under which exposure happens (the forward approach), and thus, an understanding of the spatial pattern of the endemic disease such as goiter.
However, exposure analysis is not complete without putting it in the health science context. Here we have simplified these aspects to include health effects, susceptibility, and exposure indicators (Figure 1). Early work established iodine as an essential nutrient required for normal thyroid function and that its deficiencies cause goiter. Young children (particularly girls) are more susceptible to iodine deficiencies as well as women who are pregnant. A low iodine concentration in affected thyroids was a clear indication of exposure.
In summary, solutions to the endemic goiter issue in the United States were based on medical science research that showed 1) that iodine deficiency could lead to goiter, 2) evidence of exposure through iodine deficiency in enlarged thyroids, and 3) development of well established criteria for diagnosis; and on environmental science research that showed 1) the forms and pathways of iodine in the environment, 2) the route of entry through ingestion of water and food, and 3) that endemic goiter spatially coincided with soil and rock types naturally deficient in iodine or sites where iodine had been removed by various environmental processes. The results of environmental science and health science research on endemic goiter have established two important factors in understanding the exposure, how exposure occurs, and the spatial pattern of the disease. The strong support for exposure from both environmental and health science perspectives confirms the role of iodine in the cause of endemic goiter. Public health measures such as the addition of iodine to diets in iodine deficient areas has significantly reduced the magnitude of endemic goiter. The history of endemic goiter in Croatia is a good example (22). However, goiter still remains a global problem, indicating there is less than a full understanding of the etiology of the disease (23).
Exposure analysis (applications to ochratoxin A and aristolochic acid)
Ochratoxin A
Ochratoxin A is a naturally occurring toxic metabolite produced by several molds of the Aspergillus flavus and Penicillium genera, including Aspergillus ochraceus (24). The literature on ochratoxin A is large and what we present here is not intended to be a complete review. Rather, we have reviewed the literature to find those papers that lead to credible knowledge on the various aspects of environmental and health sciences aspects leading to exposure. More detailed overviews of ochratoxin A can be found elsewhere (25-27).
Studies have shown ochratoxin A to be nephrotoxic (28), genotoxic (29), and immunotoxic (30) but uncertainties in its mode of action have complicated risk assessments over the past 10 years (27,30). Although some studies show elevated ochratoxin A in hemodialysis patients (31), there is a lack of epidemiological evidence substantiating its correlation with renal disease and cancer risk in human populations at relevant exposure levels (27,32). Recent studies have also speculated on the role of genetic susceptibility to ochratoxin A (28,29).
Known physical factors that influence the production of ochratoxin A include temperature, relative humidity, and insect infestation. Chemical factors include the use of fungicides and fertilizers (33). Warm temperatures and high humidity during grain and other food storage favor ochratoxin A formation, and the ability to predict the occurrence of ochratoxin A is developing (34-36). There are some suggestions that ochratoxin A contamination of food occurs primarily in pre-harvest periods (30). It has also been shown that ochratoxin A contamination can take place in the field in climates that are temperate and humid but such conditions are not as well defined as they are for storage (37). The climate of southern Europe favors the growth of Aspergillus species over Penicillium (38).
Evidence for widespread exposure to ochratoxin A by consumption of contaminated food products is unambiguous, with the human daily intake estimated to range from 0.7 to 4.7 ng/kg body weight in Western Europe and North America (39). Although foodstuff such as grains, cheese, and coffee have received much attention, there is growing interest in the occurrence of ochratoxin A in wine (38,40-42).
There have been many investigations designed to evaluate whether higher exposures occur, as measured by higher concentrations in foodstuffs in endemic villages or in endemic households than in selected controls (43-49). The results have been mixed, with some studies reporting statistically significant differences (47), both positive and negative, and others finding no significant differences (48). It appears that all studies faced a difficult challenge in attempting to demonstrate significance, due to the high level of variability within the comparative groups, regardless of the classification scheme. This variability is such that most studies report high exposure levels, relative to risk-based standards, but these levels are not inconsistent with what has been reported in many other regions of the world. Other mycotoxins have also been suggested as possible causative agents including citrinin, fumonisin B1 and B2, zearalenone, and aflatoxin B1 and G1, but these have received considerably less research attention and the few reports available suggest similar variance problems (46,50,51).
Attention has also been devoted to identifying and quantifying biomarkers in Balkan endemic nephropathy patients. One group reported two different studies identifying ochratoxin A in blood and urine samples of individuals from the Vratza endemic region of Bulgaria, but they apparently did not find differences between Balkan endemic nephropathy patients and non-patients (52,53). They also did not find a correlation with the consummation of ochratoxin A and recommended against using blood or urine levels as markers of exposure. Investigations reporting identification of DNA-ochratoxin A adducts (54,55) have been controversial, and more than one research group suggested that true-adduct formation had not been substantiated (56,57) or that putative adducts did not play a role in Balkan endemic nephropathy (58).
From the literature review we can begin to address the various issues in the model for the exposure analysis for ochratoxin A. Environmental science research has shown the potential for human exposure to ochratoxin A in both endemic and non endemic regions. In the agent box, it is well established that ochratoxin A is an organic toxin produced from microorganisms and has potential risks to human health. Sources for ochratoxin A are natural and occur mainly in food but can occur in soil. Human activities also influence the formation of ochratoxin A, for example poor grain storage practices. The media of occurrence is food and ochratoxin A is known to contaminate food that people eat in endemic and non endemic areas. Ochratoxin A essentially appears in particle-bound form through its association with fungi. A key factor in relating ochratoxin A to the distribution of Balkan endemic nephropathy is understanding the environmental process(es) that control its occurrence. As noted, efforts are being made along these lines. Temperature, humidity, and climate are known to influence the production of ochratoxin A but not with enough quantification to predict occurrence and thus potential differences between endemic and non-endemic environments. In addition, human behavioral patterns that might lead to differential exposure to ochratoxin A are not clear. Therefore, the reasons why exposure might differ between endemic and non endemic areas is ill defined. At the present time, the evidence does not support ochratoxin A as causative of Balkan endemic nephropathy. The critical issue is the need to establish a pattern of exposure differences between endemic and non-endemic areas. It does not appear to be as simple as the presence or absence of ochratoxin A as it is probably ubiquitous. Rather, differences might be related to either environmental processes and/or to human behaviors resulting in exposures that are not strictly reflective of environmental levels. It is possible that insights can be gained from research in other locales, such as that in Tunisia (28,59).
Health science research has shown ochratoxin A to be a health risk to humans. Results have also shown that humans have been exposed to ochratoxin A. Whether there is a significant difference in those exposed between non-endemic and endemic areas is unclear. In addition, a lack of accepted biomarkers to eliminate the uncertainties in the mechanism of action precludes understanding the role of ochratoxin A in causing Balkan endemic nephropathy. Until these questions are answered, health science cannot support exposure to ochratoxin A as the cause of Balkan endemic nephropathy.
In sum, limitations in both environmental and health science elements of exposure to ochratoxin A do not allow us to conclude that it is linked to Balkan endemic nephropathy. However, through this analysis, two questions that need to be answered to confirm the role of ochratoxin A in Balkan endemic nephropathy are made clear:
1. Given the debate on the influence of ochratoxin A on Balkan endemic nephropathy, what biomarkers can be identified that clearly establish the mechanism by which ochratoxin A causes Balkan endemic nephropathy?
2. Given the apparent widespread nature of ochratoxin A, what are the factors that cause differential exposure to ochratoxin A consistent with the disease pattern? For example can differential exposure be related to differences in food or food consumption patterns (42), job exposure (60), or a threshold value that is necessary before health effects are realized (61,62)
Finally, as noted, ochratoxin A is one of many types of mycotoxins and perhaps others need to be considered for their role(s) in Balkan endemic nephropathy (63).
Aristolochic acid
Evidence of exposure to aristolochic acid in an endemic nephropathy patients was first reported by Arlt who detected DNA adducts (as well as “ochratoxin A-related adducts”) in a small number of individuals from the Slavonski Brod region in Croatia (64). This evidentiary case was strengthened by Shibutani et al (65) who verified and quantified the presence of adducts in patients from the same region using 32P-postlabeling approaches, with confirmation of adduct structure by mass spectroscopy. To the best of our knowledge, statistically significant differences between Balkan endemic nephropathy patients and non-patients from endemic regions or residents of similar but non-endemic regions have not been reported at this time.
It appears that an exposure pathway for aristolochic acid was first proposed by Kazantizis (66) in 1967 who suggested ingestion of bread baked from flour that became contaminated as a result of the plant growing in wheat fields. Ivic (67) refined this hypothesis by reporting a series of field observations in the South Morava endemic region of Serbia in 1969. He indicated that Aristolochia clematitis was widespread in the region, and frequently but unevenly, co-mingled with cultivated wheat. He suggested that it was most prevalent in fields with poor soil, less cultivation, and more flooding, and those sown with unselected wheat grain. Ivic also reports that standard harvest practices were to reap the wheat with sickles, and bundle it into sheaves, with no attempt to separate the Aristolochia. He observed a variety of threshing techniques, but claims that Aristolochia seeds would not be separated from the grain, and would likely contaminate wheat. Although no methodology or data are provided, Ivic reports observing and counting Aristolochia seeds in wheat before milling. Hranjec et al (68) collected information on harvesting and milling techniques over the past 80 years in the Slavonski Brod region in Croatia and conclude that dietary exposure via bread made from contaminated wheat flour was plausible, especially with older, less selective, threshing and milling techniques.
The first apparent attempt to test this hypothesis was reported 36 years later by Hranjec et al (69) in 2005. This group conducted a case-control survey involving three groups in an endemic region of Croatia: EN patients, patients with other renal insufficiencies, and non-patients. It was reported that EN patients recall observing Aristolochia clematitis 20-30 ago more frequently than other groups, while all three groups recall decreases in A. clematitis prevalence in meadows and wheat fields, increases in herbicide use, and decreases in flooding. It was also reported that a greater percentage of individuals in endemic villages purchased their flour from local sources and baked their own bread than those from non-endemic villages. Analysis of A. clematitis seeds showed the presence of aristolochic acid at higher levels than in A. fangchi and A. manchurensis, which have been implicated in cases of renal failure resulting from herbal therapies.
It is clear from both animal and human studies that Aristolochia species plant material represents an agent exhibiting nephrotoxic and carcinogenic effects similar to those observed in Balkan endemic nephropathy. Aristolochic acid has been identified as the specific chemical toxin. Sources of aristolochic acid are abundant in endemic locales, as the plant is frequently found in disturbed landscapes, especially at interfaces, such as at the edges of agricultural fields, along roadways, and in gardens. This observation of prevalence does not alone substantiate its role in Balkan endemic nephropathy, however, because it is commonly found in many other areas, including locales proximate to the endemic areas where Balkan endemic nephropathy is not found. The overriding question is what causes the spatial pattern of the disease, and that if aristolochic acid is responsible, does this result from differences in prevalence, or is one of the subsequent elements of the model responsible for the exposure differences. Attention to date has focused on food, specifically wheat flour, as the medium, in which aristolochic acid is contained in the form of residual plant material, such as seeds. Environmental process and human behavior evidence is consistent with this pathway, as Aristolochia clematitis is frequently found growing within wheat fields, and a plausible argument has been offered that aristolochia seeds can be harvested with the wheat grain and would not necessarily be separated during milling such that they are incorporated into the flour. The route of entry would thus be ingestion of bread made from this flour.
This hypothesis, which was first articulated by Ivic, is well supported by a set of observations demonstrating its plausibility. The extent to which it has been critically tested is limited, however. We suggest that to establish wheat flour as the likely exposure pathway, a number of questions need to be addressed in studies designed to critically test elements of the hypothesis.
a) Can we verify the presence of aristolochic acid in either flour or bread to verify that this is a possible pathway?
b) Hranjec et al report differences in recollections of prevalence by residents of endemic and control villages in Croatia (69). Can this be substantiated in larger studies, studies in other endemic areas, and on the basis of more objective measures such as differences in soil quality, flooding, and seed purity, as proposed by Ivic (67)? Are there other factors not mentioned by Ivic, such as herbicide and fertilizer use, that likely affect prevalence and could be objectively measured?
c) Alternatively, if human behavior differences are responsible for the observed pattern of disease, can we find evidence to support behavior patterns that would lead to exposure differences? These could include considerations of harvest schedule, harvesting procedures, threshing and milling techniques, amounts and sources of flour consumed, and dietary habits.
d) Our recent observations in the endemic areas of Croatia and Bulgaria (our unpublished data) are not entirely consistent with Ivic’s hypothesis, and these inconsistencies need to be resolved. For example, we found that wheat is typically harvested in mid-summer when Aristolochia seeds are immature, contained within a large pulpy seed bulb that would be easily separated from wheat grain; that combine harvesters have been used in some villages for many years and that these would easily screen out the immature bulbs; and that many villages have had modern mechanized mills with sophisticated separation technologies available for many years.
We suggest that, given the strength of the evidence linking aristolochic acid exposure to disease, other potential pathways should also be considered. Unfortunately, there is little to be found in the literature on the environmental behavior of aristolochic acid to guide such an investigation. A number of possibilities derive from our field observations and the more general literature on Aristolochia sp
a) Could aristolochic acid exposure occur as a result of ingestion of Aristolochia clematitis by animals and consumption of animal products by people? There are previous reports of kidney disease in horses (70,71) but anecdotal comments by residents in our field investigation suggest that grazing animals avoid consuming this plant.
b) Could aristolochic acid, a by-product or related agent, be exuded by Aristolochia plants and taken up by food plants? The foods most likely would be those where Aristolochia grows in close proximity, which in our observations, include wheat and grapes. We are not aware of experimentally-determined parameters reflective of environmental mobility, but it is estimated that aristolochic acid is slightly soluble, and partitions very little to soil at pH 6 or above (72). This suggests that release and uptake by other plants could occur.
c) Could aristolochic acid exposure occur via dermal contact by field workers? We note an anecdotal report from Bulgaria indicating that nearly all of the workers on one particular farm developed nephritis.
d) It has apparently been a long-standing practice in this region to burn the wheat fields following the harvest. Could aristolochic acid exposure to field workers occur via the smoke, for example by inhalation, ingestion, or dermal contact?
e) Oral ingestion as a medicinal herb remains a possibility. There is a long history throughout the world of using Aristolochia sp for various purposes (73) and there are a few reports of such use in endemic areas (68,74).
Summary
Exposure analysis attempts to relate an environmental agent (eg, chemical, microbe) to the cause of a particular disease through the environmental sciences that examine how and if exposure can occur, and health sciences that examine evidence of exposure having taken place. A particular challenge for the environmental sciences in the case of endemic diseases such as Balkan endemic nephropathy is to account for the spatial pattern (ie, factors that cause differential exposure). We have discussed how exposure analysis confirms the role of iodine in endemic goiter.
Exposure analysis showed that neither ochratoxin A or aristolochic acid could be confirmed to cause Balkan endemic nephropathy. The analysis did reveal environmental and health sciences aspects that need study before the roles of either agent can be fully understood. For both agents, how differential exposure can occur is not known. Further, showing how ochratoxin A is involved in the diseases through biomarkers (health sciences) and how exposure to aristolochic acid happens through understanding process (environmental sciences) is needed.
Acknowledgments
This study was supported by grant 2 D43 TW00-641 from the Fogarty International Center, National Institutes of Health, USA, to the Institute of International Health at Michigan State University. We would like to thank our colleagues who attended the recent workshop on endemic nephropathy in Zagreb, Croatia (ref. 3) for their insights, and Bradley Detjen for his assistance with the literature review.
References
- 1.Voice TC, Long DT, Radovanovic Z, Atkins JL, McElmurry SP, Niagolova ND, et al. Critical evaluation of environmental exposure agents suspected in the etiology of Balkan endemic nephropathy. Int J Occup Environ Health. 2006;12:369–76. doi: 10.1179/oeh.2006.12.4.369. [DOI] [PubMed] [Google Scholar]
- 2.Stefanovic V, Toncheva D, Atanasova S, Polenakovic M. Etiology of Balkan endemic nephropathy and associated urothelial cancer. Am J Nephrol. 2006;26:1–11. doi: 10.1159/000090705. [DOI] [PubMed] [Google Scholar]
- 3.Maver H, Rudan P, editors. Recent advances in endemic nephropathy. Zagreb: Collegium Antropol. 2006; 30 Suppl l. [PubMed] [Google Scholar]
- 4.Ott W. Total human exposure: an emerging science focuses on humans as receptors of environmental pollution. Environ Sci Technol. 1985;19:880–6. [Google Scholar]
- 5.Ott WR, Steinemann AC, Wallace LA, editors. Exposure analysis. Ann Arbor: CRC Press; 2006. [Google Scholar]
- 6.Lioy PJ. Measurement methods for human exposure analysis. Environ Health Perspect. 1995;103(Suppl 3):35–43. doi: 10.1289/ehp.95103s335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rangan U, Hedli C, Gallo M, Lioy P, Snyder R. Exposure and risk assessment with respect to contaminated soil: significant biomarkers and bioavailability. Int J Toxicol. 1997;16:419–32. [Google Scholar]
- 8.Weis BK, Balshaw D, Barr JR, Brown D, Ellisman M, Lioy P, et al. Personalized exposure assessment: promising approaches for human environmental health research. Environ Health Perspect. 2005;113:840–8. doi: 10.1289/ehp.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sos J. Geographical distribution and the relevant pathogenetical relationship of endemic goiter. Geogr Med. 1969-70;1:11–4. [Google Scholar]
- 10.Greenwald I. The significance of the history of goiter for the etiology of the disease. Am J Clin Nutr. 1960;8:801–7. doi: 10.1093/ajcn/6.2.801. [DOI] [PubMed] [Google Scholar]
- 11.Schiel JB, Jr, Wepfer AJ. Distributional aspects of endemic goiter in the United States. Econ Geogr. 1976;52:116–22. [Google Scholar]
- 12.McClendon J, Williams A. Simple goiter as a result of iodine deficiency. JAMA. 1923;80:600–1. [Google Scholar]
- 13.Courtois B. Discovery of a new substance in the kelp. Ann Chim. 1813;88:304–10. [In French] [Google Scholar]
- 14.Coindet J.Discovery of a new remedy against goiter[In French]Ann Clin Phys 182049 [Google Scholar]
- 15.McClendon J. The distribution of iodine with special reference to goiter. Physiol Rev. 1927;7:25–30. [Google Scholar]
- 16.Lin NF, Tang J, Bian JM. Geochemical environment and health problems in China. Environ Geochem Health. 2004;26:81–8. doi: 10.1023/b:egah.0000020987.74065.1d. [DOI] [PubMed] [Google Scholar]
- 17.Fordyce FM, Johnson CC, Navaratna UR, Appleton JD, Dissanayake CB. Selenium and iodine in soil, rice and drinking water in relation to endemic goitre in Sri Lanka. Sci Total Environ. 2000;263:127–41. doi: 10.1016/s0048-9697(00)00684-7. [DOI] [PubMed] [Google Scholar]
- 18.Fuge R, Johnson C. The geochemistry of iodine: a review. Environ Geochem Health. 1985;8:31–54. doi: 10.1007/BF02311063. [DOI] [PubMed] [Google Scholar]
- 19.Booth N, Krabbenhoft D, Saltman T. Mapping modeled national-scale vulnerability to mercury loading across the United States: a new use for nationally available and consistent aquatic and terrestrial databases. In: Eighth International Conference on Mercury as a Global Pollutant. Aug 6-11, 2006. Madison (WI): University of Wisconsin; 2006. [Google Scholar]
- 20.de Benoist B, Andersson M, Egli I, Takkouche B, Allen H, editors. Iodine status worldwide. Geneva: World Health Organization; 2004. [Google Scholar]
- 21.Ezaki H, Ebihara S, Fujimoto Y, Iida F, Ito K, Kuma K, et al. Analysis of thyroid carcinoma based on material registered in Japan during 1977-1986 with special reference to predominance of papillary type. Cancer. 1992;70:808–14. doi: 10.1002/1097-0142(19920815)70:4<808::aid-cncr2820700415>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 22.Kusic Z, Jukic T. History of endemic goiter in Croatia: from severe iodine deficiency to iodine sufficiency. Coll Antropol. 2005;29:9–16. [PubMed] [Google Scholar]
- 23.McNeil DG Jr. In raising the world's I.Q., the secret's in the salt. New York Times. Dec 16, 2006. Available from: http://www.nytimes.com/2006/12/16/health/16iodine.html?ex=1323925200&en=8774496ea20281f7&ei=5090 Accessed: February 26, 2007.
- 24.van der Merwe KJ, Steyn PS, Fourie L, Scott DB, Theron JJ. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature. 1965;205:1112–3. doi: 10.1038/2051112a0. [DOI] [PubMed] [Google Scholar]
- 25.Moss MO. Mycotoxin review – 1. Aspergillus and Penicillium. Mycologist. 2002;16:116–9. [Google Scholar]
- 26.Fung F, Clark RF. Health effects of mycotoxins: a toxicological overview. J Toxicol Clin Toxicol. 2004;42:217–34. doi: 10.1081/clt-120030947. [DOI] [PubMed] [Google Scholar]
- 27.Clark HA, Snedeker SM. Ochratoxin a: its cancer risk and potential for exposure. J Toxicol Environ Health B Crit Rev. 2006;9:265–96. doi: 10.1080/15287390500195570. [DOI] [PubMed] [Google Scholar]
- 28.Hassen W, Abid-Essafi S, Achour A, Guezzah N, Zakhama A, Ellouz F, et al. Karyomegaly of tubular kidney cells in human chronic interstitial nephropathy in Tunisia: respective role of Ochratoxin A and possible genetic predisposition. Hum Exp Toxicol. 2004;23:339–46. doi: 10.1191/0960327104ht458oa. [DOI] [PubMed] [Google Scholar]
- 29.Lebrun S, Golka K, Schulze H, Follmann W. Glutathione S-transferase polymorphisms and ochratoxin A toxicity in primary human urothelial cells. Toxicology. 2006;224:81–90. doi: 10.1016/j.tox.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 30.Al-Anati L, Petzinger E. Immunotoxic activity of ochratoxin A. J Vet Pharmacol Ther. 2006;29:79–90. doi: 10.1111/j.1365-2885.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- 31.Ozcelik N, Kosar A, Soysal D. Ochratoxin A in human serum samples collected in Isparta-Turkey from healthy individuals and individuals suffering from different urinary disorders. Toxicol Lett. 2001;121:9–13. doi: 10.1016/s0378-4274(00)00291-5. [DOI] [PubMed] [Google Scholar]
- 32.Walker R, Larsen JC. Ochratoxin A: previous risk assessments and issues arising. Food Addit Contam. 2005;22(Suppl 1):6–9. doi: 10.1080/02652030500309343. [DOI] [PubMed] [Google Scholar]
- 33.Hussein HS, Brasel JM. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology. 2001;167:101–34. doi: 10.1016/s0300-483x(01)00471-1. [DOI] [PubMed] [Google Scholar]
- 34.Pardo E, Marin S, Sanchis V, Ramos AJ. Prediction of fungal growth and ochratoxin A production by Aspergillus ochraceus on irradiated barley grain as influenced by temperature and water activity. Int J Food Microbiol. 2004;95:79–88. doi: 10.1016/j.ijfoodmicro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Pardo E, Sanchis V, Ramos AJ. Impact of relative humidity and temperature on visible fungal growth and OTA production of ochratoxigenic Asperigillus ochraceus isolated on grapes. Food Microbiol. 2005;22:383–9. [Google Scholar]
- 36.Lindblad M, Johnsson P, Jonsson N, Lindqvist R, Olsen M. Predicting noncompliant levels of ochratoxin A in cereal grain from Penicillium verrucosum counts. J Appl Microbiol. 2004;97:609–16. doi: 10.1111/j.1365-2672.2004.02332.x. [DOI] [PubMed] [Google Scholar]
- 37.Elmholt S. Ecology of the ochratoxin A producing Penicillium verrucosum: occurence in field soil and grain with special attention to farming system and on-farm drying practices. Biol Agric Hortic. 2003;20:311–37. [Google Scholar]
- 38.Blesa J, Soriano JM, Molto JC, Manes J. Factors affecting the presence of ochratoxin A in wines. Crit Rev Food Sci Nutr. 2006;46:473–8. doi: 10.1080/10408390500215803. [DOI] [PubMed] [Google Scholar]
- 39.Kuiper-Goodman T, Scott PM. Risk assessment of the mycotoxin ochratoxin A. Biomed Environ Sci. 1989;2:179–248. [PubMed] [Google Scholar]
- 40.Battilani P, Pietri A, Bertuzzi T, Languasco L, Giorni P, Kozakiewicz Z. Occurrence of ochratoxin A-producing fungi in grapes grown in Italy. J Food Prot. 2003;66:633–6. doi: 10.4315/0362-028x-66.4.633. [DOI] [PubMed] [Google Scholar]
- 41.Otteneder H, Majerus P. Occurrence of ochratoxin A (OTA) in wines: influence of the type of wine and its geographical origin. Food Addit Contam. 2000;17:793–8. doi: 10.1080/026520300415345. [DOI] [PubMed] [Google Scholar]
- 42.Stefanaki I, Foufa E, Tsatsou-Dritsa A, Dais P. Ochratoxin A concentrations in Greek domestic wines and dried vine fruits. Food Addit Contam. 2003;20:74–83. doi: 10.1080/0265203021000031537. [DOI] [PubMed] [Google Scholar]
- 43.Krogh P, Hald B, Plestina R, Ceovic S. Balkan (endemic) nephropathy and foodborn ochratoxin A: preliminary results of a survey of foodstuffs. Acta Pathol Microbiol Scand [B] 1977;85:238–40. doi: 10.1111/j.1699-0463.1977.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 44.Petkova-Bocharova T, Castegnaro M. Ochratoxin A contamination of cereals in an area of high incidence of Balkan endemic nephropathy in Bulgaria. Food Addit Contam. 1985;2:267–70. doi: 10.1080/02652038509373555. [DOI] [PubMed] [Google Scholar]
- 45.Radic B, Fuchs R, Peraica M, Lucic A. Ochratoxin A in human sera in the area with endemic nephropathy in Croatia. Toxicol Lett. 1997;91:105–9. doi: 10.1016/s0378-4274(97)03877-0. [DOI] [PubMed] [Google Scholar]
- 46.Vrabcheva T, Usleber E, Dietrich R, Martlbauer E. Co-occurrence of ochratoxin A and citrinin in cereals from Bulgarian villages with a history of Balkan endemic nephropathy. J Agric Food Chem. 2000;48:2483–8. doi: 10.1021/jf990891y. [DOI] [PubMed] [Google Scholar]
- 47.Puntaric D, Bosnir J, Smit Z, Skes I, Baklaic Z. Ochratoxin A in corn and wheat: geographical association with endemic nephropathy. Croat Med J. 2001;42:175–80. [PubMed] [Google Scholar]
- 48.Abouzied MM, Horvath AD, Podlesny PM, Regina NP, Metodiev VD, Kamenova-Tozeva RM, et al. Ochratoxin A concentrations in food and feed from a region with Balkan Endemic Nephropathy. Food Addit Contam. 2002;19:755–64. doi: 10.1080/02652030210145036. [DOI] [PubMed] [Google Scholar]
- 49.Vrabcheva T, Petkova-Bocharova T, Grosso F, Nikolov I, Chernozemsky IN, Castegnaro M, et al. Analysis of ochratoxin A in foods consumed by inhabitants from an area with balkan endemic nephropathy: a 1 month follow-up study. J Agric Food Chem. 2004;52:2404–10. doi: 10.1021/jf030498z. [DOI] [PubMed] [Google Scholar]
- 50.Pfohl-Leszkowicz A, Petkova-Bocharova T, Chernozemsky IN, Castegnaro M. Balkan endemic nephropathy and associated urinary tract tumours: a review on aetiological causes and the potential role of mycotoxins. Food Addit Contam. 2002;19:282–302. doi: 10.1080/02652030110079815. [DOI] [PubMed] [Google Scholar]
- 51.Domijan AM, Peraica M, Zlender V, Cvjetkovic B, Jurjevic Z, Topolovec-Pintaric S, et al. Seed-borne fungi and ochratoxin A contamination of dry beans (Phaseolus vulgaris L.) in the Republic of Croatia. Food Chem Toxicol. 2005;43:427–32. doi: 10.1016/j.fct.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Petkova-Bocharova T, Chernozemsky IN, Castegnaro M. Ochratoxin A in human blood in relation to Balkan endemic nephropathy and urinary system tumours in Bulgaria. Food Addit Contam. 1988;5:299–301. doi: 10.1080/02652038809373707. [DOI] [PubMed] [Google Scholar]
- 53.Castegnaro M, Canadas D, Vrabcheva T, Petkova-Bocharova T, Chernozemsky IN, Pfohl-Leszkowicz A. Balkan endemic nephropathy: role of ochratoxins A through biomarkers. Mol Nutr Food Res. 2006;50:519–29. doi: 10.1002/mnfr.200500182. [DOI] [PubMed] [Google Scholar]
- 54.Pfohl-Leszkowicz A, Castegnaro M. Further arguments in favour of direct covalent binding of Ochratoxin A (OTA) after metabolic biotransformation. Food Addit Contam. 2005;22(Suppl 1):75–87. doi: 10.1080/02652030500309400. [DOI] [PubMed] [Google Scholar]
- 55.Manderville RA. A case for the genotoxicity of ochratoxin A by bioactivation and covalent DNA adduction. Chem Res Toxicol. 2005;18:1091–7. doi: 10.1021/tx050070p. [DOI] [PubMed] [Google Scholar]
- 56.Mally A, Dekant W. DNA adduct formation by ochratoxin A: review of the available evidence. Food Addit Contam. 2005;22(Suppl 1):65–74. doi: 10.1080/02652030500317544. [DOI] [PubMed] [Google Scholar]
- 57.Turesky RJ. Perspective: ochratoxin A is not a genotoxic carcinogen. Chem Res Toxicol. 2005;18:1082–90. doi: 10.1021/tx050076e. [DOI] [PubMed] [Google Scholar]
- 58.Arlt VM, Pfohl-Leszkowicz A, Cosyns J, Schmeiser HH. Analyses of DNA adducts formed by ochratoxin A and aristolochic acid in patients with Chinese herbs nephropathy. Mutat Res. 2001;494:143–50. doi: 10.1016/s1383-5718(01)00188-7. [DOI] [PubMed] [Google Scholar]
- 59.Abid S, Hassen W, Achour A, Skhiri H, Maaroufi K, Ellouz F, et al. Ochratoxin A and human chronic nephropathy in Tunisia: is the situation endemic? Hum Exp Toxicol. 2003;22:77–84. doi: 10.1191/0960327103ht328oa. [DOI] [PubMed] [Google Scholar]
- 60.Halstensen AS, Nordby KC, Elen O, Eduard W. Ochratoxin A in grain dust – estimated exposure and relations to agricultural practices in grain production. Ann Agric Environ Med. 2004;11:245–54. [PubMed] [Google Scholar]
- 61.Kovalsky VV. Geochemical ecology and problems of health. Philos Trans R Soc Lond B Biol Sci. 1979;288:185–91. doi: 10.1098/rstb.1979.0100. [DOI] [PubMed] [Google Scholar]
- 62.EFSA Opinion of the CONTAM Panel related to ochratoxin A in food. Available from: http://www.efsa.europa.eu/en/science/contam/contam_opinions/1521.html Accessed: December 17, 2006.
- 63.Knasmuller S, Cavin C, Chakraborty A, Darroudi F, Majer BJ, Huber WW, et al. Structurally related mycotoxins ochratoxin A, ochratoxin B, and citrinin differ in their genotoxic activities and in their mode of action in human-derived liver (HepG2) cells: implications for risk assessment. Nutr Cancer. 2004;50:190–7. doi: 10.1207/s15327914nc5002_9. [DOI] [PubMed] [Google Scholar]
- 64.Arlt VM, Ferluga D, Stiborova M, Pfohl-Leszkowicz A, Vukelic M, Ceovic S, et al. Is aristolochic acid a risk factor for Balkan endemic nephropathy-associated urothelial cancer? Int J Cancer. 2002;101:500–2. doi: 10.1002/ijc.10602. [DOI] [PubMed] [Google Scholar]
- 65.Shibutani S, Suzuki N, Fernandes A, Leko N, Jelakovic B, Grollman AP. Balkan endemic nephropathy: A preventable environmental disease. Cancer Epidemiol Biomarkers Prev. 2005;14:2717S–2717S. [Google Scholar]
- 66.Kazantzis G. Comment in the general discussion: possible nephrotoxic agents. In: Wolstenholme GE, Knight J, editors. The Balkan nephropathy. Boston (MA): Ciba Foundation; 1967. p. 114. [Google Scholar]
- 67.Ivic M. Etiology of endemic nephropathy. Lijec Vjesn. 1969;91:1273–81. [in Croatian] [PubMed] [Google Scholar]
- 68.Hranjec T. KovacPejic A, Kos J, Dika Z, Brzic I, Mao WY, et al. Dietary exposure to Aristolochia clematitis is a risk factor for endemic nephropathy. In: Maver H, Rudan P, editors. Recent advances in endemic nephropathy 2006; Zagreb: Collegium Antropol. 2006; 30 Suppl 1:37. [Google Scholar]
- 69.Hranjec T, Kovac A, Kos J, Mao W, Chen JJ, Grollman AP, et al. Endemic nephropathy: the case for chronic poisoning by aristolochia. Croat Med J. 2005;46:116–25. [PubMed] [Google Scholar]
- 70.Martincic M. Toxic effects of Aristolochia clematitis on horses' kidneys. Vetererinarski Arhiv. 1957;27:51–9. [in German] [Google Scholar]
- 71.Dumic A. Horse poisoning with Aristolochia clematitis [in Croatian]. In: Zagreb: Izdanje Vojno tehnickog glasnika; 1954. p. 3-45. [Google Scholar]
- 72.Chemical Abstracts Registry. Aristolochic acid (electronic number cas 313-67-7). [database on the Internet]. American Chemical Society. 2007. Available from: http:www.cas.org/EO/regsys.html. Accessed: February 7, 2007.
- 73.Scarborough J. Kidney Disease among the Romans. In: Maver H, Rudan P, editors. Recent advances in endemic nephropathy; 2006; Zagreb, Croatia: Collegium Antropol. 2006;30 Suppl 1:5-6. [Google Scholar]
- 74.Ploetz KL. An ethnobotanical study of wild herb use in Bulgaria [M.S. thesis]. Houghton (MI): Michigan Technological University; 2000. [Google Scholar]