Abstract
Aim
To evaluate the cardioprotective effect of sevoflurane on a beating heart in patients undergoing coronary artery bypass grafting with normal preoperative left ventricular function.
Methods
The randomized controlled study included 32 patients induced with sevoflurane and then randomized to receive either 1 minimal alveolar concentration (MAC) end-tidal concentration of sevoflurane (n = 16) or propofol (n = 16) 2 to 3 mg kg-1 hour-1. The acceleration of the aortic blood flow, cardiac index, heart rate, mean arterial pressure, and central venous pressure were measured 5 minutes after anesthesia induction, at the beginning of ischemia, 15 minutes after ischemia, and 15 minutes after sternum closure.
Results
There were no differences in heart rate, mean arterial pressure, and central venous pressure within each group and between groups during surgery. Acceleration increased in the sevoflurane group 15 minutes after ischemia (10.3 ± 3.5 m/s2; P = 0.004) and 15 minutes after sternum closure (10.7 ± 3.9 m/s2; P<0.001). Acceleration in the propofol group decreased from the beginning of ischemia (P<0.001) and remained lower 15 minutes after sternum closure (P = 0.001 and P = 0.024, respectively). Acceleration was higher in the sevoflurane group at the beginning of ischemia and 15 minutes after sternum closure (P = 0.017 and P = 0.046, respectively). There were no significant differences in cardiac index values within the sevoflurane group. In the propofol group, significant decreases in cardiac index were seen at the beginning of ischemia (P<0.001). There were between-group differences in cardiac index values at the beginning of ischemia and 15 minutes after ischemia (P = 0.002, and P = 0.011, respectively).
Conclusion
Cardiac function was better preserved in the patients anesthetized with sevoflurane than in patients anesthetized with propofol.
Trial Registration ClinicalTrials.gov Identifier
Ischemic-reperfusion event occurs in many clinical situations, especially in cardiac and vascular surgery, neurosurgery, and transplant surgery (1).
Ischemic “preconditioning,” as originally described by Murry et al (2), is defined as a rapid, adaptive response to a brief ischemic insult that improves the tolerance of the myocardium to a subsequent period of prolonged ischemia. During cardiac surgery, ischemic preconditioning of the heart can be utilized as an effective adjunct to myocardial protection, but may not be getting widespread use. Volatile anesthetics are another effective adjunct, which provide protection against reperfusion injury (postconditioning) (3).
In vitro studies and in vivo animal experiments have shown that halogenated volatile anesthetics have a protective effect on the ischemic myocardium (4). In clinical settings, however, anesthetic preconditioning may be of more interest. The underlying mechanisms are still under investigation, but it seems that protection of myocytes is mediated through an effect on mitochondrial and sarcolemmal adenosine triphosphate-regulated potassium (KATP) channels (5,6).
Studies have been performed on human patients undergoing coronary artery bypass grafting (CABG) surgery with cardiopulmonary bypass (CPB) (7,8). Only a few studies, however, have evaluated the effects of volatile anesthetics during coronary artery bypass grafting on a beating heart (OPCABG), with conflicting results as far as cardiac biomarker release is concerned (9,10).
Because CPB is known to have a profound impact on cardiac function, studies performed on patients scheduled for off-pump coronary artery bypass grafting could evaluate more specifically the effects of the anesthetic agents themselves. Patients undergoing off-pump coronary artery bypass grafting have a predictable and predefined ischemic zone during surgery and represent an extremely interesting and safe model for the study of ischemia and cardiac damage in humans (11).
Presently, there is still no consensus on the method of administration of volatile anesthetics, including the time to begin administration, its duration, the dosage, and selection of volatile anesthetics.
The aim of our study was to evaluate the cardioprotective effect of sevoflurane in patients undergoing off-pump coronary artery bypass grafting surgery. We proposed that a cardioprotective effect of sevoflurane would save myocardial function, which we measured as acceleration of the aortic blood flow by esophageal Doppler and cardiac index with bolus thermodilution methods, both during brief ischemia and reperfusion.
Patients and methods
Patients
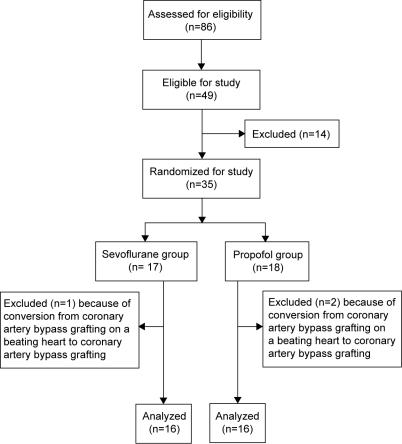
The study was conducted at Clinical Department of Anesthesiology, Reanimatology, and Intensive Care Medicine, University Hospital Dubrava, Zagreb, between August 1, 2006 and December 31, 2006. Out of 86 patients admitted to the department, 49 patients were eligible for the study as they had the diagnosis of coronary artery disease. Out of 49 eligible patients, 35 met the inclusion criteria and were randomized into two groups (Figure 1). The inclusion criteria were as follows: the degree I or II of Cardiac Anesthesia Risk Evaluation score (12), angiographically verified coronary artery disease, and left ventricular ejection fraction higher than 40%. There were 24 male and 11 female patients, all younger than 75 years (Table 1). Patients were excluded from the study if they had any kind of heart disease other than coronary artery disease, congenital heart disease, atrioventricular conduction disturbances, evidence of previously ventricular arrhythmias requiring antiarrhythmic treatment, atrial fibrillation with rapid ventricular response, and any kind of valvular heart disease which required using CPB. Patients with myocardial infarction or stroke within 6 months, diabetes mellitus, end-stage of obstructive or restrictive pulmonary disease, or sepsis, and patients taking antiarrhythmic or digoxin therapy or if required inotropic support before surgery were excluded from the study. Exclusion criteria during surgery were myocardial ischemia (depression or elevation of the ST-segment of more than 1 mm on 12-lead electrocardiography) and hemodynamic instability (heart rate >100 beats/min, systolic blood pressure <90 mm Hg, or need for cardiac pacing). Two patients randomized into the propofol group and one patient randomized in the sevoflurane group were subsequently excluded from the investigation and data analysis because they met the exclusion criteria during surgery (extreme hemodynamic instability that required conversion from off-pump coronary artery bypass grafting to coronary artery bypass grafting surgery) (Figure 1).
Figure 1.
The flow diagram of patients through the study.
Table 1.
Preoperative and operative characteristics of patients underwent coronary artery bypass grafting on a beating heart
| Treatment (mean±SD)* |
|||
|---|---|---|---|
| Characteristics | sevoflurane (n = 16) | propofol (n = 16) | P† |
| Preoperative data: | |||
| age (years) | 61.3 ± 5.9 | 64.8 ± 6.1 | 0.091 |
| body surface area (m2) | 1.9 ± 0.2 | 1.9 ± 0.1 | 0.564 |
| Operative data: | |||
| number of grafts | 2.7 ± 1.0 | 2.5 ± 0.7 | 0.532 |
| duration of coronary occlusion‡ | 15.8 ± 5.7 | 16.9 ± 6.3 | 0.534 |
*Abbreviations: SD – standard deviation.
†ANOVA test.
‡The sum of single coronary occlusions.
The study protocol was approved by the institutional review board (hospital ethical committee) and was consistent with the principles of the Declaration of Helsinki. All patients were informed about the investigation and signed an informed consent form before surgery.
Study design
The patients in a single blinded study were randomized into two groups by an anesthesiologist who drew patients’ numbers from a hat on the day before surgery. The same anesthesiologist performed anesthesia procedure in all randomized patients. After canulation of the radial artery, induction with anesthesia was performed, and intubation and mechanical ventilation were applied. The anesthesiologist then inserted the pulmonary artery catheter and esophageal Doppler probe. Measurements were performed at the following intervals: 0) 5 minutes after anesthesia induction; 1) at the beginning of ischemia, when the left anterior descending coronary artery was occluded – ischemic stage; 2) 15 minutes after ischemia, when occlusion of left anterior descending coronary artery was terminated – reperfusion stage; and 3) 15 minutes after sternum closure. In both groups, measurements were performed simultaneously with the esophageal Doppler and pulmonary artery catheter. During surgery, vital signs were followed, and in the event that the mean arterial pressure dropped lower than 60 mm Hg, phenylephrine was applied as an intravenous bolus. During surgery, Ringer’s-lactate (Croatian Institute for Transfusion Medicine, Zagreb, Croatia) or 500 mL hydroxyethylstarch 6% solution (HAES-sterile 6% in saline 0.9%; Fresenius Kabi, Bad Homburg, Germany) was administered in all patients to optimize preload.
Anesthesia procedure
Patients received their cardiac medications on the morning of surgery. Long-acting medications (calcium antagonists and angiotensin converting enzyme inhibitors) were discontinued a day before the beginning of the study. All patients were premedicated with morphine at a dose of 0.1 mg/kg IM (Morphine Merck®, Merck KgaA, Darmstadt, Germany) one hour before surgery. Anesthesia was induced by 8% vol. sevoflurane (Sevorane®, Abbott Laboratories S.A., Abbott Park, IL, USA) with high 100% oxygen flow (7 L/min) until the patient lost consciousness. Sevoflurane was administered with a Dräger vaporizer (Dräger, Lübeck, Germany). After endotracheal intubation, the lungs were mechanically ventilated by positive pressure (tidal volume of 8 mL/kg and ventilatory frequency of 12/min) (Primus; Dräger). After induction, patients were randomized into two groups. In the sevoflurane group anesthesia was maintained at 1 minimal alveolar concentration (MAC) of sevoflurane. In the propofol group, sevoflurane was switched to continuous infusion of propofol (Diprivan®, Fresenius Kabi, Graz, Austria) and anesthesia was maintained with doses between 2 to 3 mg kg-1 h-1. In both groups, remifentanil (Ultiva®, GlaxoSmithKline, Auckland, New Zealand) was administrated to provide adequate analgesia at a dose of 0.3 μg kg-1 h-1 via target-controlled infusion. For muscular relaxation, all patients received pancuronium-bromide (Pavulon®, N.V. Organon, Oss, the Netherlands) at a bolus-dose of 0.1 mg/kg intravenously. Additional doses of pancuronium-bromide (0.1 mg/kg) were administered as required to maintain neuromuscular blockade during surgery.
Hemodynamic monitoring
American Society of Anesthesiologists (ASA) standard monitors were applied to the patients on admission to the operating room (13). In the operating room, an arterial catheter (Arrow International, Reading, PA, USA) was inserted into the left radial artery at an angle of 35% to the plane of the wrist, to measure direct arterial blood pressure. Seldinger’s technique (14) was used for the central venous catheter placement and for the introduction of a 5-lumen, 7.5 French pulmonary artery catheter (Arrow International) into the right internal jugular vein. Insertion of the pulmonary artery catheter via the jugular vein through the heart and into a pulmonary artery branch was controlled by a monitor (Hewlett Packard Viridia CMS; Böblingen, Germany). Pulmonary artery catheter was fixed after being placed into the pulmonary capillary wedge position, usually 55-60 cm from the internal jugular vein puncture site. Central venous, radial, and pulmonary arterial pressure transducers (Peter von Berg, Kirschseeon, Germany) were zeroed at the level of the left atrium. Electrocardiographic leads II and V5, heart rate, central venous, radial, and pulmonary artery pressure curves and values were monitored on the same monitor in the same way throughout the study.
Thermodilution
Bolus thermodilution via the pulmonary artery catheter is currently the method of choice for measuring cardiac output in the clinical setting. By this technique, multiple cardiac output measurements can be obtained at intervals, using an inert indicator. A bolus of cold fluid was injected into the right atrium and the resulting temperature change was detected by the thermistor in the pulmonary artery. During the patient’s exhalation, an indicator (10 mL of 5% glucose at room temperature) was injected over 4 seconds into the central venous part of the pulmonary artery catheter. The thermodilution curve was monitored on a thermodilution monitor (Cardiac Output Computer; Arrow International, Reading, PA, USA). Five consecutive measurements were done. The cardiac output mean value was calculated from the three of five measurements not differing reciprocally by more than 10%.
Esophageal Doppler
Just after the induction of anesthesia, the transducer of the esophageal Doppler device (HemoSonic 100; Arrow International) was inserted through the nose and positioned in the esophagus. Prior to the transducer insertion, the intended insertion depth for the ultrasound sensors must be estimated on the basis of external anatomy. This is achieved by placing the dual sensor assembly onto the patient’s chest at the level of the third intercostal juxtasternal space, approximately 35 cm from the incisors in a tracheally intubated patient. Under these conditions, ultrasonic transducers are located approximately between the fifth and sixth thoracic vertebra, where the esophagus and the aorta run parallel for about 5 cm. Finally, the transducer handle is secured by a fixed arm attached to the operating table.
Statistical analysis
For the analysis between groups and time points, a two-way analysis of variance (ANOVA) was performed. Data were analyzed using the software program Statistica, version 4.5 for Windows (StatSoft, Inc. Tulsa, OK, USA) and P<.05 was considered statistically significant.
Results
There were no significant differences in patients' ages, body surface area, and numbers of grafts. In both groups of patients, the duration of coronary occlusion was almost equal and did not have statistical significance (Table 1).
Statistical analysis of hemodynamic parameters is shown in Table 2. There was no statistical significance within each group and between groups for heart rate, mean arterial, and central venous pressures.
Table 2.
Hemodynamic parameters (mean ± standard deviation) of patients who underwent coronary artery bypass grafting on a beating heart*
| Measurement point |
||||
|---|---|---|---|---|
| Parameter | 5 min after induction | on the beginning of ischemia | 15 min after ischemia | 15 min after sternum closure |
| ACC (m/s2): | ||||
| sevoflurane | 9.3 ± 3.1 | 9.8 ± 3.3‡ | 10.3 ± 3.5† | 10.7 ± 3.9†‡ |
| propofol | 9.9 ± 2.9 | 7.3 ± 1.8† | 8.3 ± 2.6† | 8.4 ± 2.8† |
| CI (L min-1 m-2): | ||||
| sevoflurane | 2.2 ± 0.4 | 2.2 ± 0.2‡ | 2.3 ± 0.3‡ | 2.3 ± 0.3 |
| propofol | 2.1 ± 0.3 | 1.8 ± 0.4† | 2.0 ± 0.4 | 2.1 ± 0.3 |
| HR (beats/min): | ||||
| sevoflurane | 71.6 ± 14.7 | 75.3 ± 9.1 | 77.3 ± 8.7 | 80.3 ± 9.0 |
| propofol | 71.1 ± 7.6 | 74.4 ± 9.3 | 76.2 ± 9.6 | 74.7 ± 9.0 |
| MAP (mmHg): | ||||
| sevoflurane | 79.0 ± 8.7 | 78.7 ± 10.3 | 80.7 ± 10.0 | 80.1 ± 9.4 |
| propofol | 82.3 ± 5.7 | 81.1 ± 8.9 | 81.2 ± 7.7 | 85.8 ± 7.7 |
| CVP (mmHg): | ||||
| sevoflurane | 13.7 ± 1.5 | 13.8 ± 2.3 | 14.4 ± 1.7 | 14.3 ± 1.9 |
| propofol | 13.8 ± 1.5 | 14.3 ± 1.7 | 14.9 ± 1.6 | 14.2 ± 1.3 |
*Abbreviations: ACC – acceleration; CI – cardiac index; HR – heart rate; MAP – mean arterial pressure; CVP – central venous pressure.
†P<0.05 within group vs “5 minutes after induction” (t test).
‡P<0.05 between groups in the same measurement (ANOVA).
Compared to the baseline measurements, acceleration values within the sevoflurane group showed a consistent increase during surgery, but significant increases in acceleration values were observed 15 minutes after ischemia and 15 minutes after sternum closure (t test, P = 0.004 and P<0.001, respectively). In contrast to these results, the propofol group demonstrated a statistically significant decrease of acceleration values in all measurements, compared to their baseline measurements. The peak decrease was observed at the beginning of ischemia, as compared to baseline measurements (from 9.9 ± 2.9, 95% confidence interval (CI), 8.4-11.4 to 7.3 ± 1.8 m/s2, 95% CI, 6.4-8.3). In the further two measurements, 15 minutes after ischemia and 15 minutes after sternum closure, values of acceleration increased, but they did not return to the baseline values and remained significantly lower (P = 0.001 and P = 0.024, respectively).
When we compared differences of acceleration between the sevoflurane and propofol groups, acceleration had significantly higher values in the sevoflurane group in the second and fourth measurement (P = 0.017 and P = 0.046, respectively). The value for acceleration in the third measurement was also higher in the sevoflurane group than in the propofol group, but did not reach statistical significance (P = 0.071).
There were no significant differences in cardiac index values within the sevoflurane group. Contrary to these results, a significant decrease of cardiac index at the beginning of ischemia, compared to baseline in the propofol group was observed (P<0.001). In comparison to the propofol group, values of cardiac index at the beginning of ischemia and 15 minutes after ischemia were higher in the sevoflurane group (P = 0.002 and P = 0.011, respectively) (Table 2).
Discussion
In the present study, we observed cardioprotective effect of sevoflurane on left ventricular performance during off-pump coronary artery bypass grafting surgery as measured by with acceleration and cardiac index.
In the sevoflurane group, compared to baseline, acceleration showed a permanent increasing trend during the whole period of ischemia until the 15 minutes after sternum closure, when it reached the highest value (10.7 m/s2). In the propofol group, compared to baseline, there was a strong decrease in acceleration at the beginning of ischemia (from 9.9 to 7.3 m/s2). In the ensuing two measurements, a slight trend of recovery of acceleration was noted, but its value remained definitely below baseline. This shows that propofol, in our investigation, did not have a cardioprotective effect on left ventricular performance.
Although values for cardiac index did not show a significant increasing trend in sevoflurane group compared to baseline, its values between the groups were significantly higher in the sevoflurane group at the beginning of ischemia (2.2 vs 1.8 L min-1 m-2) and 15 minutes after myocardial ischemia (2.3 vs 2.0 L min-1 m-2). Similarly as with acceleration, the propofol group, compared to baseline, displayed a noticeable decrease in cardiac index at the beginning of ischemia (2.1 vs 1.8 L min-1 m-2). In contrast to the esophageal Doppler, bolus thermodilution in this study was a less precise method for estimating myocardial cardioprotection. Myocardial ischemia and reperfusion result in injury of coronary microvasculature and contractile dysfunction. This contractile dysfunction usually resolves within 24-48 hours and is independent of left and right ventricular filling pressures (15). Because of that, along with other usual hemodynamic parameters, we used acceleration, a parameter which is in good correlation with contractility, but is independent of filling pressure (15). Due to the preload of the heart, which was similar between the groups, the acceleration improvement might result from less myocardial dysfunction and better recovery of contractility.
Guarracino et al (9), in the first multicenter randomized controlled study, used another volatile anesthetic, desflurane, in estimating the biomarker of myocardial injury, troponin I. He demonstrated a cardioprotective effect and shorter length of stay in the intensive care unit and hospital, compared with the control group in which intravenous anesthetic was administrated. De Hert et al (8) in a large number of patients tried to confirm the hypothesis that cardioprotective effect of sevoflurane in patients with coronary artery disease depended on the duration of its administration. Sevoflurane was administrated before ischemia, after ischemia, and during whole procedure. Following the biomarkers for myocardial damage, the authors concluded that the cardioprotective effects of sevoflurane were clinically most apparent when it was administrated throughout the operation. According to these results, and in contrast to the protocol of De Hert, in which sevoflurane was administrated after sternotomy, we wanted to prolong sevoflurane administration. In order to prolong the administration, our protocol started its administration during induction. This enabled us to manage sevoflurane during the unstable period of anesthesia.
Some authors did not find a cardioprotective effect of sevoflurane. Law-Koune et al (11) in a small number of patients undergoing off-pump coronary artery bypass grafting surgery did not confirm a cardioprotective effect of sevoflurane. Sevoflurane administration was adjusted according to the values of the bispectral index to between 40 and 60, which resulted in relatively low administrated end-tidal sevoflurane concentrations. These low concentrations could explain the results in his study. Kendall et al (16) reported that troponin T concentration were not significantly different in patients randomly allocated to receive propofol and isoflurane or isoflurane and high thoracic epidural analgesia.
Off-pump coronary artery bypass grafting procedure is a clinical model for a controlled short-lasting and completely reversible cardiac ischemia in humans. As the ischemic period is limited, a sublethal ischemic stress is applied to the myocardial cells at risk, which is usually not sufficient to provoke sustained cell damage (17). Therefore, a marked increase in biochemical markers of myocardial cell damage cannot be expected in most cases and, therefore, was not measured in our study.
Bein et al (18) analyzed myocardial function with echocardiography, myocardial performance index, and with early atrial filling velocity ratio, which were significantly altered in the group of patients who received propofol anesthesia. In patients that received sevoflurane, there were no changes in the parameters, such as biomarkers troponin I and myocardial fraction of the creatine kinase (CK-MB). Interestingly, even though echocardiography demonstrated better myocardial function in the sevoflurane group, the final values of the parameters showed poor myocardial function relative to their initial values. This is opposite to our findings in which acceleration values in the last measurement were significantly higher than at baseline in the sevoflurane group. Furthermore, in contrast to results of Bein, we did not have greater necessity for the use of vasopressors in the sevoflurane group.
This study has some limitations. It included a relatively small number of patients. As patients with preoperative ejection fraction less than 40% were excluded, the results are not applicable to this subgroup of patients. Further studies will have to evaluate the impact of sevoflurane-mediated cardioprotection in patients with lower preoperative ejection fractions. This particular group of patients most frequently requires administration of inotropes, which additionally attenuate any positive influence of cardioprotection. Because coronary artery disease and myocardial infarction occur with increased frequencies among diabetic patients, cardioprotection of the diabetic myocardium may differ considerably from cardioprotection of non-diabetic myocardium. In this study, cardioprotective effect of sevoflurane was observed while administrating the same dose of 1 MAC to all patients. Because cardioprotective effect of sevoflurane may depend on MAC, our results cannot be extrapolated to different sevoflurane concentrations (19).
Our protocol followed the early cardioprotective effect of sevoflurane, which is more important for the perioperative period. We did not follow possible later cardioprotective effects, which are more important in the immediate postoperative stage.
Further studies should be conducted to bring additional explanations about mechanisms of cardioprotective effect of volatile anesthetics. These explanations will improve the quality of administration and choice of anesthetic. They will have to investigate the effect of inhalational anesthetics on cardioprotection with regards to patients' outcome, primarily on perioperative morbidity and mortality. There is an increasing number of patients with coronary artery disease who will be undergoing general surgery. Previous studies have shown that sevoflurane can have protective properties against ischemic injury even in that group of patients with coronary artery disease who will undergo non-cardiac surgery. Investigation of the cardioprotective effect of sevoflurane in this group of patients could be of clinical importance. Just as protection against ischemia is possible for the myocardium during surgery, a new concept to ensure perioperative protection of other organs is emerging.
In conclusion, by measuring acceleration and cardiac index, we proved early cardioprotective effect in the sevoflurane group.
References
- 1.Schlack W. What should the anaesthesist know about ischaemia-reperfusion injury? In: Refresher course lectures Euroanaesthesia; 2004 June 05-08; Lisbon (Portugal): Publishing by the European Society of Anaesthesiologists; 2004. p. 19-25. [Google Scholar]
- 2.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 3.De Hert SG. Anesthetic preconditioning: how important is it in today's cardiac anesthesia? J Cardiothorac Vasc Anesth. 2006;20:473–6. doi: 10.1053/j.jvca.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Kohro S, Hogan QH, Nakae Y, Yamakage M, Bosnjak ZJ. Anesthetic effects on mitochondrial ATP-sensitive K channel. Anesthesiology. 2001;95:1435–40. doi: 10.1097/00000542-200112000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Stadnicka A, Kwok WM, Warltier DC, Bosnjak ZJ. Protein tyrosine kinase-dependent modulation of isoflurane effects on cardiac sarcolemmal K(ATP) channel. Anesthesiology. 2002;97:1198–208. doi: 10.1097/00000542-200211000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto K, Bosnjak ZJ, Kwok WM. Isoflurane-induced facilitation of the cardiac sarcolemmal K(ATP) channel. Anesthesiology. 2002;97:57–65. doi: 10.1097/00000542-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 7.De Hert SG, Van der Linden PJ, Cromheecke S, Meeus R, ten Broecke PW, De Blier IG, et al. Choice of primary anesthetic regimen can influence intensive care unit length of stay after coronary surgery with cardiopulmonary bypass. Anesthesiology. 2004;101:9–20. doi: 10.1097/00000542-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 8.De Hert SG, Van der Linden PJ, Cromheecke S, Meeus R, Nelis A, Van Reeth V, et al. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology. 2004;101:299–310. doi: 10.1097/00000542-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Guarracino F, Landoni G, Tritapepe L, Pompei F, Leoni A, Aletti G, et al. Myocardial damage prevented by volatile anesthetics: a multicenter randomized controlled study. J Cardiothorac Vasc Anesth. 2006;20:477–83. doi: 10.1053/j.jvca.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Conzen PF, Fischer S, Detter C, Peter K. Sevoflurane provides greater protection of the myocardium than propofol in patients undergoing off-pump coronary artery bypass surgery. Anesthesiology. 2003;99:826–33. doi: 10.1097/00000542-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Law-Koune JD, Raynaud C, Liu N, Dubois C, Romano M, Fischler M. Sevoflurane-remifentanil versus propofol-remifentanil anesthesia at a similar bispectral level for off-pump coronary artery surgery: no evidence of reduced myocardial ischemia. J Cardiothorac Vasc Anesth. 2006;20:484–92. doi: 10.1053/j.jvca.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Dupuis JY, Wang F, Nathan H, Lam M, Grimes S, Bourke M. The cardiac anesthesia risk evaluation score: a clinically useful predictor of mortality and morbidity after cardiac surgery. Anesthesiology. 2001;94:194–204. doi: 10.1097/00000542-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 13.American Society of Anesthesiologists. Standards for basic anesthetic monitoring (Approved by the ASA House of Delegates on October 21, 1986, and last amended on October 25, 2005). Available from: http://www.asahq.org/publicationsAndServices/standards/02.pdf. Accessed: March 30, 2006.
- 14.Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta Radiol. 1953;39:368–76. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- 15.Kloner RA, Przyklenk K, Kay GL. Clinical evidence for stunned myocardium after coronary artery bypass surgery. J Card Surg. 1994;9(3) Suppl:397–402. doi: 10.1111/jocs.1994.9.3s.397. [DOI] [PubMed] [Google Scholar]
- 16.Kendall JB, Russell GN, Scawn ND, Akrofi M, Cowan CM, Fox MA. A prospective, randomised, single-blind pilot study to determine the effect of anaesthetic technique on troponin T release after off-pump coronary artery surgery. Anaesthesia. 2004;59:545–9. doi: 10.1111/j.1365-2044.2004.03713.x. [DOI] [PubMed] [Google Scholar]
- 17.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001;104:3158–67. doi: 10.1161/hc5001.100039. [DOI] [PubMed] [Google Scholar]
- 18.Bein B, Renner J, Caliebe D, Scholz J, Paris A, Fraund S, et al. Sevoflurane but not propofol preserves myocardial function during minimally invasive direct coronary artery bypass surgery. Anesth Analg. 2005;100:610–6. doi: 10.1213/01.ANE.0000145012.27484.A7. [DOI] [PubMed] [Google Scholar]
- 19.Obal D, Preckel B, Scharbatke H, Mullenheim J, Hoterkes F, Thamer V, et al. One MAC of sevoflurane provides protection against reperfusion injury in the rat heart in vivo. Br J Anaesth. 2001;87:905–11. doi: 10.1093/bja/87.6.905. [DOI] [PubMed] [Google Scholar]