Abstract
Aim
To evaluate the histomorphological features of veins in normal and transplanted kidneys.
Methods
Between 1992 and 1997 at the Institute of Pathology in Ljubljana, we semiquantitatively evaluated histomorphological changes in veins in nephrectomy specimens of 29 renal allografts with rejection and in 31 control kidneys. The structure of different segments of renal veins was additionally analyzed.
Results
Small interlobular veins were composed of endothelium and basement membrane, similar to capillaries, while the walls of large interlobular and arcuate veins had smooth muscle cell bundles forming the medial layer, similar to large extrarenal veins. In the control group, only focal mononuclear infiltration around small interlobular veins was found (8/31). In rejected kidney allografts, the veins were frequently infiltrated with inflammatory cells, predominantly T lymphocytes and macrophages (29/29). Other changes included thrombosis (16/29), fibrinoid necrosis (7/29), and sclerosis (9/29), and in one case an intimal lipid deposition.
Conclusion
This study, performed on whole explanted kidney specimens, revealed that rejection vasculitis often involved extrarenal and intrarenal veins, showing a whole spectrum of histopathological changes similar to those in arteries. Since large intrarenal veins have a muscle wall, we believe that the term »rejection phlebitis« could be used in renal transplant pathology.
Little has been written on the normal structure of intrarenal veins. In anatomy an histology textbooks (1,2) and even in a specialized book on blood vessels of the kidney (3) there are no details about the structure of intrarenal veins. It has been reported that they consist only of an endothelium resting on a basement membrane (4). No systematic study of renal veins in kidney allograft rejection has so far been published. Arcuate and interlobular phlebitis in renal allografts was specifically studied only in a single study (5), which defined its clinicopathologic characteristics and possible impact on graft outcome. In a limited number of histopathological studies of kidney allograft rejection, mononuclear infiltration of extrarenal (6) and intrarenal veins (7-9) has been reported. On the other hand, some investigators have even denied the existence of intrarenal vein rejection, except when small renal veins are involved in leukocyte transmigration to the interstitium, as is found in capillaries and venules (10).
The aim of our study was to evaluate the histomorphological features of various extrarenal and intrarenal segments of veins in normal and transplanted kidneys.
Material and methods
The study included nephrectomy specimens obtained from 29 patients with transplanted kidney. There were 19 male and 10 female patients with the median age (range) of 44 (8 to 55) years. Control group consisted of kidney tissue samples taken during autopsy, conducted as a part of routine work at the Institute for Pathology in Ljubljana, from 31 age-matched patients. In the control group, there were 19 male and 12 female patients with the median age (range) of 46 (5 to 59) years and without any clinically and histologically proven kidney disease and without hypertension in whom we assessed the normal morphology of various segments of renal veins.
The type of rejection was classified according to the Banff scheme (11). Out of 29 renal allografts, 8 showed acute rejection, while 21 showed chronic rejection accompanied by signs of acute rejection in 19 allografts. In most cases in the latter group, acute rejection was caused by a withdrawal of immunosuppressive therapy prior to transplantectomy.
In the acute rejection group, allograft nephrectomy was performed 1 to 6 months after transplantation. Three patients underwent transplantectomy because of persistent graft intolerance syndrome, 3 because of thrombosis of large extrarenal allograft vessels, and 2 because of life-threatening infection. In the chronic rejection group, transplantectomy was performed 6 months to 12 years after transplantation. In 13 patients it was performed because of persistent graft intolerance syndrome, 4 because of thrombosis of large extrarenal allograft vessels, 3 because of life-threatening infection, and 1 because of persistent nephrotic syndrome due to the recurrence of focal and segmental glomerulosclerosis.
Anatomy of renal vasculature
The veins of the kidney are situated near the corresponding arteries. The main renal artery and vein divide into several segmental vessels just before reaching the hilum of the kidney. Within the renal sinus, segmental vessels divide into interlobar arteries and veins, which enter the kidney substance. At the junction between the cortex and medulla, the interlobar vessels divide dichotomously into arcuate vessels, which follow a curved course between the cortex and medulla. A series of interlobular vessels rises from each of these arcuate vessels, which after additional division, ascend radially through the cortex (4). Some authors divide interlobular arteries into large (>200-500 μm) and small (>50-200 μm) (12) on the basis of diameter. In order to evaluate the histologic structure of intrarenal veins in detail, we also divided corresponding interlobular veins into large and small. The diameter was assessed using an ocular micrometer.
Standard light microscopy
Nine tissue samples were taken from both rejection and control kidneys, from different segments of renal arteries and veins, including main renal, segmental, interlobar, arcuate, and interlobular arteries and veins. Tissue samples were fixed in 10% buffered formalin, embedded in Paraplast (McCormick Scientific, St. Louis, MO, USA), and cut into 4µm sections. For histochemical and immunohistochemical studies, consecutive sections (two for each staining) were cut from each tissue block. Deparaffinized tissue sections were stained with the following methods: hematoxylin-eosin, Masson trichrome, periodic acid-Schiff, periodic acid-silver Azan, and van Gieson-Weigert. Cell infiltration, necrosis, thrombosis, elastica disruption, sclerosis, and lipid deposits were evaluated semiquantitavely using a scale 0 to 3+ (0 – no change, 1 – mild, 2 – moderate, and 3 – severe change).
Immunohistochemical studies. Sections of formalin fixed, paraffin-embedded tissues were labeled using the standard streptavidin-biotin technique and commercially available reagents (LSAB kit, DAKO, Glostrup, Denmark). Tissue sections were incubated with primary antibodies to CD3 (dilution 1:50), CD79a (dilution 1:50), CD68 (dilution 1:100), CD31 (dilution 1:20), alpha-smooth muscle actin (SMA) (dilution 1:200) (DAKO), and elastin (dilution 1:40) (Sigma Chemical Co., St. Louis, MO, USA). Bound peroxidase was visualized using 0.05% 3,3-diaminobenzidine tetrahydrochloride as a chromogenic substrate for 10 minutes at room temperature. The slides were counterstained with hematoxylin, dehydrated, and mounted. Negative controls included omission of the primary antibodies from the staining procedure.
Histomorphologic changes and results of immunohistochemical stainings were evaluated in a blinded, random fashion by two independent renal pathologists (V.J. and D.F.).
Results
Morphology of normal veins
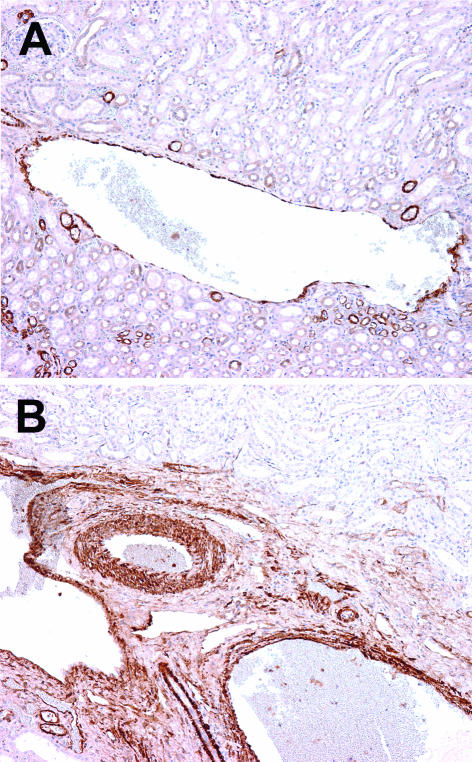
In normal healthy kidneys, small interlobular veins had the structure of capillaries and consisted of endothelium and basement membrane (Figure 1). Similar to extrarenal veins, the walls of large interlobular and arcuate veins were clearly composed of alpha- smooth muscle actin-positive bundles, forming a fairly distinct muscle layer of varying thickness (Figures 2A and 2B). The internal elastica was absent in small interlobular veins, discontinuous in large intrarenal veins and interlobar veins, and continuous in segmental renal veins. Focal mononuclear infiltration around small interlobular veins was found in 8/31 cases associated with global glomerulosclerosis, interstitial fibrosis, and arteriosclerotic changes.
Figure 1.
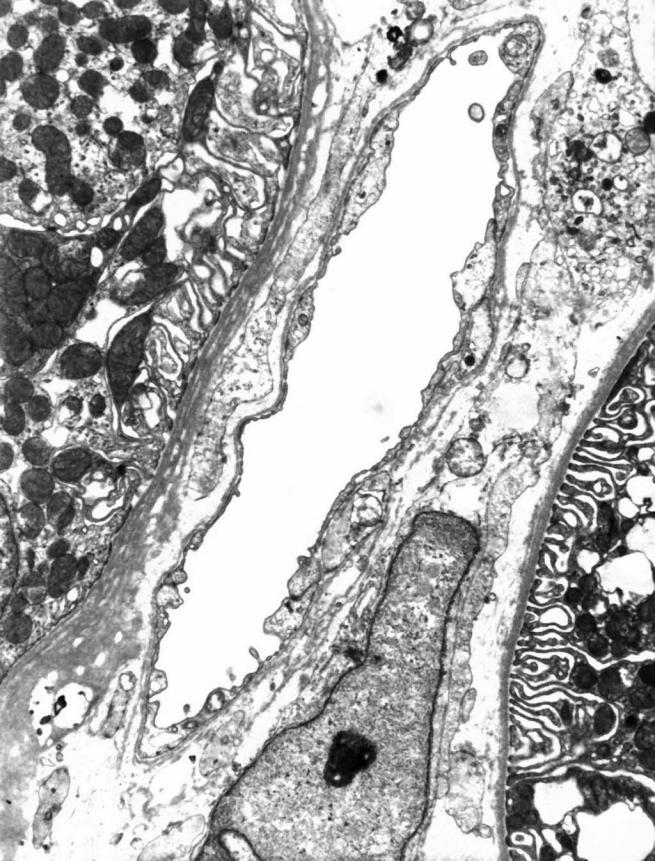
Small interlobular vein that consists of endothelium and basement membrane. There are no smooth muscle cells. Samples for electron microscopy were fixed in osmium tetraoxid (OsO4), embeded in Epon, and contrasted with lead citrate and uranil acetate; original magnification ×5000.
Figure 2.
Positivity for smooth muscle actin in control kidney. A, Large interlobular vein with muscle layer of varying thickness. B, Arcuate vein with well-developed muscle wall. The wall of the corresponding artery is thicker, with densely packed muscle cells. Streptavidin-biotin peroxidase; original magnification ×100.
Veins in renal allografts
In allografts, mononuclear inflammatory infiltrate consisted of CD3-positive T lymphocytes, CD68-positive macrophages, rare CD79- positive B lymphocytes, and plasma cells. In 8 allografts with histologic signs of acute rejection and in 19 allografts with signs of both acute and chronic rejection (11), we found vessel wall infiltration predominantly with T lymphocytes in extrarenal veins (Figure 3A) and arteries, arcuate, and large interlobular veins (Figure 3B) and arteries, and small interlobular arteries. In two allografts with pure chronic rejection, only rare CD68-positive macrophages were found in the walls of the arteries and veins.
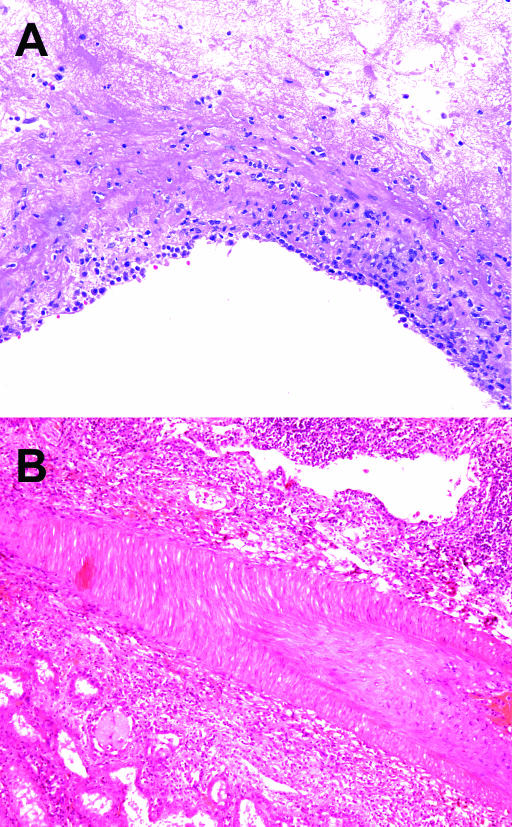
Figure 3.
Rejection phlebitis. A, Segmental vein with transmural inflammatory cell infiltration. Hematoxylin-eosin; original magnification ×200. B, Large interlobular vein with severe inflammatory infiltration, while infiltration of the corresponding artery is mild, predominantly intimal. Hematoxylin-eosin; original magnification ×100.
Focal infiltration of T lymphocytes around small interlobular veins was found in all 29 allografts. Interstitial hemorrhages were found in 17/29 allografts.
In veins, cell infiltration was transmural (Figures 3A and 3B), while in arteries the intima was found to be most intensively affected (Figure 3B).
The frequencies of inflammatory infiltration, thrombosis, fibrinoid necrosis, sclerosis, and lipid deposits in extrarenal and intrarenal veins and arteries in allografts and in control kidneys are shown in Table 1. Sclerosis and lipid deposits were found only in allografts with chronic rejection.
Table 1.
Histomorphologic changes in arteries and veins in 29 kidney allografts and 31 control kidneys
| No. of kidneys |
||||
|---|---|---|---|---|
| Histomorphologic change | control (n = 31) |
allografts (n = 29) |
||
| veins | arteries | veins | arteries | |
| Inflammatory infiltration of extrarenal vessels | 0 | 0 | 26 | 27 |
| Inflammatory infiltration of intrarenal vessels | 8 | 0 | 29 | 27 |
| Thrombi | 0 | 0 | 15 | 18 |
| Fibrinoid necrosis | 0 | 0 | 7 | 18 |
| Sclerosis | 0 | 8 | 9 | 21 |
| Lipid deposits in extrarenal vessels | 0 | 6 | 1 | 12 |
| Lipid deposits in intrarenal vessels | 0 | 0 | 0 | 11 |
Discussion
Our investigation of the structure of intrarenal veins revealed that the walls of large intrarenal veins are composed of smooth muscle cell bundles and not only of endothelium and basement membrane, as reported by others (4). We identified a fairly distinct muscle layer in arcuate and large interlobular veins, but not in small interlobular veins, which are actually venules. Like capillaries, the latter have no smooth muscle cells or elastic membrane in their walls, which suggests that they play a similar role in the transmigration of inflammatory cells. It is known that the migration of inflammatory cells through the endothelium of capillaries and venules is an important part of inflammatory and rejection processes (13).
The finding of focal mononuclear inflammatory cell infiltration around intrarenal small veins in kidney transplants seems nonspecific, since it was also frequently observed in common aging-related nephroangiosclerosis. However, we found a capillary injury with consequent interstitial hemorrhage only in cases of rejection. Interstitial hemorrhage was already reported as a prognostically significant finding in acute rejection (9).
The involvement of veins was not addressed in several important, otherwise excellent, review articles and books dealing with renal transplant pathology (12,14). Some authors deny the existence of intrarenal vein rejection, and claim that the term “phlebitis” should not be used in kidney transplant pathology (10). On the other hand, Torbenson and Randhawa (5) found that arcuate and interlobular veins had well developed muscle walls, and that inflammation at this level could significantly alter the renal hemodynamics. However, they did not distinguish between large and small interlobular veins. Our study showed that large interlobular veins had muscle walls, while small did not.
In comparison to other studies (Table 2), our study systematically assessed the involvement of various segments of renal veins in transplant rejection and showed that histomorphological changes in renal arteries were often accompanied by changes in the corresponding veins. In contrast to the opinion of some authors (10), we believe that the term “phlebitis” in kidney transplant pathology is disputable only in relation to small interlobular veins, which have the structure of capillaries and serve for the transmigration of inflammatory cells. In large interlobular veins and arcuate veins with a well-developed muscle layer, phlebitis is a sign of acute rejection. The incidence of rejection phlebitis in the study by Torbenson and Randhawa (5) was very low, probably due to the infrequent presence of veins in the bioptic material. According to our findings, veins were nearly as frequently involved as the corresponding arteries.
Table 2.
Comparison of results from this study with other studies
| Histology parameters | This study | Other studies |
|---|---|---|
| Morphology and function of small intrarenal veins | Small intrarenal veins have a thin wall with no smooth muscle cells or elastic membrane. They serve for transmigration of inflammatory cells, so the presence of these cells around small veins cannot be called phlebitis. | - |
| Presence of muscle layer in intrarenal veins | Walls of large intrarenal veins contain smooth muscle cell bundles. | Lemley and Kriz (4): walls of all intrarenal veins consist only of endothelium and basement membrane. |
| Incidence of rejection phlebitis | High (study performed on whole explanted kidneys). | Torbenson and Randhawa (5): low incidence (study performed on small bioptic samples). |
| Extension of rejection phlebitis | Inflammatory infiltrate involves both intrarenal and extrarenal veins. | Vollmer and Roessner (6): inflammatory infiltrate involves extrarenal veins, intrarenal veins were not studied. |
| Comparison of rejection phlebitis and rejection arteritis | Rejection phlebitis is histologically similar to arteritis, but is often transmural, probably due to thinness and discontinuity of the media and internal elastic membrane. | - |
The diagnosis of acute rejection arteritis is based on lymphocytic infiltration of the vessel wall (11). Predominant inflammatory cells are T lymphocytes, but other cells, such as macrophages, eosinophils, plasma cells, and B lymphocytes may also be present (8). Infiltration of large extrarenal but not intrarenal veins with T lymphocytes and, less extensively, by macrophages has already been described (6). Our immunohistochemical study of the composition of inflammatory infiltrate is in accordance with previous studies. In addition, we found that inflammatory infiltrate of intrarenal veins is as frequent as that of extrarenal veins. To some extent, this is in accordance with other reports, in which inflammatory infiltration of intrarenal veins has been mentioned, but has not been studied in detail (7,8).
We found that infiltration in veins was transmural, while in arteries it was mostly limited to the intimal layer. The reason for transmural vein involvement may be the thinness and discontinuity of the vein media and internal elastic membrane, allowing inflammatory cells to infiltrate the whole thickness of the vessel wall.
The hallmark of chronic rejection is sclerosis with progressive luminal occlusion of arteries (15). Histopathologic changes in veins in chronic rejection have not yet been specifically studied. According to our findings, they were similar as in arteries and included sclerosis, infiltration of vessel wall with rare CD68-positive macrophages, and lipid deposition.
In conclusion, our study showed that rejection vasculitis often involves extrarenal and intrarenal veins. While inflammatory infiltration in arteries is mostly intimal, there is transmural involvement of the corresponding veins. We propose that the term “rejection phlebitis” should be used in renal transplant pathology.
References
- 1.Williams PL, Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE, et al, editors. Gray’s Anatomy. New York (NY): Churchill Livingstone; 1995. [Google Scholar]
- 2.Sternberg SS, editor. Histology for pathologists. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 3.Fourman J, Moffat DB. The blood vessels of the kidney. Oxford (UK): Blackwell Scientific; 1971. [Google Scholar]
- 4.Lemley KV, Kriz W. Structure and function of the renal vasculature. In: Tisher CC, Brenner BM, editors. Renal pathology with clinical and functional correlations. Philadelphia (PA): JB Lippincott; 1989. p. 926-60. [Google Scholar]
- 5.Torbenson M, Randhawa P. Arcuate and interlobular phlebitis in renal allografts. Hum Pathol. 2001;32:1388–91. doi: 10.1053/hupa.2001.29669. [DOI] [PubMed] [Google Scholar]
- 6.Vollmer E, Roessner A. Parallels between renal transplant arteriopathy and atherosclerosis in respect of functional morphology. Curr Top Pathol. 1993;87:223–51. doi: 10.1007/978-3-642-76849-1_8. [DOI] [PubMed] [Google Scholar]
- 7.Croker BP, Salomon DR. Pathology of the renal allograft. In: Tisher CC, Brenner BM, editors. Renal pathology with clinical and functional correlations. Philadelphia (PA): JB Lippincott; 1989. p. 1518-54. [Google Scholar]
- 8.Feingold RE, Churg J. Transplant vasculitis. In: Churg A, Churg J, editors. Systemic vasculitides. New York (NY): Igaku-Shoin; 1991. p. 351-7. [Google Scholar]
- 9.Nadadsy T. Transplantation. In: Silva FG, D’Agati VD, Nadasdy T, editors. Renal biopsy interpretation. New York (NY): Churchill Livingstone; 1996. p. 373-421. [Google Scholar]
- 10.Colvin RB. The renal allograft biopsy. Kidney Int. 1996;50:1069–82. doi: 10.1038/ki.1996.410. [DOI] [PubMed] [Google Scholar]
- 11.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–23. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 12.Nishi T, Bond C, Brown G, Solez K, Heptinstall RH. A morphometric study of arterial intimal thickening in kidneys of dialyzed patients. Am J Pathol. 1979;95:597–610. [PMC free article] [PubMed] [Google Scholar]
- 13.Risau W. Differentiation of endothelium. FASEB J. 1995;9:926–33. [PubMed] [Google Scholar]
- 14.Racusen LC, Solez K, Olsen S. Pathology of kidney transplantation. In: Solez K, Racusen LC, Billingham ME, editors. Solid organ transplant rejection. New York (NY): Marcel Dekker Inc; 1996. p. 207-96. [Google Scholar]
- 15.Hostetter TH. Chronic transplant rejection. Kidney Int. 1994;46:266–79. doi: 10.1038/ki.1994.269. [DOI] [PubMed] [Google Scholar]