Abstract
Aim
Based on previous observations of strain-related alterations in sensitivity to anesthetics, this study used a newly established genetic rat model to identify differences in cardiovascular sensitivity to the commonly used, clinically relevant, anesthetic propofol and to correlate such differences with specific chromosomal substitutions.
Methods
Cardiovascular sensitivity to propofol was compared in groups of normotensive Dahl Salt Sensitive (SS) and Brown Norway (BN) inbred rats, as well as in a unique panel of consomic strains based on these SS and BN parentals. The consomics were produced by introgression of individual BN chromosomes into an otherwise unchanged SS genetic background. Cardiovascular sensitivity was assessed by measuring the infusion rate of propofol required to reduce mean arterial blood pressure by 50% and cause cardiovascular collapse in each parental and consomic strain.
Results
Significantly lower propofol infusion rates caused both a 50% reduction in mean arterial pressure and ultimate cardiovascular collapse in SS compared to BN. Substitution of BN chromosome 13, but not of any other BN chromosome, reversed the enhanced propofol sensitivity in SS rats to the level of BN rats.
Conclusions
Differential propofol sensitivity exhibited by SS and BN rat strains is associated with chromosome 13. This is consistent with earlier findings and represents the first complete screening of all rat autosomes for their relationship to anesthetic sensitivity. Initial localization of this sensitivity reversal to chromosome 13 provides a basis upon which additional, more selective genetic screening studies can be applied. Such studies may serve to identify specific regions of the genome responsible for different physiological responses to various anesthetic agents.
All general anesthetics produce analgesia, altered consciousness, and a general physiologic state that is favorable for the tolerance of painful stimuli. Despite decades of investigation, the wide diversity of effects that anesthetic agents have on cellular function has made it difficult to determine which of these effects are mechanistically significant for desired anesthetic action, major side effects, or both. As basic science research has increasingly focused on potential genetic determinants of cause and effect, research into the mechanisms and side effects of anesthetic action has likewise taken a similar turn. Since the genetic code is ultimately responsible for the synthesis of the receptors and other proteins that are thought to be modified by anesthetic agents, identifying genetic components associated with the sites and methods of anesthetic action is a promising endeavor. A genetically focused investigative schema may represent a way to accurately study anesthetic mechanisms while avoiding the confounding influence of superfluous effects on experimental systems (1).
We have previously demonstrated that Dahl Salt Sensitive rats (SS/JrHsdMcwi, abbreviated as SS) exhibit significantly greater cardiovascular sensitivity to both volatile and parenteral anesthetics than do rats of the Brown Norway strain (BN/NhsdMcwi, abbreviated as BN), and further, that these sensitivity differences remain intact even when SS rats are normotensive and maintained on a low salt diet (2,3). Our objective for the current study was to identify which regions of the rat genome are responsible for this cardiovascular sensitivity. For this task we employed a unique consomic methodology, which allowed us to investigate the effects of single chromosome substitutions on cardiovascular sensitivity to anesthetic agents.
In this consomic model, single SS chromosomes were substituted with their BN equivalents, while leaving the otherwise inbred parental genetic background unchanged (4). Once identified, any chromosome(s) associated with anesthetic sensitivity differences can be subdivided further, providing genetic insight into the mechanisms of anesthetic action. This strategy is becoming an increasing familiar model and is often referred to as functional genomics (5).
The hypothesis for this study is that there is an identifiable genetic basis which could explain the observed, strain specific differences in cardiovascular sensitivity to the clinical anesthetic propofol, and that these observed sensitivity differences result from genetically determined alterations in cardiovascular functions. If the genes involved in these sensitivity differences are localized to specific chromosomes, then selective chromosomal substitutions from BN rats to SS rats will reverse the anesthetic sensitive phenotype of the SS to the non-sensitive phenotype of the BN. In this way, we can identify candidates of interest for the ultimate identification and mapping of specific genes.
Methods
Experimental animals
The methodology for the present study was similar to that used previously (3), with some modifications. First, in addition to the parental animal strains SS and BN, consomic animal strains SS.1BN-SS.20BN were studied. This refers to the homozygous substitution of single BN chromosomes, 1 through 20, into an otherwise unchanged SS genetic background (4). Second, propofol, rather than a volatile anesthetic, was used as the experimental agent. All animals were produced from inbred parental strains, under the direction of the Human and Molecular Genetics Center at the Medical College of Wisconsin.
Each consomic rat strain used in our study represented a single chromosome substitution. These consomic animals are a reliable and reproducible model, bred from progenitors with differing anesthetic sensitivity profiles. The animals were developed over a 4-year period by conducting a series of successive backcrosses. Genotyping was used to identify breeders from each generation, and crosses continued until the strain was homozygous at all loci. In this way, singly substituted animals representing all 20 SS autosomes and both sex chromosomes were developed (4). Once produced, SS.1BN-SS.20BN animals were maintained in a transgenic facility located within the Medical College of Wisconsin’s Biological Resource Center, and were 8-12 weeks old when studied. A total of 93 animals were studied. All studies were approved by the Animal Care and Use Committee.
Animal preparation
During each experiment, spontaneously ventilating animals were anesthetized with 3% isoflurane and a tracheotomy was performed. Following tracheotomy placement, isoflurane was reduced to 2%, and both femoral arterial and femoral venous catheters were placed (6). Following catheter placement, the concentration of administered isoflurane was reduced to 0.5% and maintained until an attached infrared capnograph (Criticare POET 2, Critcare Systems Inc. Waukesha, WI, USA) indicated that end tidal isoflurane concentration had decreased to 0.6%. At this point, intravenous propofol infusion was started at a rate of 550 µg kg-1 min-1 and isoflurane was discontinued. The animal was then equilibrated for 10 minutes or until the measured end tidal isoflurane concentration reached 0.2%.
Propofol sensitivity measurements
The propofol infusion rate was initially increased by 30 µg kg-1 min-1 every 3 minutes, until the animal no longer demonstrated extremity or tail movement in response to tactile stimulation. Following this loss of response, the sequential dose increase was raised from 30 µg kg-1 min-1 to 300 µg kg-1 min-1, with each dose adjustment again separated by a 3-minute equilibration interval. The propofol infusion rate at which the mean arterial blood pressure was reduced by 50% was recorded and then the sequential propofol dosage increases were raised to 3 mg kg-1 min-1. This pattern was maintained until the point of cardiovascular collapse. This was defined as the time at which CO2 no longer was detectable on the end tidal medical gas analyzer and represented the cessation of all effective cardiac pumping.
Anesthetics and equipment
Throughout all experiments, inspired gas consisted of a 30% O2/70% N2 mixture, which was used to reduce any possibility of hypoxia-induced effects on the anesthetic responses. All of the animals were ventilated with a model 680 rodent respirator (Harvard Apparatus Co., South Natick, MA, USA). Respiratory rate and tidal volumes were adjusted in such a way that end tidal CO2 was 35-40 mm Hg. End tidal CO2 and anesthetic concentrations were measured with a POET 2 infrared capnograph and end tidal agent monitor (Criticare Systems). Isoflurane was administered using an Ohio Medical Products vaporizer (Airco Inc., Madison, WI, USA). Propofol was purchased from Zenica Pharmaceuticals (Wilmington, DE, USA) and was infused using a Harvard model 22-syringe infusion pump (Harvard Apparatus Co., South Natick, MA, USA).
Measurements and statistics
For each strain, the following two response points were of interest: mean dose of propofol required to reduce mean arterial blood pressure by 50% and mean dose of propofol required to produce cardiovascular collapse.
Both mean infusion rate of propofol required to reduce mean arterial blood pressure by 50% and mean infusion rate of propofol required to produce cardiovascular collapse were determined for the SS and BN parental strains and for each of the 20 autosomally substituted consomic strains. These results were examined for any tendencies suggesting differential propofol sensitivity. The BN parental group and the SS.13BN consomic were clearly less sensitive than the SS. Therefore, at least 10 studies for each of these three groups were completed. In contrast, there were no tendencies toward differences between SS and any of the other consomics. Therefore, all of the remaining consomic data was pooled and analyzed in aggregate. The propofol infusion rates that reduced mean arterial blood pressure and the propofol infusion rates that produced cardiovascular collapse in SS, BN, SS.13BN and the pooled consomics were compared using analysis of variance and the Bonferroni post hoc test. We used Statistical Software for the Social Sciences, version 11.0.4 for Macintosh computers (SPSS. Inc, Chicago, IL, USA), with a significance level of P≤0.05.
Results
Reduction in mean arterial blood pressure
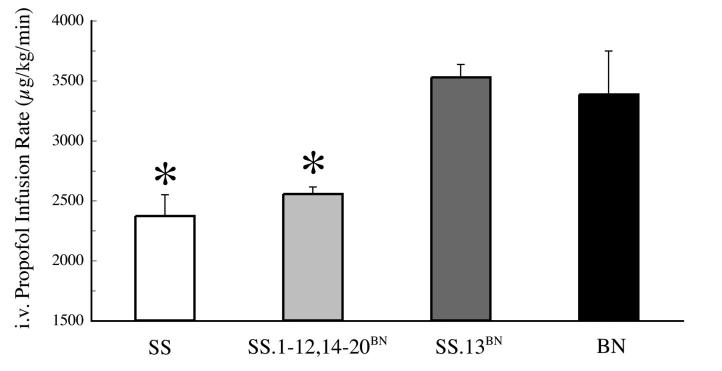
Mean ± standard error of the mean (SEM) infusion rate of propofol required to reduce mean arterial blood pressure by 50% was significantly different between the two parental strains (SS and BN). In BN (n = 10) this rate was 3.4 ± 0.36 mg kg-1 min-1, compared with a rate of 2.4 ± 0.18 µg kg-1 min-1 in SS (n = 10). When compared to the parental strains, significant differences were also observed in the consomic groups. The infusion rate of propofol required to reduce mean arterial pressure by 50% in the SS.13BN strain (n = 11) was 3.5 ± 0.36 µg kg-1 min-1. This was similar to the rate in BN and significantly different from that in SS (P≤0.001). In contrast, in all of the other consomic groups studied (n = 3-5 for each chromosome 1-12, 14-20), the infusion rate of propofol required to reduce mean blood pressure by 50% did not significantly differ from the SS. When pooled (ntotal = 62), this rate was 2.5 ± 0.35 µg kg-1 minute-1, representing a statistically significant deviation from BN and SS.13BN (P≤0.001) (Figure 1).
Figure 1.
Enhanced effect of IV administration of propofol in Dahl Salt Sensitive (SS) and pooled consomic strains (SS.1-20BN excluding 13) vs Brown Norway (BN) and SS.13BN (n = 10, 62, 10 and 11, respectively) in attenuating mean arterial blood pressure by 50%. Data shown are means±SEM of propofol infusion rates that attenuate mean arterial blood pressure by 50%. Asterisk indicates significant difference between BN and SS.13BN. There were no apparent differences between any of the other consomic strains and SS.
Cardiovascular collapse
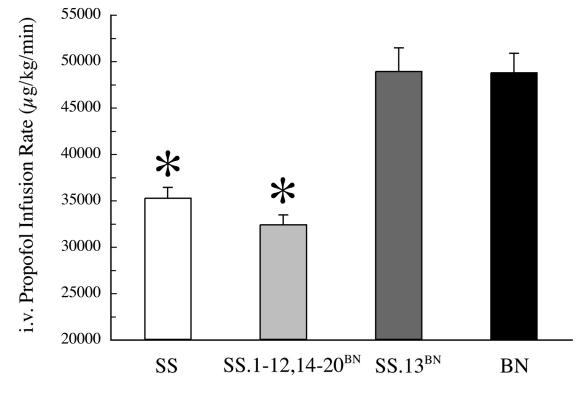
When propofol infusions were increased to the point of cardiovascular collapse, observed strain differences followed the same pattern as was observed for 50% reduction in mean arterial blood pressure. The mean±SEM infusion rate of propofol that produced cardiovascular collapse was 35 ± 1.2 mg kg-1 minute-1 in SS, which was significantly less than the 49 ± 2.1 mg kg-1 minute-1 required to cause the same effect in BN (P≤0.001). Likewise, all of the consomics except for the SS.13BN developed cardiovascular collapse at an infusion rate of propofol similar to the SS. Mean±SEM rate for this pooled group was 32 ± 0.8 mg kg-1 min-1. In contrast, in SS.13BN this rate was 49 ± 2.5 mg kg-1 min-1, which was similar to BN and significantly greater than both the SS and the pooled consomics (Figure 2).
Figure 2.
Earlier cardiovascular collapse in Dahl Salt Sensitive (SS) and consomic Strains (SS.1-20BN excluding 13) vs Brown Norway (BN) and SS.13BN (n = 10, 62, 10 and 11, respectively) in response to IV administration of propofol. Data shown are means±SEM of propofol doses that produce cardiovascular collapse. Asterisk indicates significant difference between BN and SS.13BN. There were no apparent differences between any of the other consomic strains and SS.
Discussion
The results of the present study demonstrate that SS rats exhibited greater cardiovascular sensitivity to propofol than BN rats. It further demonstrates that this increased sensitivity can be reversed by substituting BN chromosome 13 into an otherwise genotypically intact SS background. The fact that this reversal is not observed in any of the other consomic strains strongly suggests that the genetic factors responsible for these sensitivity differences are associated with chromosome 13.
Although consomic methodology is a relatively new approach (4), the study of a genetic basis for differences in anesthetic sensitivity has been an area of interest for many years. Over thirty years ago, the first evidence of a genetic basis for altered anesthetic sensitivity was reported with the identification of an ether-resistant strain of Drosophila. Since that time, a large number of mutations in this species correlating with enhanced or reduced sensitivity to different anesthetics have been identified (7). More recently, naturally occurring differences in measured minimum alveolar concentration (MAC) values of halothane, isoflurane, and desflurane between different stains of mice have been identified (8). Similarly, a specific quantitative trait locus (QTL) accounting for the observed differences in emergence times from propofol injections in mice has been identified and mapped (9). A similar genetic approach has also been used to successfully demonstrate that in vivo differences in vasoreactivity during cardiopulmonary bypass in humans correlates with polymorphism of the angiotensin converting enzyme gene (10). Novel use of knockout genes in mice has demonstrated a difference in anesthetic sensitivity, compared with control, linked to the GABAA receptor (11). Finally, in the soil nematode C. elegans, a number of mutations associated with altered anesthetic sensitivity have been identified and mapped via cross breeding followed by positional cloning of QTLs (12).
The chromosomal substitution technique in the present study represents a powerful alternative to either extensive screening studies of existing strains or the development of new strains via phenotypically selective inbreeding (13). Even though a single consomic strain can only be used to study the effects of a single chromosomal substitution, an entire consomic panel, by design, greatly facilitates the study of potentially multifactorial traits. Multifactorial traits are much more common than single gene traits, but their biologic complexity makes them difficult to study with traditional knockout or ethyl nitrosourea mutagenesis methodologies.
Perhaps the greatest strength of the present consomic model is the fact that SS and BN parental strains are inbred, and should thus be, genetically identical. Hence, as a result of this inbreeding in the parental strains, the consomic animals have isogenic backgrounds. In models that do not utilize inbred parental strains, it is difficult to distinguish whether an observed difference in response to an anesthetic is truly a differential gene/chromosome effect or is simply a result of background heterogenicity. Heterogenicity which may, in fact, modulate anesthetic response in some unidentified fashion. In contrast, within the context of our consomic model, the SS parental strain represents a “true control” for the SS.13BN. This is because any differences in anesthetic sensitivity are necessarily related to mechanisms associated only with the chromosomal substitution, since the remainder of the genetic background is isogenic. In a sense, this model is analogous to comparing single chromosomal substitution effects in inbred animals vs outbred animals – ie, identical vs non-identical twins. In this way, the complexity of significant background allelic variation is eliminated.
The results of the present study establish a correlation between the observed anesthetic sensitivity and chromosome 13. Although our results do not completely exclude the possibility that other influential chromosomes will be identified, the fact that we did not detect any such chromosomes in our survey of the full consomic panel suggests that this is unlikely. However, one issue which does need clarification is the possibility that the enhanced sensitivity of the SS was somehow related to the development of salt sensitive hypertension. We attempted to control for this possibility by maintaining all of the studied animals on low salt diets (0.4% NaCl). This diet has been shown to delay the development of hypertension in salt sensitive animals beyond the 8-12 week age range that we used (14). Moreover, in preliminary data, we observed no significant differences in pooled baseline mean arterial pressures between SS, BN, SS.13BN and representative consomic animals (142 ± 16, 133 ± 21, 139 ± 18, and 137 ± 14 mm Hg, respectively). Accordingly, anesthetic sensitivity and mean arterial pressure appear to be unrelated in this animal model. In our present study, we demonstrate that substitution of BN chromosome 13 into the SS isogenic background produces a reversal of SS associated anesthetic sensitivity, but substitution of any other BN chromosome into the SS isogenic background does not. Thus, only the SS.13BN animals demonstrate an anesthetic sensitivity profile similar to the BN parentals, whereas all other consomics demonstrate an anesthetic sensitivity profile similar to the SS.
Alone, our current results do not explain the mechanistic links between chromosome 13 and propofol sensitivity. However, the application of our methodology to a unique class of anesthetic agents, as represented by the current study, is an interesting and necessary step toward elucidating mechanistic detail. Given that our methodology has now been applied to different classes of anesthetic compounds with similar results, the current study serves to generalize the observed sensitivity differences across the borders of drug class, and to narrow the potential field of mechanistic possibilities. For example, a class specific or drug specific response would tend to suggest a specific receptor alteration or a modification of a drug metabolism pathway. In contrast, the observed similarity in cardiovascular response across drug class is more likely to suggest an underlying physiological difference such as altered sympathetic tone or ion channel density.
Although it is tempting to propose that a common mechanism related to chromosome 13 is involved in both 50% MAP reduction and in total cardiovascular collapse, and that total cardiovascular collapse represents a progression of earlier blood pressure changes, this cannot be supported without additional data. However, as more correlation between genotype and phenotype becomes available from sources such as the National Institutes of Health-sponsored Rat Genome Project (15), investigators will gain the ability to correlate traits localized to chromosomes, or portions of chromosomes, with known markers. Similarly, because our consomic animals have inbred genomic backgrounds, chromosomes of interest can be subdivided further by developing congenic substrains on the same genomic background. By separating whole chromosomes into smaller substitutions, the congenic approach allows for further characterization and localization of the genetic basis for complex genotypic traits. It is hoped that data such as ours will eventually contribute to a greater understanding of anesthetic sensitivity in humans.
This understanding will progress if known linkages in rats can be compared with existing libraries of sequences already known from the human and mouse genome projects. Thus, these results will provide the basis upon which we can apply additional and more selective genetic screening studies in order to identify the regions of the rat genome responsible for different responses to various anesthetic agents. Characterization of these differences, on both a phenotypic and genotypic level, will contribute to an enhanced understanding of the coupling between the mechanisms of anesthetic action on vascular smooth muscle and overall cardiac stability during anesthesia. Such an understanding is important in developing improved anesthetic techniques, particularly for individuals with demonstrated sensitivity to anesthetic agents.
Acknowledgment
This work was supported in part by the General Medicine Institute grant No. 068725 and by the PhysGen Program for Genomic Applications, National Heart, Lung, and Blood Institute grant No. U01 HL66579, both of which are through the National Institutes of Health, Bethesda, Maryland, USA.
References
- 1.Kayser B, Rajaram S, Thomas S, Morgan PG, Sedensky MM. Control of anesthetic response in C. elegans. Toxicol Lett. 1998;100-101:339–46. doi: 10.1016/s0378-4274(98)00204-5. [DOI] [PubMed] [Google Scholar]
- 2.Stekiel TA, Contney SJ, Bosnjak ZJ, Kampine JP, Roman RJ, Stekiel WJ. Chromosomal substitution-dependent differences in cardiovascular responses to sodium pentobarbital. Anesth Analg. 2006;102:799–805. doi: 10.1213/01.ane.0000195582.22822.e7. [DOI] [PubMed] [Google Scholar]
- 3.Stekiel TA. J Contney S, Bosnjak ZJ, Kampine JP, Roman RJ, Stekiel WJ. Reversal of minimum alveolar concentrations of volatile anesthetics by chromosomal substitution. Anesthesiology. 2004;101:796–8. doi: 10.1097/00000542-200409000-00032. [DOI] [PubMed] [Google Scholar]
- 4.Cowley AW, Jr, Liang M, Roman RJ, Greene AS, Jacob HJ. Consomic rat model systems for physiological genomics. Acta Physiol Scand. 2004;181:585–92. doi: 10.1111/j.1365-201X.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- 5.Schwinn DA, Booth JV. Genetics infuses new life into human physiology: implications of the human genome project for anesthesiology and perioperative medicine. Anesthesiology. 2002;96:261–3. doi: 10.1097/00000542-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Stekiel TA, Contney SJ, Kokita N, Bosnjak ZJ, Kampine JP, Stekiel WJ. Mechanisms of isoflurane-mediated hyperpolarization of vascular smooth muscle in chronically hypertensive and normotensive conditions. Anesthesiology. 2001;94:496–506. doi: 10.1097/00000542-200103000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Gamo S, Dodo K, Matakatsu H, Tanaka Y. Molecular genetical analysis of Drosophila ether sensitive mutants. Toxicol Lett. 1998;100-101:329–37. doi: 10.1016/s0378-4274(98)00203-3. [DOI] [PubMed] [Google Scholar]
- 8.Sonner JM, Gong D, Eger EI., II Naturally occurring variability in anesthetic potency among inbred mouse strains. Anesth Analg. 2000;91:720–6. doi: 10.1097/00000539-200009000-00042. [DOI] [PubMed] [Google Scholar]
- 9.Simpson VJ, Rikke BA, Costello JM, Corley R, Johnson TE. Identification of a genetic region in mice that specifies sensitivity to propofol. Anesthesiology. 1998;88:379–89. doi: 10.1097/00000542-199802000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Lasocki S, Iglarz M, Seince PF, Vuillaumier-Barrot S, Vicaut E, Henrion D, et al. Involvement of renin-angiotensin system in pressure-flow relationship: role of angiotensin-converting enzyme gene polymorphism. Anesthesiology. 2002;96:271–5. doi: 10.1097/00000542-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Homanics GE, Xu Y, Tang P. Integrated approaches to the action of general anesthetics and alcohol. Physiol Behav. 2002;77:495–9. doi: 10.1016/s0031-9384(02)00910-1. [DOI] [PubMed] [Google Scholar]
- 12.Gamo S. Studies on target genes of general anesthetics. Curr Drug Targets. 2002;3:31–41. doi: 10.2174/1389450023348118. [DOI] [PubMed] [Google Scholar]
- 13.Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet. 2000;24:221–5. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- 14.Iwai J, Knudsen KD, Dahl LK, Tassinari L. Effects of adrenalectomy on blood pressure in salt-fed, hypertension-prone rats. Failure of hypertension to develop in absence of evidence of adrenal cortical tissue. J Exp Med. 1969;129:663–78. doi: 10.1084/jem.129.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pennisi E. Genomics. Rat genome off to an early start. Science. 2000;289:1267–9. [PubMed] [Google Scholar]