Abstract
Aim
To elucidate the mechanisms involved in apoptosis of psoriatic keratinocytes by examining the expression of pro-apoptotic (Bak, Bax) and anti-apoptotic (Bcl-2, Bcl-X) Bcl-2 family of proteins, as well as the expression of p53 and Ki-67 proteins in normal skin, and uninvolved and involved psoriatic skin.
Methods
A total of 90 skin samples (30 cases of involved and uninvolved psoriatic skin and normal skin) were examined immunohistochemicaly to determine the protein expression of p53, Ki-67, Bcl-2, Bcl-X, Bax, and Bak. The results were quantified and expressed as a percentage of positive keratinocytes.
Results
There was a significant increase in Ki-67 (17.05 vs 3.65; P<0.001), Bcl-X (40.21 vs 13.97; P<0.001), Bak (89.46 vs 73.36; P<0.001), and Bax (50.00 vs 29.25; P<0.001) expression and a decrease in Bcl-2 (3.23 vs 6.25; P=0.008) expression in involved psoriatic skin, as well as an increase in Bcl-X (25.13 vs 13.97; P<0.001) expression in uninvolved psoriatic skin, when compared to normal skin. Samples with higher percentage of Ki-67 positive cells showed a higher percentage of p53 positive cells (correlation coefficient r = 0.75 in involved psoriatic samples, P<0.001; r = 0.88 in uninvolved psoriatic samples, P<0.001; and r = 0.85 in normal skin samples, P<0.001). Samples with higher percentage of p53 positive cells expressed pro-apoptotic Bak and Bax in higher percentage of cells; the correlation coefficients were r = 0.74 and r = 0.68 in involved psoriatic samples (P<0.001 for both), r = 0.75 and r = 0.69 in uninvolved psoriatic samples (P<0.001, for both), and r = 0.87 and r = 0.70 in normal skin samples (P<0.001, for both).
Conclusion
Increased expression of Bcl-X protein was associated with psoriatic epidermal hyperplasia. Strong Bax and Bak expression in involved psoriatic skin are probably inhibitory mechanisms counteracting intensive proliferation.
Psoriasis is a chronic, relapsing, inflammatory, and hyperproliferative skin disorder characterized by well-circumscribed erythematosquamous lesions (1). Clinically, psoriasis is characterized by significant epidermal keratinocyte hyperplasia with proliferation, keratinocyte maturation, and turn-over rates as important mechanisms in its pathogenesis (2,3).
In normal human skin, keratinocytes in the superficial layer of the epidermis undergo apoptosis and regulate proliferation of cells in the basal layer (4). As opposed to normal skin, keratinocytes derived from psoriatic plaques were shown to be resistant to apoptosis (5). Numerous TUNEL-positive keratinocytes were also positive for proliferating nuclear antigen and Ki-67, which are indicative of proliferating cells (5). Inappropriate regulation of apoptosis was proposed as a possible explanation for epidermal thickening in hyperproliferative inflammatory skin disorders (6).
The list of molecular mediators influencing apoptosis is rapidly expanding with Bcl-2 and its homologous proteins, emerging as one of the most important regulators of programmed cell death and playing a crucial role in the balance between cell survival and cell death. Protein p53 is a well described tumor suppressor that plays a central role in the initiation of apoptosis and cell cycle control (2,7,8).
The mechanisms involved in the psoriatic plaque formation are not completely elucidated. In the era of biological therapy, better understanding of different apoptotic cell cycle regulatory mechanisms involved in this process would enable a development of novel specific therapeutic approaches for treating psoriatic patients. In this study, we examined the expression and distribution of the pro-apoptotic (Bak, Bax) and anti-apoptotic (Bcl-2, Bcl-X) Bcl-2 family proteins, as well as the expression of p53 protein in psoriatic epidermis and normal human skin. High TUNEL-positive rate in psoriatic keratinocytes was linked to high proliferation rate, so staining of Ki-67 was also performed.
Materials and methods
Patients and specimens
Thirty adult patients (older than 18) of both sexes diagnosed with psoriasis at the Department of Dermatovenerology, Rijeka University Hospital, between 2003 and 2005 were enrolled in the study. Diagnosis of psoriasis was established on the basis of typical clinical features and histology. Patients who had undergone psoralen photochemotherapy or received oral therapy up to 10 weeks before biopsy and used topical medications up to 6 weeks before biopsy were excluded. Biopsy samples of involved and uninvolved psoriatic skin were taken from patients. Uninvolved skin specimens were taken at least 1 cm away from the psoriatic lesion. Thirty samples of normal skin surrounding fibromas or hemangiomas, surgically resected for cosmetic reasons, served as a control group. Informed consent was obtained from all patients before the biopsy.
The median (range) age of psoriatic patients was 60.5 (38.0-89.0) years and 65.5 (39.0-87.0) years of healthy controls (P = 0.901). There was no difference in sex between the group of patients and controls (P = 1.000). All specimens were fixed in 10% buffered formaldehyde and embedded in paraffin. Specimen sections, 4 μm thick, were stained with hematoxylin-eosin, and two different pathologists examined each slide independently.
Immunohistochemical analysis
The following antibodies and dilutions were used: mouse monoclonal antihuman p53 antibody, clone DO7 (1:50, DAKO A/S, Glostrup, Denmark), rabbit monoclonal antihuman Ki-67 antibody, clone MIB-1 (1:50, DAKO A/S), mouse monoclonal antihuman Bcl-2 antibody, clone 124 (1:50, DAKO A/S), mouse monoclonal antihuman Bcl-X antibody, clone A 35-10 (1:50, DAKO A/S), rabbit polyclonal antihuman Bax antibody, clone A 3533 (1:1000, DAKO A/S), rabbit polyclonal antihuman Bak antibody, clone A 3538 (1:250, DAKO A/S). Specimens of gastric cancer, oral mucosa, lymph node, and mammary carcinoma served as positive control for p53, Ki-67, Bcl-2, and Bax, respectively. Additional sections were ran in parallel with omission of the primary antibodies and served as negative control.
Paraffin-embedded tissue sections were deparaffinized in xylene and rehydrated by washing in absolute and diluted ethyl alcohol and distilled water. Staining of p53, Ki-67, and Bcl-2 protein was carried out after sections were treated with antigen retrieval solution (DAKO Chem Mate, Glostrup, Denmark) at a dilution of 1:9 and placed in microwave for 20 minutes for antigen retrieval. For antigen retrieval in cases of Bcl-X, Bax, and Bak protein, staining sections were treated with TRIS/EDTA, pH 9, and incubated in water bath at 98°C for 20 minutes. This was followed by the standard avidin-biotin complex (ABC) procedure for 2 hours and 10 minutes in DAKO Techmate Immunostainer (Techmate Horizon, serial No. 30097, LJL Biosystems Inc., Sunnyvale, CA, USA). Immunostaining results of p53, Ki-67, Bax, Bak, Bcl-2, and Bcl-X were quantified by counting positive keratinocytes in 1000 cells in each section and expressed as a percentage of positive cells. Also, cell-staining intensity for Bax, Bak, Bcl-2, and Bcl-X was assessed and graded as follows: 0 (negative), 1+ (weakly positive), 2+ (moderately positive), and 3+ (intensely positive), as described previously (5). Counting fields were randomly selected to minimize a possible bias.
Statistical analysis
Differences in patients’ age between the groups were analyzed using Mann-Whitney test. Differences in immunostaining results between the groups were analyzed using Kruskal-Wallis test or analysis of variance, while differences in frequency of cell staining intensity between the groups were analyzed using χ2-test. The association of parameters for immunostaining expression was tested using Pearson coefficient. Two-tailed P values of less than 0.05 were considered statistically significant.
Results
Cell proliferation in psoriasis
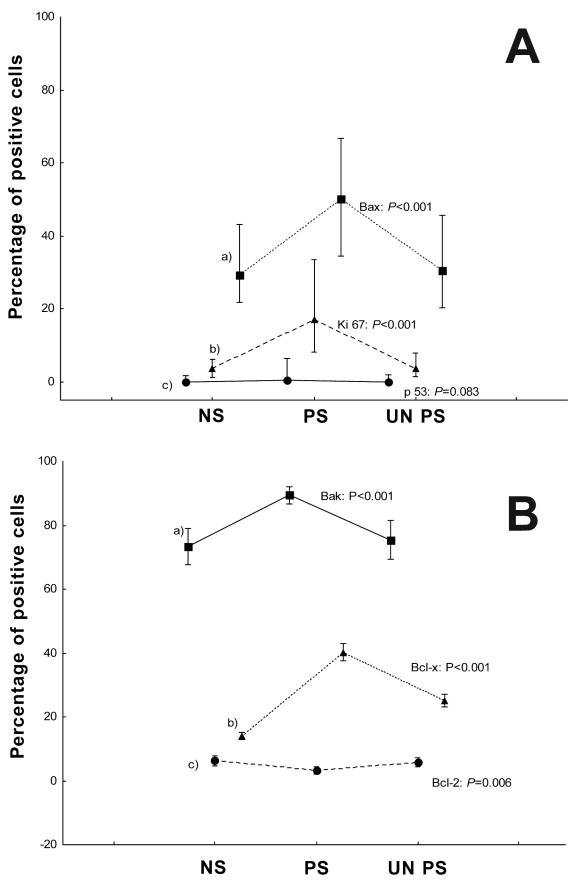
Nuclear staining for p53 was detected in 13/30 (43%) cases of normal skin, 12/30 (40%) cases of uninvolved psoriatic skin, and 15/30 (50%) cases of involved psoriatic skin. p53 positive cells were predominately located in the basal layer of the normal and uninvolved psoriatic skin, while in psoriatic skin they were located in the superbasal layers (distribution results not shown). Statistically significant increase in Ki-67 nuclear expression was detected in involved psoriatic skin, as compared to normal (P<0.001) and uninvolved psoriatic skin (P<0.001). Percentages of p53, Ki-67, Bax, Bak, Bcl-2, and Bcl-X positive cells are shown in Figure 1A and 1B. Distribution of Ki-67 positive cells was similar to p53 staining (distribution results not shown).
Figure 1.
Expression of p53, Ki-67, Bak, Bax, Bcl-X, and Bcl-2 proteins in psoriasis. (A) Expression of Bax (a), Ki-67 (b), and p53 (c) in normal skin (NS), involved psoriatic (PS), and uninvolved psoriatic skin (UNPS) presented with median and 5-95th percentile because the distribution of expression values was not normal. (B) Expression of Bak (a), Bcl-X (b), and Bcl-2 (c) in normal skin, involved psoriatic and uninvolved psoriatic skin presented with mean and 95% confidence intervals (CI) due to normal distribution of expression values.
Bcl-2 and Bcl-X expression in psoriasis
The Bcl-2 immunostaining was identified in all the examined cases of normal skin, 25/30 (83.3%) cases of uninvolved psoriatic skin, and 23/30 (76.6%) cases of involved psoriatic skin. The suprabasal keratinocytes were uniformly Bcl-2 negative in involved and uninvolved psoriatic, as well as in normal skin samples. Bcl-2 protein expression was significantly decreased in involved psoriatic skin samples, as compared to normal (3.23 vs 6.25; P = 0.008) and uninvolved psoriatic skin samples (P = 0.014) (Figure 1B). There was a significant difference in Bcl-2 protein staining intensity between the groups (χ2 = 18.51; P = 0.001), showing the lowest staining intensity in psoriatic plaque keratinocytes (Table 1). Positive cells were predominately identified in the basal layer of psoriatic and normal skin.
Table 1.
Immunostaining intensity for Bak, Bax, Bcl-2, and Bcl-X*
| Type of skin (No.) |
|||||
|---|---|---|---|---|---|
| Immunostaining intensity | normal | involved psoriatic | uninvolved psoriatic | χ2 | P |
| Bak: | |||||
| +1 | 3 | 0 | 4 | 6.60 | 0.164 |
| +2 | 9 | 5 | 8 | ||
| +3 | 18 | 25 | 18 | ||
| Bcl-2: | |||||
| 0 | 0 | 7 | 0 | 18.51 | 0.001 |
| +1 | 26 | 23 | 28 | ||
| +2 | 4 | 0 | 2 | ||
| Bax: | |||||
| +1 | 16 | 0 | 15 | 31.03 | <0.001 |
| +2 | 11 | 13 | 11 | ||
| +3 | 3 | 17 | 4 | ||
| Bcl-X: | |||||
| +1 | 25 | 7 | 9 | 19.95 | <0.001 |
| +2 | 5 | 13 | 16 | ||
| +3 | 0 | 10 | 5 | ||
*Immunostaining intensity: 0 (negative), 1+ (weakly positive), 2+ (moderately positive), and 3+ (intensely positive).
The Bcl-X expression was detected in all samples of normal skin samples and involved and uninvolved psoriatic skin samples, predominantly in the upper layers of the epidermis. The expression in normal skin was slight, mainly in upper granular layers (Figure 2A). Uninvolved and especially involved psoriatic skin keratinocytes displayed strong and diffuse expression of Bcl-X with marked perinuclear accentuation (Figure 2B, 2C). Bcl-X expression was significantly increased in involved (40.21 vs 13.97; P<0.001) and uninvolved psoriatic skin (25.13 vs 13.97; P<0.001), as compared to normal skin samples (Figure 1B). There was also a significant difference between involved and uninvolved psoriatic skin in Bcl-X expression (P<0.001). We found a significant difference in Bcl-X cell staining intensity between the groups (χ2 = 29.95; P<0.001), with the lowest staining intensity detected in normal keratinocytes (Table 1).
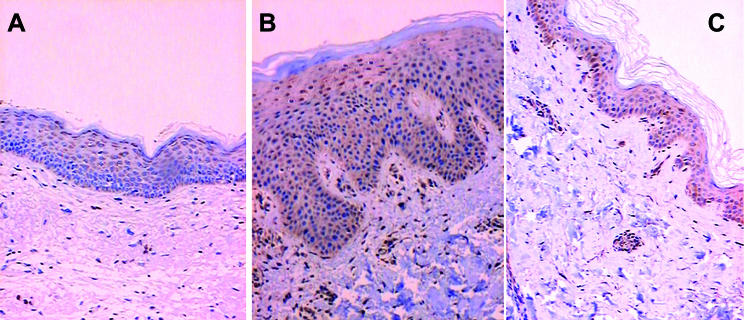
Figure 2.
Immunohistochemical staining for Bcl-X. (A) Bcl-X positive cells in upper granular layers of normal skin ( × 200). (B) Strong diffuse Bcl-X staining in involved psoriatic skin ( × 200). (C) Intense diffuse Bcl-X staining in uninvolved psoriatic skin ( × 200).
Bak and Bax expression in psoriasis
The expression of Bak and Bax was detected in all examined cases of normal, involved, and uninvolved psoriatic skin samples (Figure 3A, 3B, 3C). In normal skin and uninvolved psoriatic skin samples Bak protein was expressed as a brown, homogenous cytosolic punctuate staining, usually distributed in the suprabasal region with granular layer being stained slightly stronger than the spinous and basal layers. In some cases, staining was diffuse with positive cells detected in all layers, including the basal layer of normal skin epidermis. In involved psoriatic skin, Bak expression was mainly diffuse and present in all epidermal layers. Bak protein expression was significantly increased in involved psoriatic skin, as compared to uninvolved psoriatic (P<0.001) and normal skin samples (89.46 vs 73.36; P<0.001) (Figure 1B). There was no significant difference in Bak staining intensity between the groups (χ2 = 6.60; P = 0.164), but the strongest staining intensity was detected in psoriatic plaque keratinocytes (Table 1).
In normal skin (Figure 4A) and uninvolved psoriatic skin samples (Figure 3C), Bax protein was expressed as a brown cytosolic punctuate staining, with suprabasal keratinocytes being stained more strongly than the basal cell layer, which stained markedly weak for Bax. Psoriatic skin specimens revealed mainly a diffuse positive staining, especially in the suprabasal layers (Figure 4B), with distribution similar to normal skin. Bax protein expression was significantly increased in involved psoriatic skin, as compared to normal skin (50.00 vs 29.25; P<0.001) and uninvolved psoriatic skin samples (P<0.001) (Figure 1A). There was a significant difference in Bax staining intensity between the groups (χ2 = 31.03; P<0.001), with the strongest staining intensity detected in psoriatic plaque keratinocytes (Table 1).
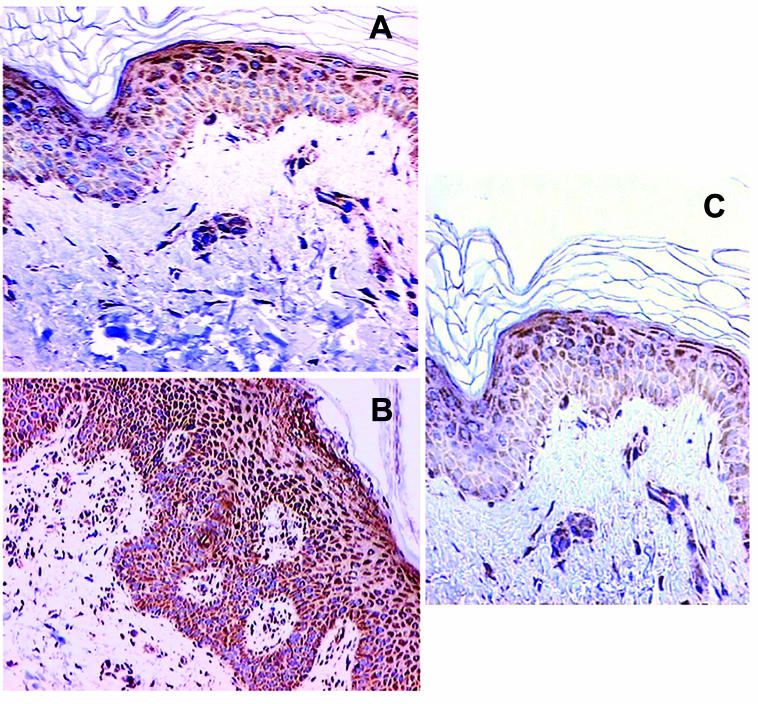
Figure 4.
Immunohistochemical staining for Bax. (A) Weak Bax staining mainly in suprabasal layers of normal skin ( × 400). (B) Strong diffuse Bax staining in psoriatic skin ( × 200). (C) Weak Bax staining predominately in suprabasal layers of uninvolved psoriatic skin ( × 200).
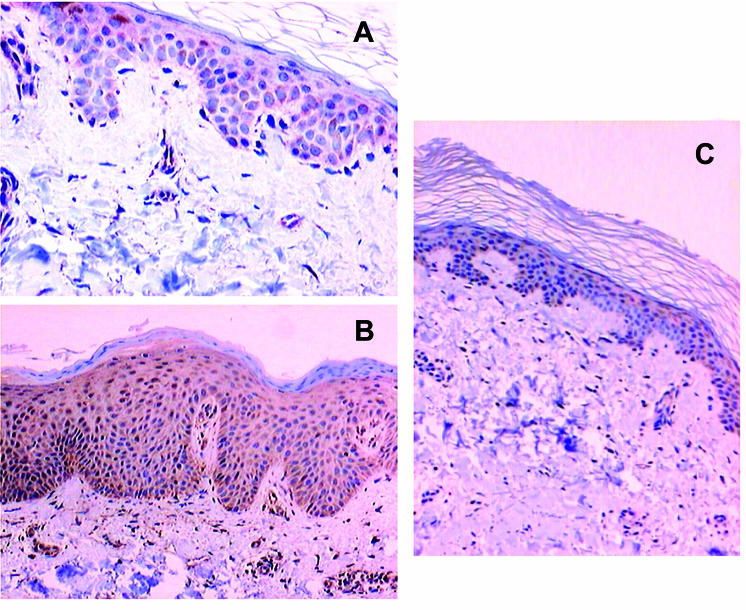
Figure 3.
Immunohistochemical staining for Bak. (A) Intense Bak staining mainly in suprabasal layers of normal skin with granular layer being stained slightly stronger than the spinous and basal layers ( × 400). (B) Strong diffuse Bak staining in involved psoriatic skin with granular layer being stained slightly stronger than the spinous and basal layers ( × 200). (C) Intense Bak staining predominately in the suprabasal layers of uninvolved psoriatic skin, with accentuation in upper layers ( × 400).
Correlation between p53 and Ki-67 and Bcl-2 family proteins
The coefficient of correlation between p53 and Ki-67 protein expression was positive in all the examined skin samples; the correlation coefficient was r = 0.75 in involved psoriatic skin samples (P<0.001), r = 0.88 in uninvolved psoriatic skin samples (P<0.001), and r = 0.85 in normal skin samples (P<0.001). Also, in all groups samples with higher percentage of p53 positive cells expressed pro-apoptotic Bak and Bax proteins in a higher percentage of cells; correlation coefficients were r = 0.74 and r = 0.68 in involved psoriatic skin samples (P<0.001, for both r = 0.75, and r = 0.69 in uninvolved psoriatic skin samples (P<0.001, for both), and r = 0.87 and r = 0.70 in normal skin samples (P<0.001, for both).
Involved psoriatic skin samples with higher p53 protein expression showed lower Bcl-X protein expression; correlation coefficient was r = -0.37 (P = 0.042).
In all the groups, samples with higher cell proliferation, evaluated by Ki-67 expression, expressed pro-apoptotic Bak and Bax proteins in the higher percentage of cells; correlation coefficients were r = 0.75 and r = 0.81 in involved psoriatic skin samples (P<0.001, for both), r = 0.70 and r = 0.76 in uninvolved psoriatic skin samples (P<0.001, for both), and r = 0.82 and r = 0.79 in normal skin samples (P<0.001, for both).
Involved psoriatic skin samples with higher Bcl-X protein expression showed a lower cell proliferation; the coefficient of correlation was r = -0.32 (P = 0.009).
In all examined groups, samples with higher Bak protein expression expressed Bax protein in higher percentage of cells; the correlation coefficient was r = 0.57 in involved psoriatic skin samples (P<0.001), r = 0.62 in uninvolved psoriatic skin samples (P<0.001), and r = 0.62 in normal skin samples (P<0.001). The coefficient of correlation between Bak and Bcl-X (r = -0.43, P = 0.017) or Bax and Bcl-X (r = -0.21, P = 0.036) was negative in involved psoriatic skin.
Discussion
We found a deregulated expression of pro- and anti-apoptotic proteins in psoriasis. There was a significant increase in Ki-67, Bcl-X, Bak, and Bax, together with a decrease in Bcl-2 protein expression in involved psoriatic skin and an increase in Bcl-X expression in uninvolved psoriatic skin samples, when compared to normal skin. We found a positive correlation between p53 expression, keratinocyte hyperproliferation, as well as Bak and Bax expression in normal skin and involved, and uninvolved psoriatic epidermis.
Bcl-2 protein protects cell from apoptosis by binding to the Bax and Bak protein (4,9,10). In normal skin, we detected Bcl-2 expression confined mainly to the basal cell layer and the outer root sheat, as shown in some previous studies (6,11), protecting the proliferative compartment from apoptotic stimuli (12). Expression of Bcl-2 protein was significantly decreased in involved psoriatic skin, which was in accordance with minimal or absent Bcl-2 expression detected by previous studies (Bcl-2 mRNA and Western blot analysis) (4,6,13). This unexpected finding could be a result of increased p53 protein expression in involved psoriatic skin and suggests that Bcl-2 plays no role in the anti-apoptotic mechanisms proposed to operate in psoriasis. Also, decreased Bcl-2 expression could be a result of intense proliferation, probably secondary to inflammatory stimuli in psoriasis, since no changes in Bcl-2 expression have been detected in uninvolved psoriatic skin, when compared to normal skin.
Bcl-X is a Bcl-2 independent regulator of apoptosis. Its predominant form expressed by keratinocytes in vitro and in vivo, the long form (Bcl-X L), inhibits apoptosis (4). Contrary to Bcl-2, Bcl-X was expressed in the upper epidermal, mainly granular, layers of normal skin, which is in agreement with previous studies (4,6,14). Some authors found diffusely cytosolic Bcl-X staining within the basal keratinocytes, perinuclear staining in keratinocytes of spinous layer, but no staining in the granular and cornified layers (11). As shown previously (1,4,6,13), we observed a strong and diffuse Bcl-X expression in almost all involved and uninvolved psoriatic skin samples, significantly higher than in the normal epidermis (4,6,14). These findings suggest a major contributory role of Bcl-X protein in the anti-apoptotic mechanisms and epidermal hyperplasia in psoriasis. Bcl-X expression is elevated early in psoriasis before plaque formation and probably represents an aberrant physiological response secondary to locally produced Th1 cytokines (4,15). Therefore, increased Bcl-X expression may represent one of the earliest events in disregulation of apoptotic process and might contribute to the longevity of psoriatic keratinocytes (5). Overabundance of Bcl-X suppresses the ability of cells that sustain DNA strand breaks to complete the complex multistep process of apoptosis (4,6). On the other hand, changes in the expression of other molecules occur in the later stages of plaque formation.
Bax, considered a principal molecular inducer of apoptosis (6,10,16), forms heterodimers with a number of homologous anti-apoptotic proteins (Bcl-2, Bcl-X, and Bad), which are then inactivated to accelerate apoptosis (9,10,13,16). As shown previously, we found that Bax expression was up-regulated in the suprabasal layers of normal skin epidermis and mainly showed no overlap with the distribution of Bcl-2 (6,13,16). There was a significant increase and strong Bax protein expression in involved psoriatic skin, as compared to uninvolved psoriatic and normal skin (6,13,16).
Bak was reported to induce apoptosis by opposing the protecting function of Bcl-2 and Bcl-XL (9), preferentially interacting with Bcl-XL (17). As shown previously (12), Bak expression was detected in uninvolved psoriatic and normal skin keratinocytes from basal to granular layer, with enhanced expression in the granular layers. As opposed to this, other authors found only rare Bak-positive keratinocytes in granular layer, cytosolic staining in the basal layer, and perinuclear staining in the spinous layer (11). In comparison to normal skin, our study showed pro-apoptotic protein Bak to be strongly expressed and significantly increased in involved psoriatic skin (6,12,13).
Increased Bak and Bax expression in involved psoriatic skin is probably a result of intensive proliferation seen in psoriasis, since we detected no changes in uninvolved psoriatic skin and it was suggested that proliferation stimulates apoptosis (18). We also found a positive correlation between p53, Ki-67, Bak, and Bax expression in uninvolved and involved psoriatic and normal skin.
p53 protein is essential for the regulation of cell proliferation. Increased p53 protein expression, coupled with intensive proliferation in involved psoriatic skin and similar distribution of p53 and Ki-67 positive cells, supports the hypothesis that increased proliferation induces enhanced wild-type p53 protein synthesis (2). A positive correlation found between p53 protein expression and Ki-67, Bak, and Bax expression speaks in favor of p53 pathway acting as a possible inhibitory mechanism of enhanced cell proliferation (2). Furthermore, p53 overexpression in involved psoriatic skin could be a result of enhanced wild-type protein synthesis as a response to increased number of DNA double-strand breaks in psoriatic lesions due to active DNA replication (19).
Consistent with the previous results, this study showed a deregulated expression of pro- and anti-apoptotic proteins in psoriasis (4,12,16). The results of this study suggest the possible role of Bcl-X protein in pathogenesis of psoriasis. Its increased expression was detected in clinically unchanged psoriatic skin and could contribute to the anti-apoptotic mechanisms and epidermal thickening in psoriatic epidermis. Strong Bax and Bak expression in involved psoriatic skin probably represent the only inhibitory mechanisms counteracting intensive proliferation. We believe that the increased expression of anti-apoptotic Bcl-X protein has a contributory role in psoriatic epidermal hyperplasia.
References
- 1.Karasek MA. Progress in our understanding of the biology of psoriasis. Cutis. 1999;64:319–22. [PubMed] [Google Scholar]
- 2.Batinac T, Zamolo G, Jonjic N, Gruber F, Petrovecki M. p53 protein expression and cell proliferation in non-neoplastic and neoplastic proliferative skin diseases. Tumori. 2004;90:120–7. doi: 10.1177/030089160409000124. [DOI] [PubMed] [Google Scholar]
- 3.Bowen AR, Hanks AN, Murphy KJ, Florell SR, Grossman D. Proliferation, apoptosis, and survivin expression in keratinocytic neoplasms and hyperplasias. Am J Dermatopathol. 2004;26:177–81. doi: 10.1097/00000372-200406000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrone-Smith T, Johnson T, Nelson B, Boise LH, Thompson CB, Nunez G, et al. Discordant expression of Bcl-x and Bcl-2 by keratinocytes in vitro and psoriatic keratinocytes in vivo. Am J Pathol. 1995;146:1079–88. [PMC free article] [PubMed] [Google Scholar]
- 5.Wrone-Smith T, Mitra RS, Thompson CB, Jasty R, Castle VP, Nickoloff BJ. Keratinocytes derived from psoriatic plaques are resistant to apoptosis compared with normal skin. Am J Pathol. 1997;151:1321–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Kocak M, Bozdogan O, Erkek E, Atasoy P, Birol A. Examination of Bcl-2, Bcl-X and bax protein expression in psoriasis. Int J Dermatol. 2003;42:789–93. doi: 10.1046/j.1365-4362.2003.01821.x. [DOI] [PubMed] [Google Scholar]
- 7.Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis – the p53 network. J Cell Sci. 2003;116:4077–85. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 8.Wrone-Smith T, Bergstrom J, Quevedo ME, Reddy V, Gutierrez-Steil C, Nickoloff BJ. Differential expression of cell survival and cell cycle regulatory proteins in cutaneous squamoproliferative lesions. J Dermatol Sci. 1999;19:53–67. doi: 10.1016/s0923-1811(98)00052-8. [DOI] [PubMed] [Google Scholar]
- 9.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 10.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–6. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delehedde M, Cho SH, Sarkiss M, Brisbay S, Davies M, El-Naggar AK, et al. Altered expression of bcl-2 family member proteins in nonmelanoma skin cancer. Cancer. 1999;85:1514–22. [PubMed] [Google Scholar]
- 12.Tomkova H, Fujimoto W, Arata J. Expression of bcl-2 antagonist bak in inflammatory and neoplastic skin diseases. Br J Dermatol. 1997;137:703–8. [PubMed] [Google Scholar]
- 13.Takahashi H, Manabe A, Ishida-Yamamoto A, Hashimoto Y, Iizuka H. Aberrant expression of apoptosis-related molecules in psoriatic epidermis. J Dermatol Sci. 2002;28:187–97. doi: 10.1016/s0923-1811(01)00162-1. [DOI] [PubMed] [Google Scholar]
- 14.Taylor JK, Zhang QQ, Monia BP, Marcusson EG, Dean NM. Inhibition of Bcl-xL expression sensitizes normal human keratinocytes and epithelial cells to apoptotic stimuli. Oncogene. 1999;18:4495–504. doi: 10.1038/sj.onc.1202836. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich M, Krammig S, Henze M, Docke WD, Sterry W, Asadullah K. Flow cytometric characterization of lesional T cells in psoriasis: intracellular cytokine and surface antigen expression indicates an activated, memory/effector type 1 immunophenotype. Arch Dermatol Res. 2000;292:519–21. doi: 10.1007/s004030000167. [DOI] [PubMed] [Google Scholar]
- 16.Tomkova H, Fujimoto W, Arata J. Expression of the bcl-2 homologue Bax in normal human skin, psoriasis vulgaris and non-melanoma skin cancers. Eur J Dermatol. 1998;8:256–60. [PubMed] [Google Scholar]
- 17.Chittenden T, Harrington EA, O'Connor R, Flemington C, Lutz RJ, Evan GI, et al. Induction of apoptosis by the Bcl-2 homologue Bak. Nature. 1995;374:733–6. doi: 10.1038/374733a0. [DOI] [PubMed] [Google Scholar]
- 18.Einspahr JG, Alberts DS, Warneke JA, Bozzo P, Basye J, Grogan TM, et al. Relationship of p53 mutations to epidermal cell proliferation and apoptosis in human UV-induced skin carcinogenesis. Neoplasia. 1999;1:468–75. doi: 10.1038/sj.neo.7900061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawashima K, Doi H, Ito Y, Shibata MA, Yoshinaka R, Otsuki Y. Evaluation of cell death and proliferation in psoriatic epidermis. J Dermatol Sci. 2004;35:207–14. doi: 10.1016/j.jdermsci.2004.05.008. [DOI] [PubMed] [Google Scholar]