Abstract
Aim
To evaluate trends in DNA typing success rates of different skeletal elements from mass graves originating from conflicts that occurred in the former Yugoslavia (Bosnia and Herzegovina and Kosovo) during the 1990s, and to establish correlation between skeletal sample age and success of high throughput short tandem repeat (STR) typing in the large data set of the International Commission on Missing Persons.
Method
DNA extraction and short tandem repeat (STR) typing have been attempted on over 25 000 skeletal samples. The skeletal samples originated from different geographical locations where the conflicts occurred and from different time periods from 1992 to 1999. DNA preservation in these samples was highly variable, but was often significantly degraded and of limited quantity. For the purpose of this study, processed samples were categorized according to skeletal sample type, sample age since death, and success rates tabulated.
Results
Well-defined general trends in success rates of DNA analyses were observed with respect to the type of bone tested and sample age. The highest success rates were observed with samples from dense cortical bone of weight-bearing leg bones (femur 86.9%), whereas long bones of the arms showed significantly lower success (humerus 46.2%, radius 24.5%, ulna 22.8%). Intact teeth also exhibited high success rates (teeth 82.7%). DNA isolation from other skeletal elements differed considerably in success, making bone sample selection an important factor influencing success.
Conclusion
The success of DNA typing is related to the type of skeletal sample. By carefully evaluating skeletal material available for forensic DNA testing with regard to sample age and type of skeletal element available, it is possible to increase the success and efficiency of forensic DNA testing.
The aftermath of the 1992-1995 conflict in the area of former Yugoslavia was marked with estimated 40 000 missing individuals. To address the issue of missing persons, the International Commission on Missing Persons (ICMP) was created in 1996 following the G-7 Summit in Lyon, France. ICMP’s mandate was expanded to also cover the DNA typing of missing persons resulting from 1999 conflict in Kosovo region.
ICMP employs a “population based, DNA-led” identification system for the identification of missing persons in the region of former Yugoslavia. On a regional scale, DNA profiles from reference samples of living relatives of missing persons are continuously compared in a batch mode to the DNA profiles obtained from mortal remains of victims. To date, more than 84 000 blood samples representing over 28 000 missing individuals have been collected, analyzed, and entered into the database. Since 2001, short tandem repeat (STR) profiles from more than 21 000 skeletal samples, representing more than 15 000 different individuals, have also been entered into the ICMP database (1). DNA matching reports of greater than 99.95% probability of identity have been issued for over 11 600 individuals.
ICMP DNA laboratories currently operate at a target rate of 105 bone or tooth extractions per day, using a silica-based extraction method (1-3). Bone and teeth samples tested are between 8 and 15 years post mortem. The quality of DNA preservation in these bones is highly variable and often substantially limited or/and degraded. This reflects the fact that the remains were buried or disposed in many different environmental contexts, with differential exposure to potentially harsh extrinsic factors such as temperature, UV radiation, humidity, and exposure to animals, insects, and microbes. Different disposal conditions are marked by burial in different soil types, complete or partial immersion of remains in water, contact with fire, or use of plastic sheeting. Microbial degradation is variably evidenced in these samples by both bone morphology and co-extraction of sometimes large amounts of microbial DNA (our unpublished observation). As always in this type of work, co-extraction of DNA inhibitors is a serious issue, and is also variable among samples.
Bone and teeth samples clearly protect DNA through their physical and/or chemical robustness to environmental degradation and/or biological attack. An elementary manifestation of this is that bone and teeth are often the only surviving material that can be tested. However, the mechanisms by which DNA is preserved in bone are not very well characterized (4). Bone tissue is primarily composed of protein and mineral. The two most abundant proteins in bone tissue are collagen and osteocalcin. Approximately 70% of the mineral portion of the bone is composed of hydroxyapatite, which includes calcium phosphate, calcium carbonate, calcium fluoride, calcium hydroxide, and citrate. Structural arrangement of bone tissue is such that the mineral portion provides structural support to the protein portion in the bone and, by doing so, physically excludes exogenous/extracellular agents/enzymes that are potentially harmful to the protein portion of the bone (4). DNA has a very strong affinity for hydroxyapatite. DNA degradation is linked to the loss of crystallinity in the hydroxyapatite, but it may also be related to the loss of collagen (5).
Overall, it seems reasonable to suppose that the characteristics of the bone that are correlated with its general long term survival, ie its resistance to morphological degradation at the macroscopic and microscopic level, would be those that contribute to the protection of DNA from degradation. Bone density, ie the extent of mineralization, is one of the most important intrinsic factors in survival of bone material. There is a significant difference in bone density between men and women, with the latter showing lower density values. The difference in bone density is also specific for different areas of the skeletal element morphology, with the highest density values noted for the mid-shaft region (6). Teeth are the hardest tissue in the human body because of the dental enamel (7).
To know which bones best preserve DNA is of fundamental importance to DNA identification casework in mass fatality incidents and mass graves from armed conflict or genocide. The question equally applies to “ancient DNA” analyses in archaeological or human molecular evolutionary investigations. Despite the logical expectation that denser, more intact bones may be preferable, there is very little empirical data published on this issue (8). We also note that a successful recovery of DNA is linked not only to the degree of protection within the bone, but also the total amount of starting DNA. One reason for the lack of precise information on the best samples for DNA testing from degraded bone is the difficulty in performing controlled experiments, with regard to the effect of relevant environmental variables, inter-individual variation (related to for example sex or age), the long periods of time involved, and the need for large sample size.
The aim of our study was to analyze DNA typing success rates from very large sample sizes of various skeletal elements from victims of conflict in the former Yugoslavia. Given the large number of variables affecting DNA preservation, a large sample size helps to average out the influences of a wide range of environmental contexts and permit general conclusions. Further, we divided our data into three time periods, with respect to time since death. This allows the analysis of the relative rate of degradation in different skeletal elements over time. These empirical data can serve as a useful guide to sampling strategies from degraded skeletal remains.
Materials and methods
Samples and success rates
Skeletal samples, including teeth, received by ICMP over a 6-year period (2001-2007) were categorized according to the type of skeletal element (when known), as well as to the conflict period when the victims were killed. In many instances graves could be attributed to particular events or time periods and we reported on samples from the following categories: individuals killed in 1992 at the outset of the conflict in Bosnia and Herzegovina (BH), individuals killed in 1995 as part of the Srebrenica-related massacres in BH, and individuals killed in 1999 in the Kosovo conflict. The skeletal remains were recovered from a wide variety of contexts relating to disposal sites, although a great majority was recovered from mass graves covered with soil. There was not a strong correlation between the date of death/initial burial and the date of exhumation/recovery. After exhumation, samples were stored by various agencies under a variety of conditions, ranging from cool storage at 10°C to relatively uniform room temperature to seasonally fluctuating temperatures including significant summer heat.
To classify success rates of DNA typing, success was defined as the recovery of genetic data from 12 or more loci of the Promega PowerPlex 16 multiplex (PP16, Promega Corporation, Mannheim, Germany), with successful results required for the amelogenin locus. For ~ 90% of the samples, this would reflect the combined results from two separate DNA extractions performed routinely, with a variable number of amplification attempts from each, but rarely exceeding three; if both attempts to extract DNA failed, this was counted as a single attempt and a single failure.
According to ICMP guidelines, different skeletal elements are sampled in a rather uniform manner for each sample type (data not shown), with the aim of including as much dense cortical bone as the specimen would allow in a ~ 5 g minimum portion. However, ICMP is directly in control of the sampling process in less than 50% of cases, so there is a potential for significant variation. Whether sampled by the ICMP or not, long bones from the limbs were nearly always sampled from dense mid-shaft regions. Favored teeth would be intact (devoid of cracks and caries) molars, premolars, and canines (in that order), followed by any intact tooth.
Bone sample preparation and extraction
Cleaning and grinding of skeletal samples, DNA extraction, quantification, and amplification set-up were conducted in a dedicated pre-amplification laboratory area with rigorous procedures for avoiding contamination. Bone/tooth processing and DNA extraction was conducted as previously reported (1), using a silica-binding DNA extraction method (3), based on modifications to the QIAamp DNA Blood Maxi kit (Qiagen, Hilden, Germany).
DNA quantitation and amplification
The amount of isolated amplifiable human DNA in a sample extract was quantified using the ABI QuantifilerTM Human DNA Quantification Kit, (Applied Biosystems, Foster City, CA, USA) using either an ABI 7000 or 7500 real time detection instrument (Applied Biosystems). DNA amplification and STR typing were performed using the commercially available Promega PowerPlex® 16 System. For samples with more than 10 pg/µL human DNA, polymerase chain reaction (PCR) was set up in a 25 µL reaction consisting of 2.5 µL of Gold ST*R 10X Buffer (Promega), 2.5 µL of PowerPlex® 16 Primer Pair Mix (Promega), 5 units of AmpliTaq Gold® polymerase (Applied Biosystems), and typically 9 µL of ddH20 and 10 µL of DNA extract (DNA extract amounts differed, depending on the response to inhibition, DNA concentration, or other factors). For samples with less than 10 pg/µL, PCR was typically set up in a 25 µL reaction consisting of 2.5 µL of Gold ST*R 10X Buffer (Promega), 2.75 µL of PowerPlex® 16 Primer Pair Mix (Promega), 7.5 unit of AmpliTaq Gold® polymerase (Applied Biosystems), 3.25 µL of double distilled H2O, and 15 µL of DNA template. Thermocycling parameters were as follows: 95°C for 11 minutes, 96°C for 1 minute, 10 cycles of 94°C for 30 seconds, 60°C for 30 seconds, 70°C for 90 seconds, 24 cycles of 90°C for 30 seconds, 60°C for 30 seconds, 70°C for 90 seconds, and 60°C for 45 minutes.
Sequencing and analysis
Detection and analysis of amplified STRs were carried out on ABI PRISM® 3100 Genetic Analyzer System using the POP-4 polymer (Applied Biosystems) and the collection software, version 1.0 or 1.1 (Applied Biosystems). Data was analyzed using GeneScan®, version 3.7, Genotyper®, version 3.7 (both Applied Biosystems), and PowerTyper 16 Macro, version 2 (Promega). Statistical analysis of 95% confidence intervals was calculated using the Wald method.
Results
The nuclear STR typing success rate from 25 361 bone or tooth samples from victims of armed conflict in the former Yugoslavia, with success defined as recovery of data from 12 or more PP16 loci, was 75.4%. The success rate differed greatly among the types of skeletal elements (Table 1, Figure 1). Results were tabulated for three different events/time periods as follows: the 1992 conflict in Bosnia and Herzegovina, the 1995 fall of Srebrenica, and the 1999 conflict in Kosovo. The distribution of skeletal elements received for testing was different among these events.
Table 1.
Summary of types and number of skeletal elements tested in BH 1992, Srebrenica 1995, and Kosovo 1999 sample categories
| BH 1992 |
Sreberenica 1995 |
Kosovo 1999 |
Total |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skeletal element | No. of skeletal elements tested | Reportable DNA profiles obtained | Success rate (%) | No. of skeletal elements tested | Reportable DNA profiles obtained | Success rate (%) | No. of skeletal elements tested | Reportable DNA profiles obtained | Success rate (%) | No. of skeletal elements tested | Reportable DNA profiles obtained | Success rate (with 95% confidence intervals) |
| Femur | 5729 | 4742 | 82.77 | 3459 | 3130 | 90.49 | 2168 | 1997 | 92.11 | 11 356 | 9869 | 86.91 (86.29-87.53) |
| Teeth | 4197 | 3312 | 78.91 | 1659 | 1485 | 89.51 | 1107 | 963 | 86.99 | 6963 | 5760 | 82.72 (81.83-83.61) |
| Tibia | 821 | 634 | 77.22 | 398 | 293 | 73.62 | 110 | 82 | 74.55 | 1329 | 1009 | 75.92 (73.62-78.22) |
| Fibula | 90 | 49 | 54.44 | 38 | 31 | 81.58 | 32 | 20 | 62.50 | 160 | 100 | 62.50 (55.00-70.00) |
| Scapula | 0 | 0 | 0 | 0 | 0 | 0 | 35 | 20 | 57.14 | 35 | 20 | 57.14 (40.75-73.53) |
| Mandibular body | 66 | 33 | 50.00 | 41 | 30 | 73.17 | 24 | 11 | 45.83 | 131 | 74 | 56.49 (48.00-64.98) |
| Humerus | 1329 | 582 | 43.79 | 887 | 414 | 46.67 | 199 | 120 | 60.30 | 2415 | 1116 | 46.21 (44.22-48.20) |
| Vertebra | 51 | 22 | 43.14 | 0 | 0 | 0 | 95 | 69 | 72.63 | 146 | 91 | 62.33 (54.74-69.92) |
| Ilium | 107 | 41 | 38.32 | 0 | 0 | 0 | 78 | 57 | 73.08 | 185 | 98 | 52.97 (45.78-60.16) |
| Cranium | 544 | 199 | 36.58 | 77 | 35 | 45.45 | 136 | 73 | 53.68 | 757 | 307 | 40.55 (37.06-44.04) |
| Metacarpal | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 11 | 61.11 | 18 | 11 | 61.11 (38.59-83.63) |
| Metatarsal | 91 | 28 | 30.77 | 0 | 0 | 0 | 29 | 11 | 37.93 | 120 | 39 | 32.50 (24.12-40.88) |
| Radius | 232 | 40 | 17.24 | 152 | 45 | 29.61 | 85 | 30 | 35.29 | 469 | 115 | 24.52 (20.63-28.41) |
| Ulna | 218 | 37 | 16.97 | 144 | 35 | 24.31 | 82 | 29 | 35.37 | 444 | 101 | 22.75 (18.85-26.55) |
| Clavicle | 81 | 13 | 16.05 | 0 | 0 | 0 | 47 | 20 | 42.55 | 128 | 33 | 25.78 (18.20-33.36) |
| Total | 13 556 | 9732 | 71.79 | 6855 | 5498 | 80.2 | 4245 | 3513 | 82.76 | 24 656 | 18 743 | 76.02 (75.49-76.55) |
*95% confidence intervals were calculated using the Wald method.
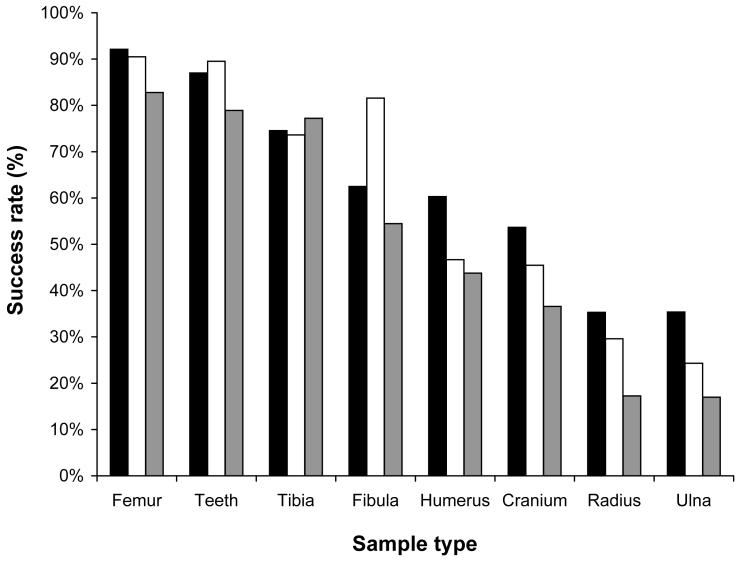
Figure 1.
Trends in DNA typing success rates for various skeletal elements dating to different years of conflict. Closed bars – Kosovo 1999; open bars – Srebrenica 1995; gray bars – Bosnia and Herzegovina 1992.
All three event/time categories showed a pronounced and consistent variation in success rates from various elements. Results for 13 556 samples from 13 different skeletal elements were observed in the BH 1992 category (Table 1). Femur and teeth samples provided the best success rates and they were also the most numerous samples in this category. The lowest success rates were observed for the samples from the clavicle, ulna, and radius. Results for 6855 samples from 9 different skeletal elements were observed in the Srebrenica 1995 category (Table 1). Again, femur and teeth samples provided the best success rates and they were also the most numerous samples in this category. The lowest success rates were observed for samples from the ulna and radius. Results for 4232 samples from 16 different skeletal elements were observed in the Kosovo 1999 category (Table 1). Femur and teeth samples, once again, provided the best success rates and they were also the most numerous samples in this category. Also, the lowest success rates were observed for samples from the ulna and radius. For long bones of the limbs (Table 2), success rates were distinctly higher for the lower body (femur, tibia, fibula) than for the upper body (humerus, radius, ulna).
Table 2.
Average DNA typing success rates for long bones of upper and lower body extremities
| Skeletal elements | Success rate (%) |
|---|---|
| Upper body: | |
| humerus | 46.2 |
| radius | 24.5 |
| ulna | 22.8 |
| Lower body: | |
| femur | 86.9 |
| fibula | 62.5 |
| tibia | 75.9 |
Overall, there was a slight but discernable decrease in the success rate with relation to the time since death of the three different victim categories (Table 3). This general trend was evident among all sample types, but was most pronounced for sample types with lower absolute success rates, such as the humerus, cranium, radius, and ulna. Figure 1 plots success rates for each age category for those bone types that were represented in large sample size for each age category.
Table 3.
Total number of samples tested for each sample age category and corresponding success rates of DNA typing*
| BH 1992 | Srebrenica 1995 | Kosovo 1999 | |
|---|---|---|---|
| Number of skeletal elements tested | 14 019 | 6948 | 4394 |
| Reportable DNA profiles obtained | 9956 | 5554 | 3602 |
| Success rate (%) | 71 | 80 | 82 |
*The totals here are different than the sum of the different sample types listed in Table 1 because this table includes samples which were of unknown bone type.
Discussion
The high success rates for nuclear STR typing reported here further confirmed that STRs could be considered a method of choice in casework with missing persons involving degraded skeletal remains. In the past, similar undertakings have primarily been considered the domain of mitochondrial DNA (mtDNA) typing due to the extreme degree of degradation routinely encountered (8,9). More recently, laboratories specializing in mtDNA analysis have successfully turned to more aggressive STR approaches (10,11). Until now, however, there has not been a large scale study to guide the selection of skeletal elements to maximize the chances for success. Such information is of particular importance to human ancient DNA studies, missing persons casework, and disaster victim identification response.
The advantage of our data set is its very large sample size, although the size is variable among different skeletal elements. Large sample size is extremely important for dealing with the difficulties in performing long term experimental studies of DNA degradation in bone and properly controlling for the effect of environmental variables. In this study, the effect of environmental variables was not controlled for at all, since the samples originated from a very wide range of contexts. For most of the skeletal elements, we considered that the sample size is large enough to average out the variable effects of the environment and show relative preservative characteristics of different bone types. However, it is not clear whether this is always true. For example, because of the lower sample size, the success rate for 47 clavicle samples (43%) from Kosovo 1999, in contrast to 81 samples from BH 1992 (16%), may reflect other factors rather than the overall rate of degradation which would be expected for clavicles over this time period. Unfortunately, there is not sufficiently tractable documentation of environmental variables associated with the submission of samples for ICMP testing to permit a more detailed analysis at this point.
Our study showed that the most successful samples for STR testing were intact teeth and mid-shaft sections of femur. Bones that performed less well tend to be less dense and/or have a greater proportion of spongy or diploic bone (eg, vertebrae, ilium, cranium). Perhaps surprisingly, though, there was a marked difference in success rates between dense compact long bones of the lower body and bones of the upper body. Upper body extremity samples were taken from the humerus, radius, and ulna, and lower body extremity samples from the femur, fibula, and tibia. These results correlate with known mid-shaft densities reported in the study by Galloway et al (6), where samples from long bones of the upper and lower body were ranked from the most to least dense: femur, tibia, humerus, radius, ulna, and fibula. The correlation between bone density and success rate of DNA typing was confirmed for all skeletal elements except the fibula. Despite its lower reported density, fibulae consistently showed a higher success rate than humeri, radii, or ulnae.
In general, our results are consistent with those reported in another study on 1021 skeletal samples subjected to mtDNA testing (8). That study involved older samples, primarily from the Vietnam and Korean War, but reported somewhat higher absolute success rates, due to the typing with high copy number mtDNA rather than nuclear DNA. To our knowledge, there is no reason why different bones would protect mtDNA differently than nuclear DNA, and the data are consistent with this expectation. The difference in success rates between long bones of the upper body and lower body were also shown by Edson et al (8). However, they listed ribs as the single most successful bone type, with 96% success. Our study did not include ribs, since they were rarely sampled because of our initial experience that they are an inferior source of DNA. Edson et al explained the high success with ribs as possible artifact of particular preservation conditions, as all 26 ribs tested in their study came from a single case.
Our results were tabulated with respect to three different periods of victim death – 1992, 1995, and 1999. This provides information not only on the relative amount of DNA present in the various bones, but on the changes in amount of DNA over time. We can, therefore, distinguish between bones that contain higher amounts of DNA at the outset and bones that are intrinsically better at protecting DNA over time. However, the interpretation of these results should be cautionary, as the recovery of remains from all time periods has occurred variably over multiple years. For example, remains from a 1992 mass grave exhumed in 1996 have spent considerably less time in the ground than remains from 1995 that were exhumed in 2006. Surely, the relevant “age” with regard to DNA degradation relates not just to time-since-death, but significantly to the conditions experienced during that time. In the field of ancient DNA analysis that considers much longer periods of time, a notion of “thermal age” of a sample has emerged, as opposed to its temporal age (12). Moreover, storage conditions after exhumation and storage duration prior to test were variable, ranging from mortuary facilities with little seasonal climate control to refrigerated morgues to tunnels in salt mines. Another qualification worth mentioning relates to variations in sex and age-at-death of the victims. The structure of bone changes with age (6). The elderly experience loss of bone mass and changes in mineralization. It has been reported that skeletal remains of elderly show decreased preservation upon burial, and men show greater preservation than women (13). We did not tabulate the age or sex distribution of the three victim categories, but 1995 Srebrenica victims are distinctive since they are almost exclusively men.
Even with the qualifications mentioned above, given our large sample sizes and the continuously ongoing exhumation process from all events/time periods, it can be expected that there is a general correlation between the time period and the “challenge” to DNA preservation experienced by the samples from that period. All samples showed a decrease in success with increasing time since burial, but this was by far most pronounced for samples with lower absolute success. Thus, the success rate of femur samples dropped from 92% in the 1999 category to 83% in the 1992 category, retaining 92% of its potential for successful typing. In contrast, the radius and ulna success rates dropped from 35% to 17% over this time, being only ~ 50% as successful in 1992 samples as in 1999 samples. Another interesting trend was observed with vertebra, ilium, and radius/ulna samples. From 1999 to 1992, vertebra success rates dropped from 73% to 43%, and ilium success rates dropped from 80% to 38%; both lost about 50% of their potential for successful typing. The same proportional decrease was seen for the radius and ulna, but these showed much lower absolute success rates in both time periods (both falling from 35% to 17%). It may be that the radius, ulna, vertebra, and ilium experience the same propensity to degrade, but that ilium and vertebrae start out with a higher amount of DNA. Ilium and vertebra are spongier and may have a higher blood and cell content due to increased vascularization, causing them to start out with larger amounts of DNA than the radius and ulna, but the spongy bone is not as protective of DNA as femur and tooth samples.
Our results provide further definitive empirical verification that the densest compact bones and teeth are the optimal samples for DNA recovery. In many instances, in disaster victim identification work or mass graves investigations, the individuals, agencies, or teams performing the sampling are not the same as the team that will eventually be tasked with DNA testing. We would like to urge that the current knowledge of preferred sources be universally applied by teams involved in sample collection. Field teams are often deployed on short notice, and find themselves working under adverse conditions. This makes it all the more imperative that proper sampling methods are put into well-established guidelines. Presently, it is not uncommon to find field teams working without clear methods and priorities for sampling or using old guidelines that are based more on convenience of sampling than on selection of what we now know are the clearly preferable sources. In many instances, it is very difficult, time consuming, or impossible to resample a skeletal element after a sample has failed the DNA extraction process. We suggest it is far better to make minor investments in equipment and training, and take some additional time during field operations than to front-load a costly and time consuming DNA identification process with less than optimal samples.
Acknowledgments
As this study reports on results from many years work at the ICMP, it is appropriate to note that this work could not have been done without the direct contributions of more than 200 ICMP staff members, past and present. In particular, we note the early contributions of Edwin Huffine, Theodore Anderson, Jon Davoren, and John Crews on establishing the DNA laboratories; the primary contribution of Jon Davoren and Daniel Vanek in development of the current ICMP extraction method; the highly expert archaeological and anthropological work undertaken by many and overseen for much of the time by Jon Sterenberg; the critical work of Asta Zinbo and Jeffrey Buenger and their staff in their respective ICMP departments of Civil Society Initiatives and Government Relations, and lastly the overarching and untiring administrative support and institutional direction provided by Kathryne Bomberger and Adam Boys. During this time, the ICMP has been supported by the governments of Canada, Denmark, Finland, France, Germany, Greece, the Holy See, Iceland, Ireland, Italy, the Netherlands, Norway, Sweden, Switzerland, the United Kingdom, and the United States, as well as the European Union and the C.S. Mott Foundation.
References
- 1.Parsons TJ, Huel R, Davoren J, Katzmarzyk C, Miloš A, Selmanović A, et al. Application of novel “mini-amplicon” STR multiplexes to high volume casework on degraded skeletal remains. Forensic Science International: Genetics. 2007;1:175–9. doi: 10.1016/j.fsigen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Davoren J, Vanek D, Konjhodzic R, Crews J, Huffine E, Parsons TJ. Highly effective DNA extraction method for nuclear STR testing of skeletal remains from mass graves. Croat Med J. 2007;48:478–85. [PMC free article] [PubMed] [Google Scholar]
- 3.Rohland N, Hofreiter M. Comparison and optimization of ancient DNA extraction. Biotechniques. 2007;42:343–52. doi: 10.2144/000112383. [DOI] [PubMed] [Google Scholar]
- 4.Collins MJ, Nielsen-Marsh CM, Hiller J, Smith CI, Roberts JP, Prigodich RV, et al. The survival of organic matter in bone: a review. Archaeometry. 2002;44:383–94. [Google Scholar]
- 5.Gotherstrom A, Collins MJ, Angerbjorn A, Liden K. Bone preservation and DNA amplification. Archaeometry. 2002;44:395–404. [Google Scholar]
- 6.Galloway A, Willey P, Snyder L. Human bone mineral densities and survival of bone elements: a contemporary sample. In: Haglund WD, Sorg MH, editors. Forensic taphonomy: the postmortem fate of human remains. Boca Raton (FL): CRC Press; 1996. p. 296-317. [Google Scholar]
- 7.Malaver PC, Yunis JJ. Different dental tissues as source of DNA for human identification in forensic cases. Croat Med J. 2003;44:306–9. [PubMed] [Google Scholar]
- 8.Edson SM, Ross JP, Coble MD, Parson TJ, Barritt SM. Naming the dead: confronting the realities of rapid identification of degraded skeletal remains. Forensic Science Review. 2004;16:63–90. [PubMed] [Google Scholar]
- 9.Ginther C, Issel-Tarver L, King MC. Identifying individuals by sequencing mitochondrial DNA from teeth. Nat Genet. 1992;2:135–8. doi: 10.1038/ng1092-135. [DOI] [PubMed] [Google Scholar]
- 10.Irwin JA, Edson SM, Loreille O, Just RS, Barritt SM, Lee DA, et al. The intersection of genetic identity: the application of multiple marker systems and new technologies to establish identity 50 years after death. J Forensic Sci. 2007 [Google Scholar]
- 11.Irwin JA, Leney MD, Loreille O, Barritt SM, Christensen AF, Holland TD, et al. Application of low copy number STR typing to the identification of aged, degraded skeletal remains. J Forensic Sci. 2007 doi: 10.1111/j.1556-4029.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith CI, Chamberlain AT, Riley MS, Stringer C, Collins MJ. The thermal history of human fossils and the likelihood of successful DNA amplification. J Hum Evol. 2003;45:203–17. doi: 10.1016/s0047-2484(03)00106-4. [DOI] [PubMed] [Google Scholar]
- 13.Walker PL, Johnson JR, Lambert PM. Age and sex biases in the preservation of human skeletal remains. Am J Phys Anthropol. 1988;76:183–8. doi: 10.1002/ajpa.1330760206. [DOI] [PubMed] [Google Scholar]