Abstract
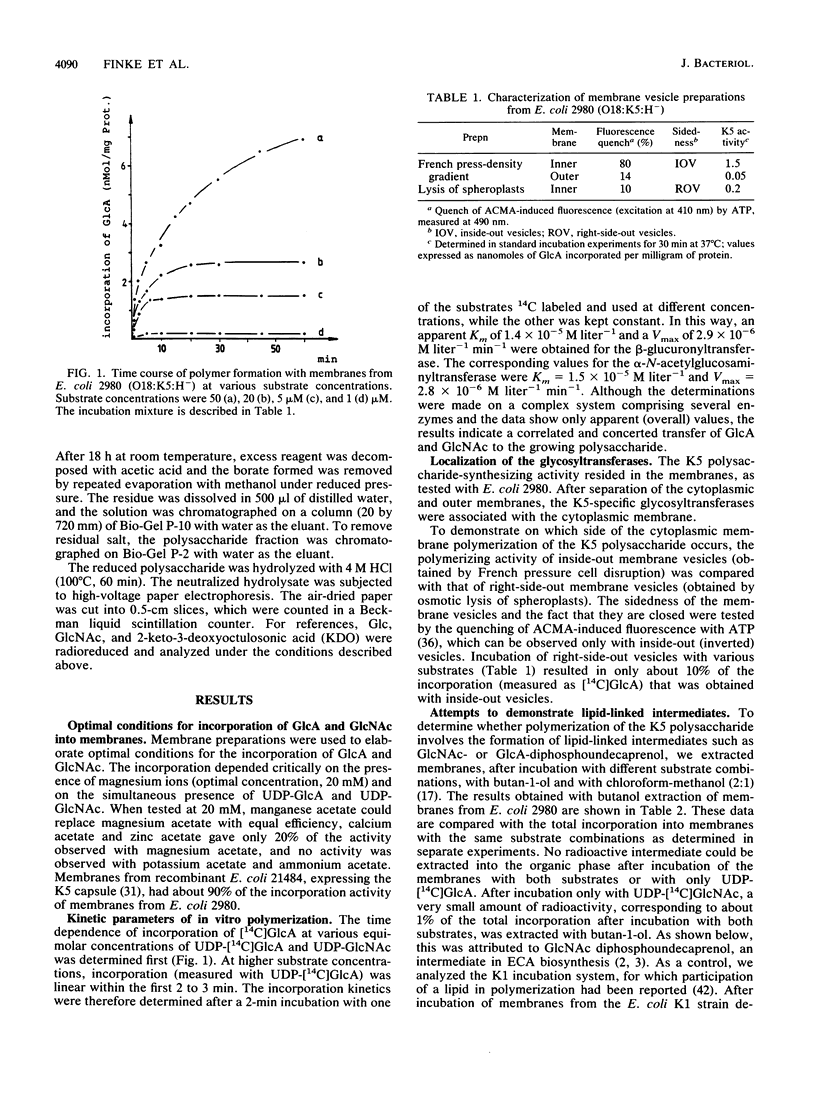
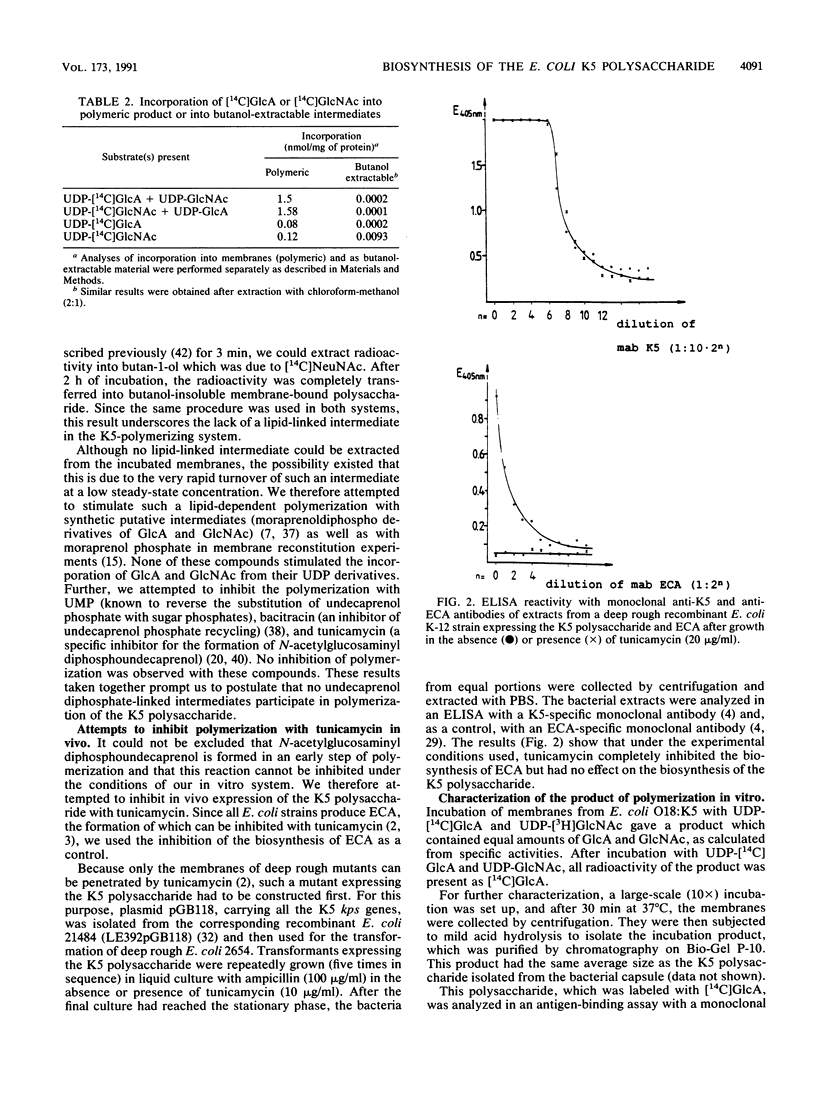
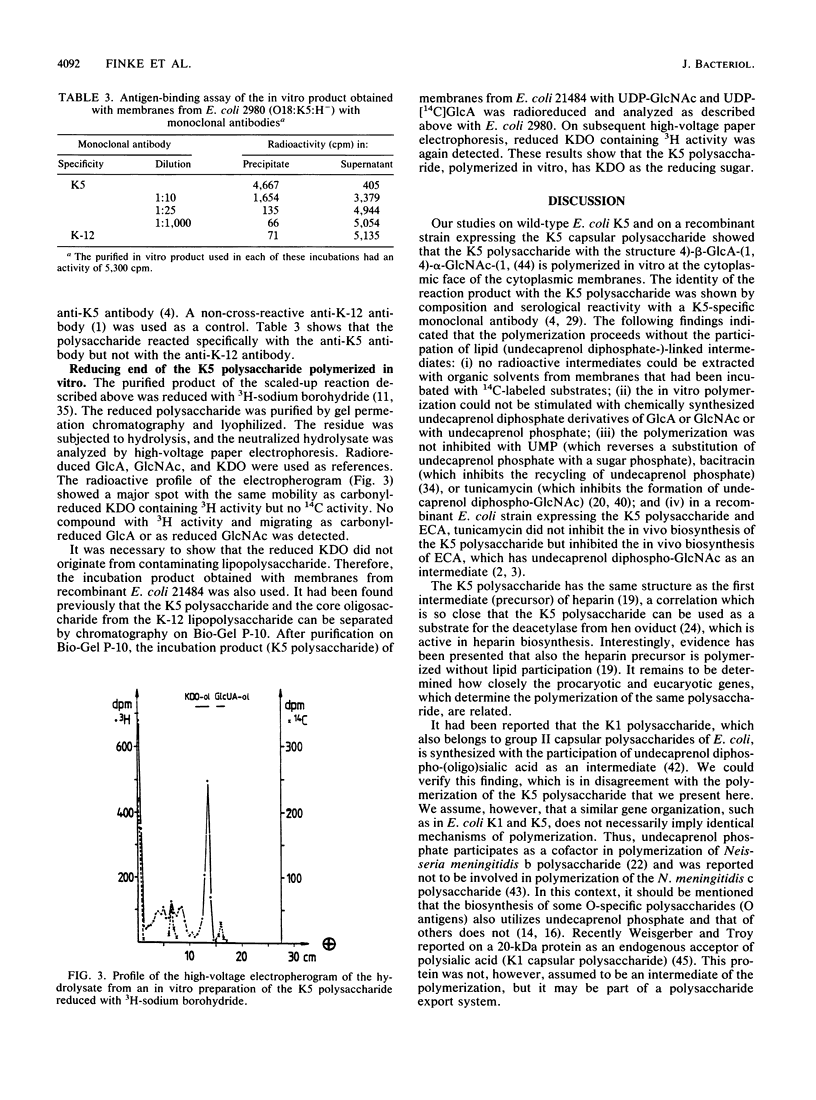
Biosynthesis of the capsular K5 polysaccharide of Escherichia coli, which has the structure 4)-beta GlcA-1,4-alpha GlcNAc-(1, was studied with membrane preparations from an E. coli K5 wild-type strain and from a recombinant K-12 strain expressing the K5 capsule. Polymerization occurs at the inner face of the cytoplasmic membrane without the participation of lipid-linked oligosaccharides. The serological K5 specificity of the in vitro product was determined with a K5-specific monoclonal antibody in an antigen-binding assay. The K5 polysaccharide, as obtained from the membranes after an in vitro incubation, has 2-keto-3-deoxyoctulosonic acid as the reducing sugar, which indicates that the polysaccharide grows by chain elongation at the nonreducing end.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr K., Rick P. D. Biosynthesis of enterobacterial common antigen in Escherichia coli. In vitro synthesis of lipid-linked intermediates. J Biol Chem. 1987 May 25;262(15):7142–7150. [PubMed] [Google Scholar]
- Barr K., Ward S., Meier-Dieter U., Mayer H., Rick P. D. Characterization of an Escherichia coli rff mutant defective in transfer of N-acetylmannosaminuronic acid (ManNAcA) from UDP-ManNAcA to a lipid-linked intermediate involved in enterobacterial common antigen synthesis. J Bacteriol. 1988 Jan;170(1):228–233. doi: 10.1128/jb.170.1.228-233.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnois G. J., Jann K. Bacterial polysaccharide capsule synthesis, export and evolution of structural diversity. Mol Microbiol. 1989 Dec;3(12):1819–1823. doi: 10.1111/j.1365-2958.1989.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Boulnois G. J., Roberts I. S. Genetics of capsular polysaccharide production in bacteria. Curr Top Microbiol Immunol. 1990;150:1–18. doi: 10.1007/978-3-642-74694-9_1. [DOI] [PubMed] [Google Scholar]
- Danilov L. L., Druzhinina T. N., Kalinchuk N. A., Maltsev S. D., Shibaev V. N. Polyprenyl phosphates: synthesis and structure-activity relationship for a biosynthetic system of Salmonella anatum O-specific polysaccharide. Chem Phys Lipids. 1989 Nov;51(3-4):191–203. doi: 10.1016/0009-3084(89)90006-6. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Finke A., Jann B., Jann K. CMP-KDO-synthetase activity in Escherichia coli expressing capsular polysaccharides. FEMS Microbiol Lett. 1990 May;57(1-2):129–133. doi: 10.1016/0378-1097(90)90426-q. [DOI] [PubMed] [Google Scholar]
- Finke A., Roberts I., Boulnois G., Pzzani C., Jann K. Activity of CMP-2-keto-3-deoxyoctulosonic acid synthetase in Escherichia coli strains expressing the capsular K5 polysaccharide implication for K5 polysaccharide biosynthesis. J Bacteriol. 1989 Jun;171(6):3074–3079. doi: 10.1128/jb.171.6.3074-3079.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming H. C., Jann K. Biosynthesis of the O9 antigen of Escherichia coli. Growth of the polysaccharide chain. Eur J Biochem. 1978 Feb 1;83(1):47–52. doi: 10.1111/j.1432-1033.1978.tb12066.x. [DOI] [PubMed] [Google Scholar]
- Jann B., Jann K. Structure and biosynthesis of the capsular antigens of Escherichia coli. Curr Top Microbiol Immunol. 1990;150:19–42. doi: 10.1007/978-3-642-74694-9_2. [DOI] [PubMed] [Google Scholar]
- Jann K., Pillat M., Weisgerber C., Shibaev V. N., Torgov V. I. Biosynthesis of the O9 antigen of Escherichia coli. Synthetic glycosyldiphosphomoraprenols as probes for requirement of mannose acceptors. Eur J Biochem. 1985 Sep 2;151(2):393–397. doi: 10.1111/j.1432-1033.1985.tb09114.x. [DOI] [PubMed] [Google Scholar]
- Kröncke K. D., Boulnois G., Roberts I., Bitter-Suermann D., Golecki J. R., Jann B., Jann K. Expression of the Escherichia coli K5 capsular antigen: immunoelectron microscopic and biochemical studies with recombinant E. coli. J Bacteriol. 1990 Feb;172(2):1085–1091. doi: 10.1128/jb.172.2.1085-1091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröncke K. D., Golecki J. R., Jann K. Further electron microscopic studies on the expression of Escherichia coli group II capsules. J Bacteriol. 1990 Jun;172(6):3469–3472. doi: 10.1128/jb.172.6.3469-3472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney W. C., Duksin D. Biological activities of the two major components of tunicamycin. J Biol Chem. 1979 Jul 25;254(14):6572–6576. [PubMed] [Google Scholar]
- Masson L., Holbein B. E. Role of lipid intermediate(s) in the synthesis of serogroup B Neisseria meningitidis capsular polysaccharide. J Bacteriol. 1985 Mar;161(3):861–867. doi: 10.1128/jb.161.3.861-867.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S., Yamada H. Isolation and characterization of two outer membrane preparations from Escherichia coli. Biochim Biophys Acta. 1975 Jan 14;375(1):44–53. doi: 10.1016/0005-2736(75)90071-1. [DOI] [PubMed] [Google Scholar]
- Navia J. L., Riesenfeld J., Vann W. F., Lindahl U., Rodén L. Assay of N-acetylheparosan deacetylase with a capsular polysaccharide from Escherichia coli K5 as substrate. Anal Biochem. 1983 Nov;135(1):134–140. doi: 10.1016/0003-2697(83)90741-8. [DOI] [PubMed] [Google Scholar]
- Orskov F., Sharma V., Orskov I. Influence of growth temperature on the development of Escherichia coli polysaccharide K antigens. J Gen Microbiol. 1984 Oct;130(10):2681–2684. doi: 10.1099/00221287-130-10-2681. [DOI] [PubMed] [Google Scholar]
- Orskov I., Nyman K. Genetic mapping of the antigenic determinants of two polysaccharide K antigens, K10 and K54, in Escherichia coli. J Bacteriol. 1974 Oct;120(1):43–51. doi: 10.1128/jb.120.1.43-51.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Prehm P., Stirm S., Jann B., Jann K., Boman H. G. Cell-wall lipopolysaccharides of ampicillin-resistant mutants of Escherichia coli K-12. Eur J Biochem. 1976 Jul 1;66(2):369–377. doi: 10.1111/j.1432-1033.1976.tb10526.x. [DOI] [PubMed] [Google Scholar]
- Roberts I. S., Mountford R., Hodge R., Jann K. B., Boulnois G. J. Common organization of gene clusters for production of different capsular polysaccharides (K antigens) in Escherichia coli. J Bacteriol. 1988 Mar;170(3):1305–1310. doi: 10.1128/jb.170.3.1305-1310.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I., Mountford R., High N., Bitter-Suermann D., Jann K., Timmis K., Boulnois G. Molecular cloning and analysis of genes for production of K5, K7, K12, and K92 capsular polysaccharides in Escherichia coli. J Bacteriol. 1986 Dec;168(3):1228–1233. doi: 10.1128/jb.168.3.1228-1233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M. L., Jann B., Jann K. Structure and serological characteristics of the capsular K4 antigen of Escherichia coli O5:K4:H4, a fructose-containing polysaccharide with a chondroitin backbone. Eur J Biochem. 1988 Oct 15;177(1):117–124. doi: 10.1111/j.1432-1033.1988.tb14351.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Aparicio L. B., Reglero A., Ortiz A. I., Luengo J. M. A protein-sialyl polymer complex involved in colominic acid biosynthesis. Effect of tunicamycin. Biochem J. 1988 Apr 15;251(2):589–596. doi: 10.1042/bj2510589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Altendorf K. Proton-conducting portion (F0) from Escherichia coli ATP synthase: preparation, dissociation into subunits, and reconstitution of an active complex. Methods Enzymol. 1986;126:569–578. doi: 10.1016/s0076-6879(86)26059-0. [DOI] [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc Natl Acad Sci U S A. 1967 Mar;57(3):767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Vann W. F., Aaronson W. Genetic and molecular analyses of Escherichia coli K1 antigen genes. J Bacteriol. 1984 Feb;157(2):568–575. doi: 10.1128/jb.157.2.568-575.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Troy F. A., McCloskey M. A. Role of a membranous sialyltransferase complex in the synthesis of surface polymers containing polysialic acid in Escherichia coli. Temperature-induced alteration in the assembly process. J Biol Chem. 1979 Aug 10;254(15):7377–7387. [PubMed] [Google Scholar]
- Troy F. A., Vijay I. K., Tesche N. Role of undecaprenyl phosphate in synthesis of polymers containing sialic acid in Escherichia coli. J Biol Chem. 1975 Jan 10;250(1):156–163. [PubMed] [Google Scholar]
- Vann W. F., Liu T. Y., Robbins J. B. Cell-free biosynthesis of the O-acetylated N-acetylneuraminic acid capsular polysaccharide of group C meningococci. J Bacteriol. 1978 Mar;133(3):1300–1306. doi: 10.1128/jb.133.3.1300-1306.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann W. F., Schmidt M. A., Jann B., Jann K. The structure of the capsular polysaccharide (K5 antigen) of urinary-tract-infective Escherichia coli 010:K5:H4. A polymer similar to desulfo-heparin. Eur J Biochem. 1981 May 15;116(2):359–364. doi: 10.1111/j.1432-1033.1981.tb05343.x. [DOI] [PubMed] [Google Scholar]
- Weisgerber C., Troy F. A. Biosynthesis of the polysialic acid capsule in Escherichia coli K1. The endogenous acceptor of polysialic acid is a membrane protein of 20 kDa. J Biol Chem. 1990 Jan 25;265(3):1578–1587. [PubMed] [Google Scholar]
- Whitfield C., Adams D. A., Troy F. A. Biosynthesis and assembly of the polysialic acid capsule in Escherichia coli K1. Role of a low-density vesicle fraction in activation of the endogenous synthesis of sialyl polymers. J Biol Chem. 1984 Oct 25;259(20):12769–12775. [PubMed] [Google Scholar]
- Whitfield C., Troy F. A. Biosynthesis and assembly of the polysialic acid capsule in Escherichia coli K1. Activation of sialyl polymer synthesis in inactivate sialyltransferase complexes requires protein synthesis. J Biol Chem. 1984 Oct 25;259(20):12776–12780. [PubMed] [Google Scholar]
- Whitfield C., Vimr E. R., Costerton J. W., Troy F. A. Protein synthesis is required for in vivo activation of polysialic acid capsule synthesis in Escherichia coli K1. J Bacteriol. 1984 Jul;159(1):321–328. doi: 10.1128/jb.159.1.321-328.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


