Abstract
Glutamine synthetase activity from Synechocystis sp. strain PCC 6803 is regulated as a function of the nitrogen source available in the medium. Addition of 0.25 mM NH4Cl to nitrate-grown cells promotes a clear short-term inactivation of glutamine synthetase, whose enzyme activity decreases to 5 to 10% of the initial value in 25 min. The intracellular levels of glutamine, determined under various conditions, taken together with the results obtained with azaserine (an inhibitor of transamidases), rule out the possibility that glutamine per se is responsible for glutamine synthetase inactivation. Nitrogen starvation attenuates the ammonium-mediated glutamine synthetase inactivation, indicating that glutamine synthetase regulation is modulated through the internal balance between carbon-nitrogen compounds and carbon compounds. The parallelism observed between the glutamine synthetase activity and the internal concentration of alpha-ketoglutarate suggests that this metabolite could play a role as a positive effector of glutamine synthetase activity in Synechocystis sp. Despite the similarities of this physiological system to that described for enterobacteria, the lack of in vivo 32P labeling of glutamine synthetase during the inactivation process excludes the existence of an adenylylation-deadenylylation system in this cyanobacterium.
Full text
PDF
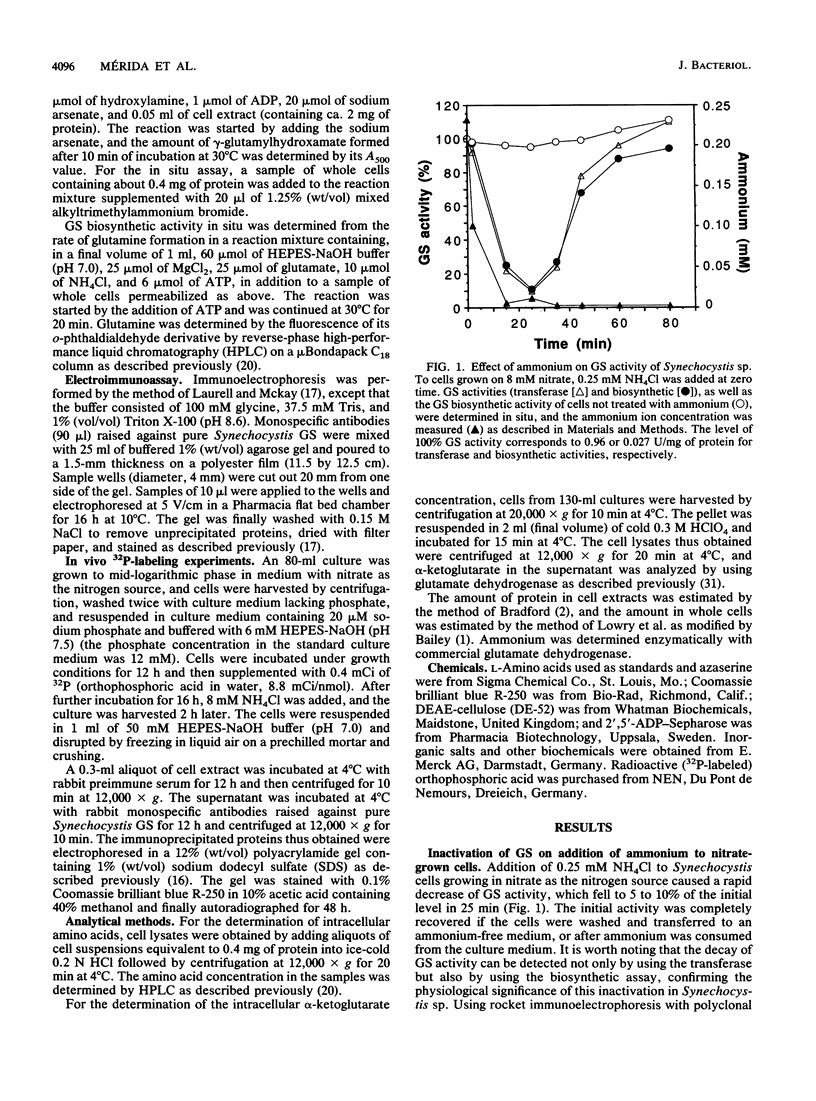
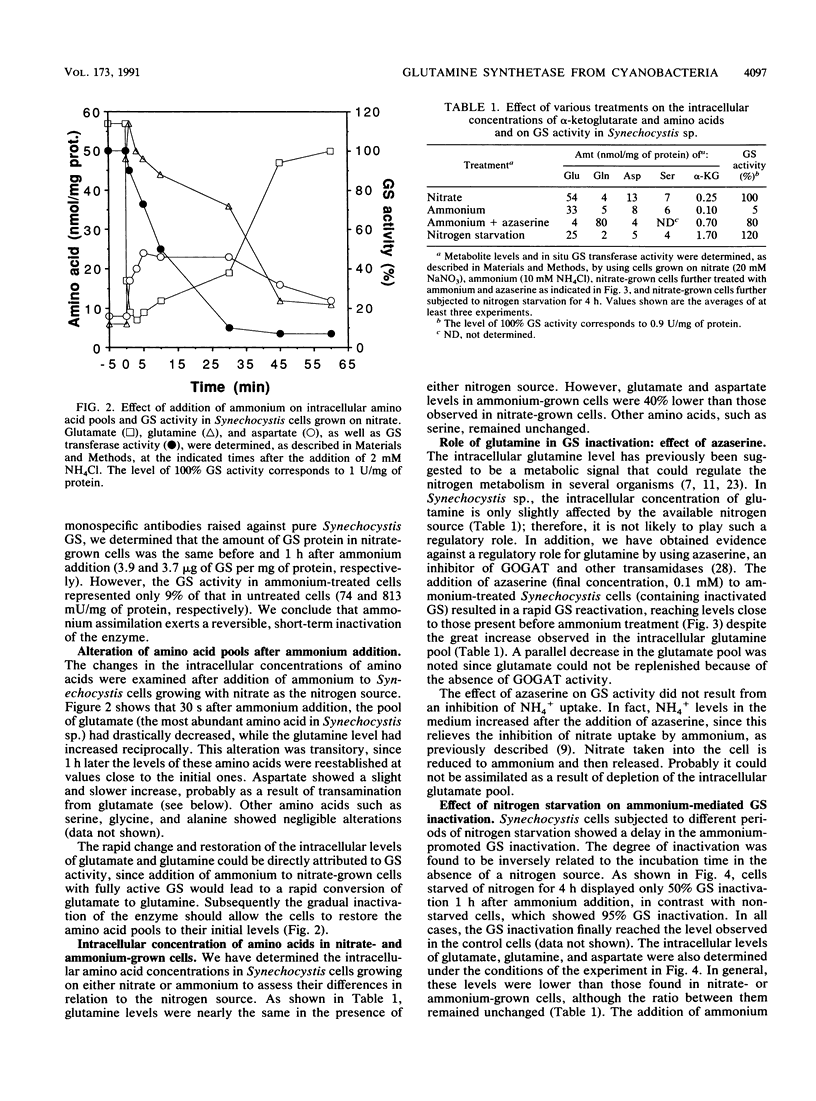
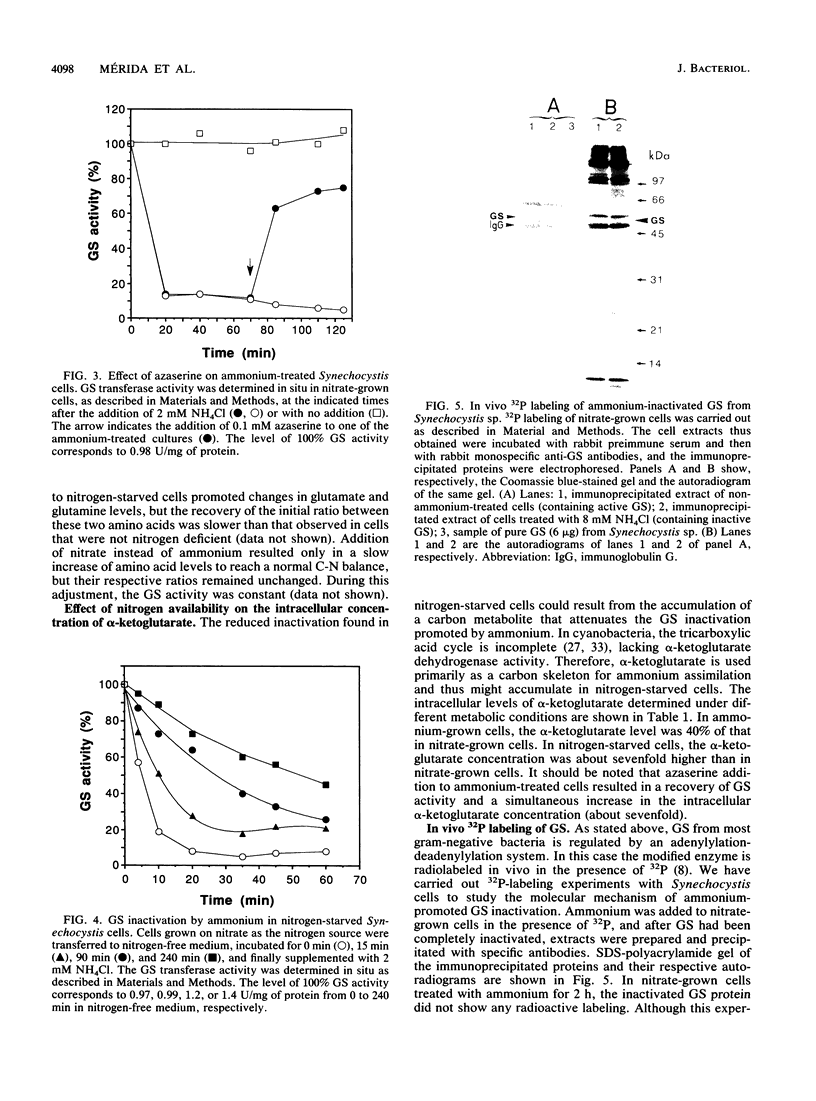


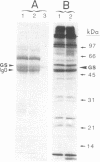
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Segal A., Stadtman E. R. Modulation of glutamine synthetase adenylylation and deadenylylation is mediated by metabolic transformation of the P II -regulatory protein. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2949–2953. doi: 10.1073/pnas.68.12.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno R., Pahel G., Magasanik B. Role of glnB and glnD gene products in regulation of the glnALG operon of Escherichia coli. J Bacteriol. 1985 Nov;164(2):816–822. doi: 10.1128/jb.164.2.816-822.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Stadtman E. R. Some kinetic properties of Bacillus subtilis glutamine synthetase. J Biol Chem. 1970 Oct 25;245(20):5206–5213. [PubMed] [Google Scholar]
- Dharmawardene M. W., Haystead A., Stewart W. D. Glutamine synthetase of the nitrogen-fixing alga Anabaena cylindrica. Arch Mikrobiol. 1973 Apr 26;90(4):281–295. doi: 10.1007/BF00408924. [DOI] [PubMed] [Google Scholar]
- Ferguson A. R., Sims A. P. The regulation of glutamine metabolism in Candida utilis: the role of glutamine in the control of glutamine synthetase. J Gen Microbiol. 1974 Jan;80(1):159–171. doi: 10.1099/00221287-80-1-159. [DOI] [PubMed] [Google Scholar]
- Fisher R., Tuli R., Haselkorn R. A cloned cyanobacterial gene for glutamine synthetase functions in Escherichia coli, but the enzyme is not adenylylated. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3393–3397. doi: 10.1073/pnas.78.6.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. K., Brill W. J. Derepression of nitrogenase synthesis in the presence of excess NH4+. Biochem Biophys Res Commun. 1974 Aug 5;59(3):967–971. doi: 10.1016/s0006-291x(74)80074-4. [DOI] [PubMed] [Google Scholar]
- Harrison M. A., Keen J. N., Findlay J. B., Allen J. F. Modification of a glnB-like gene product by photosynthetic electron transport in the cyanobacterium Synechococcus 6301. FEBS Lett. 1990 May 7;264(1):25–28. doi: 10.1016/0014-5793(90)80755-8. [DOI] [PubMed] [Google Scholar]
- Hubbard J. S., Stadtman E. R. Regulation of glutamine synthetase. II. Patterns of feedback inhibition in microorganisms. J Bacteriol. 1967 Mar;93(3):1045–1055. doi: 10.1128/jb.93.3.1045-1055.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S., Hirschman J., Meeks J. C. Adenylylation of bacterial glutamine synthetase: physiological significance. Curr Top Cell Regul. 1985;27:201–213. doi: 10.1016/b978-0-12-152827-0.50024-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Magasanik B. Reversible phosphorylation of an enhancer binding protein regulates the transcription of bacterial nitrogen utilization genes. Trends Biochem Sci. 1988 Dec;13(12):475–479. doi: 10.1016/0968-0004(88)90234-4. [DOI] [PubMed] [Google Scholar]
- Mangum J. H., Magni G., Stadtman E. R. Regulation of glutamine synthetase adenylylation and deadenylylation by the enzymatic uridylylation and deuridylylation of the PII regulatory protein. Arch Biochem Biophys. 1973 Oct;158(2):514–525. doi: 10.1016/0003-9861(73)90543-2. [DOI] [PubMed] [Google Scholar]
- Marqués S., Florencio F. J., Candau P. Ammonia assimilating enzymes from cyanobacteria: in situ and in vitro assay using high-performance liquid chromatography. Anal Biochem. 1989 Jul;180(1):152–157. doi: 10.1016/0003-2697(89)90104-8. [DOI] [PubMed] [Google Scholar]
- Meeks J. C., Wolk C. P., Lockau W., Schilling N., Shaffer P. W., Chien W. S. Pathways of assimilation of [13N]N2 and 13NH4+ by cyanobacteria with and without heterocysts. J Bacteriol. 1978 Apr;134(1):125–130. doi: 10.1128/jb.134.1.125-130.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mérida A., Leurentop L., Candau P., Florencio F. J. Purification and properties of glutamine synthetases from the cyanobacteria Synechocystis sp. strain PCC 6803 and Calothrix sp. strain PCC 7601. J Bacteriol. 1990 Aug;172(8):4732–4735. doi: 10.1128/jb.172.8.4732-4735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H., Nordlund S. Regulation of nitrogenase synthesis in intact cells of Rhodospirillum rubrum: inactivation of nitrogen fixation by ammonia, L-glutamine and L-asparagine. J Gen Microbiol. 1975 Nov;91(1):53–62. doi: 10.1099/00221287-91-1-53. [DOI] [PubMed] [Google Scholar]
- Ninfa A. J., Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr J., Haselkorn R. Regulation of glutamine synthetase activity and synthesis in free-living and symbiotic Anabaena spp. J Bacteriol. 1982 Nov;152(2):626–635. doi: 10.1128/jb.152.2.626-635.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce J., Leach C. K., Carr N. G. The incomplete tricarboxylic acid cycle in the blue-green alga Anabaena variabilis. J Gen Microbiol. 1969 Mar;55(3):371–378. doi: 10.1099/00221287-55-3-371. [DOI] [PubMed] [Google Scholar]
- Pinkus L. M. Glutamine binding sites. Methods Enzymol. 1977;46:414–427. doi: 10.1016/s0076-6879(77)46049-x. [DOI] [PubMed] [Google Scholar]
- Senior P. J. Regulation of nitrogen metabolism in Escherichia coli and Klebsiella aerogenes: studies with the continuous-culture technique. J Bacteriol. 1975 Aug;123(2):407–418. doi: 10.1128/jb.123.2.407-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey G., Van Baalen C., Tabita F. R. Nitrogen and ammonia assimilation in the cyanobacteria: regulation of glutamine synthetase. Arch Biochem Biophys. 1979 May;194(2):457–467. doi: 10.1016/0003-9861(79)90640-4. [DOI] [PubMed] [Google Scholar]
- Tuli R., Thomas J. In vivo regulation of glutamine synthetase by ammonium in the cyanobacterium Anabaena L-31. Arch Biochem Biophys. 1981 Jan;206(1):181–189. doi: 10.1016/0003-9861(81)90079-5. [DOI] [PubMed] [Google Scholar]
- Vega-Palas M. A., Madueño F., Herrero A., Flores E. Identification and cloning of a regulatory gene for nitrogen assimilation in the cyanobacterium Synechococcus sp. strain PCC 7942. J Bacteriol. 1990 Feb;172(2):643–647. doi: 10.1128/jb.172.2.643-647.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]