Abstract
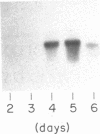
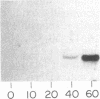
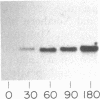
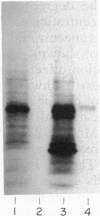
The expression of manganese peroxidase in nitrogen-limited cultures of Phanerochaete chrysosporium is dependent on Mn, and initial work suggested that Mn regulates transcription of the mnp gene. In this study, using Northern (RNA) blot analysis of kinetic, dose-response, and inhibitor experiments, we demonstrate unequivocally that Mn regulates mnp gene transcription. The amount of mnp mRNA in cells of 4-day-old nitrogen-limited cultures is a direct function of the concentration of Mn in the culture medium up to a maximum of 180 microM. Addition of Mn to nitrogen-limited Mn-deficient secondary metabolic (4-, 5-, and 6-day-old) cultures results in the appearance of mnp mRNA within 40 min. The appearance of this message is completely inhibited by the RNA synthesis inhibitor dactinomycin but not by the protein synthesis inhibitor cycloheximide. Furthermore, the amount of mnp mRNA produced is a direct function of the concentration of added Mn. In contrast, addition of Mn to low-nitrogen Mn-deficient 2- or 3-day-old cultures does not result in the appearance of mnp mRNA. Manganese peroxidase protein is detected by specific immunoprecipitation of the in vitro translation products of poly(A) RNA isolated from Mn-supplemented (but not from Mn-deficient) cells. All of these results demonstrate that Mn, the substrate for the enzyme, regulates mnp gene transcription via a growth-stage-specific and concentration-dependent mechanism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alic M., Kornegay J. R., Pribnow D., Gold M. H. Transformation by Complementation of an Adenine Auxotroph of the Lignin-Degrading Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1989 Feb;55(2):406–411. doi: 10.1128/aem.55.2.406-411.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alic M., Letzring C., Gold M. H. Mating System and Basidiospore Formation in the Lignin-Degrading Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1987 Jul;53(7):1464–1469. doi: 10.1128/aem.53.7.1464-1469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnarme P., Jeffries T. W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl Environ Microbiol. 1990 Jan;56(1):210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. A., Glenn J. K., Gold M. H. Manganese regulates expression of manganese peroxidase by Phanerochaete chrysosporium. J Bacteriol. 1990 Jun;172(6):3125–3130. doi: 10.1128/jb.172.6.3125-3130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta V. C., Hamer D. H. Fine mapping of a mouse metallothionein gene metal response element. Mol Cell Biol. 1989 Mar;9(3):1376–1380. doi: 10.1128/mcb.9.3.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fürst P., Hu S., Hackett R., Hamer D. Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell. 1988 Nov 18;55(4):705–717. doi: 10.1016/0092-8674(88)90229-2. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Akileswaran L., Gold M. H. Mn(II) oxidation is the principal function of the extracellular Mn-peroxidase from Phanerochaete chrysosporium. Arch Biochem Biophys. 1986 Dec;251(2):688–696. doi: 10.1016/0003-9861(86)90378-4. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Gold M. H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Nov 1;242(2):329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- Godfrey B. J., Mayfield M. B., Brown J. A., Gold M. H. Characterization of a gene encoding a manganese peroxidase from Phanerochaete chrysosporium. Gene. 1990 Sep 1;93(1):119–124. doi: 10.1016/0378-1119(90)90144-g. [DOI] [PubMed] [Google Scholar]
- Gold M. H., Cheng T. M. Induction of colonial growth and replica plating of the white rot basidiomycete Phanaerochaete chrysosporium. Appl Environ Microbiol. 1978 Jun;35(6):1223–1225. doi: 10.1128/aem.35.6.1223-1225.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert J., Culotta V., Fürst P., Gedamu L., Hamer D. Regulation of metallothionein gene transcription by metals. Adv Inorg Biochem. 1990;8:139–164. [PubMed] [Google Scholar]
- Keyser P., Kirk T. K., Zeikus J. G. Ligninolytic enzyme system of Phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol. 1978 Sep;135(3):790–797. doi: 10.1128/jb.135.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leisola M. S., Kozulic B., Meussdoerffer F., Fiechter A. Homology among multiple extracellular peroxidases from Phanerochaete chrysosporium. J Biol Chem. 1987 Jan 5;262(1):419–424. [PubMed] [Google Scholar]
- Müller R. M., Taguchi H., Shibahara S. Nucleotide sequence and organization of the rat heme oxygenase gene. J Biol Chem. 1987 May 15;262(14):6795–6802. [PubMed] [Google Scholar]
- Münger K., Germann U. A., Lerch K. The Neurospora crassa metallothionein gene. Regulation of expression and chromosomal location. J Biol Chem. 1987 May 25;262(15):7363–7367. [PubMed] [Google Scholar]
- Paszczyński A., Huynh V. B., Crawford R. Comparison of ligninase-I and peroxidase-M2 from the white-rot fungus Phanerochaete chrysosporium. Arch Biochem Biophys. 1986 Feb 1;244(2):750–765. doi: 10.1016/0003-9861(86)90644-2. [DOI] [PubMed] [Google Scholar]
- Pease E. A., Andrawis A., Tien M. Manganese-dependent peroxidase from Phanerochaete chrysosporium. Primary structure deduced from cDNA sequence. J Biol Chem. 1989 Aug 15;264(23):13531–13535. [PubMed] [Google Scholar]
- Pribnow D., Mayfield M. B., Nipper V. J., Brown J. A., Gold M. H. Characterization of a cDNA encoding a manganese peroxidase, from the lignin-degrading basidiomycete Phanerochaete chrysosporium. J Biol Chem. 1989 Mar 25;264(9):5036–5040. [PubMed] [Google Scholar]
- Ralston D. M., O'Halloran T. V. Metalloregulatory proteins and molecular mechanisms of heavy metal signal transduction. Adv Inorg Biochem. 1990;8:1–31. [PubMed] [Google Scholar]
- Scott R. E., Jones A., Gaucher G. M. Manganese and antibiotic biosynthesis. III. The site of manganese control of patulin production in Penicillium urticae. Can J Microbiol. 1986 Mar;32(3):273–279. doi: 10.1139/m86-053. [DOI] [PubMed] [Google Scholar]
- Singh T. J. Activation of a manganese-dependent membrane protein kinase by serine and tyrosine phosphorylation. Biochem Biophys Res Commun. 1990 Aug 31;171(1):75–83. doi: 10.1016/0006-291x(90)91358-y. [DOI] [PubMed] [Google Scholar]
- Smith T. L., Schalch H., Gaskell J., Covert S., Cullen D. Nucleotide sequence of a ligninase gene from Phanerochaete chrysosporium. Nucleic Acids Res. 1988 Feb 11;16(3):1219–1219. doi: 10.1093/nar/16.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O. Organization, expression, and evolution of genes for mercury resistance. Annu Rev Microbiol. 1986;40:607–634. doi: 10.1146/annurev.mi.40.100186.003135. [DOI] [PubMed] [Google Scholar]
- Tien M., Tu C. P. Cloning and sequencing of a cDNA for a ligninase from Phanerochaete chrysosporium. Nature. 1987 Apr 2;326(6112):520–523. doi: 10.1038/326520a0. [DOI] [PubMed] [Google Scholar]
- Walther I., Kälin M., Reiser J., Suter F., Fritsche B., Saloheimo M., Leisola M., Teeri T., Knowles J. K., Fiechter A. Molecular analysis of a Phanerochaete chrysosporium lignin peroxidase gene. Gene. 1988 Oct 15;70(1):127–137. doi: 10.1016/0378-1119(88)90111-4. [DOI] [PubMed] [Google Scholar]
- Wariishi H., Akileswaran L., Gold M. H. Manganese peroxidase from the basidiomycete Phanerochaete chrysosporium: spectral characterization of the oxidized states and the catalytic cycle. Biochemistry. 1988 Jul 12;27(14):5365–5370. doi: 10.1021/bi00414a061. [DOI] [PubMed] [Google Scholar]
- Wariishi H., Dunford H. B., MacDonald I. D., Gold M. H. Manganese peroxidase from the lignin-degrading basidiomycete Phanerochaete chrysosporium. Transient state kinetics and reaction mechanism. J Biol Chem. 1989 Feb 25;264(6):3335–3340. [PubMed] [Google Scholar]
- Yu K. T., Khalaf N., Czech M. P. Insulin stimulates a novel Mn2+-dependent cytosolic serine kinase in rat adipocytes. J Biol Chem. 1987 Dec 5;262(34):16677–16685. [PubMed] [Google Scholar]
- de Boer H. A., Zhang Y. Z., Collins C., Reddy C. A. Analysis of nucleotide sequences of two ligninase cDNAs from a white-rot filamentous fungus, Phanerochaete chrysosporium. Gene. 1987;60(1):93–102. doi: 10.1016/0378-1119(87)90217-4. [DOI] [PubMed] [Google Scholar]