Abstract
During stomatal opening potassium uptake into guard cells and K+ channel activation is tightly coupled to proton extrusion. The pH sensor of the K+ uptake channel in these motor cells has, however, not yet been identified. Electrophysiological investigations on the voltage-gated, inward rectifying K+ channel in guard cell protoplasts from Solanum tuberosum (KST1), and the kst1 gene product expressed in Xenopus oocytes revealed that pH dependence is an intrinsic property of the channel protein. Whereas extracellular acidification resulted in a shift of the voltage-dependence toward less negative voltages, the single-channel conductance was pH-insensitive. Mutational analysis allowed us to relate this acid activation to both extracellular histidines in KST1. One histidine is located within the linker between the transmembrane helices S3 and S4 (H160), and the other within the putative pore-forming region P between S5 and S6 (H271). When both histidines were substituted by alanines the double mutant completely lost its pH sensitivity. Among the single mutants, replacement of the pore histidine, which is highly conserved in plant K+ channels, increased or even inverted the pH sensitivity of KST1. From our molecular and biophysical analyses we conclude that both extracellular sites are part of the pH sensor in plant K+ uptake channels.
Plant growth, differentiation, cell and tissue polarity, as well as movements strongly depend on the formation of pH gradients across individual cell types (1–3). Stomata that are formed by two guard cells represent a unique model for plant movement. Regulation of stomatal movement in higher plants is essential for efficient uptake of CO2 at minimal water loss. Stomatal opening requires accumulation of potassium ions into guard cells. K+ uptake is mediated by K+ uptake channels, a process accompanied by the acidification of the apoplast (4–7). Due to differential pumping activity of the plasma membrane H+-pump the apoplastic pH around closed and open stomata varies between 7 and 5 (8, 9). Within this range changes in the extracellular proton concentration affect the activity of guard cell inward rectifying K+ channels (10–13). In contrast to their functional and structural animal counterparts, which are either not activated or even inhibited by protons (14, 15), the guard cell K+ channel is activated upon extracellular acidification (10–13).
The molecular cloning of plant K+ inward rectifiers revealed a structural homology to animal outward rectifying K+ channels of the Shaker gene family (12, 16, 17). Hydrophobicity analyses of their primary structure predicted six transmembrane domains (S1–S6) (12, 16, 17). Whereas S4 is likely to represent the voltage sensor of these voltage-dependent channels, the amphiphilic linker between S5 and S6 (P) forms the conductive pore (18). Based on the current molecular model of the Shaker channel we were able to relate structural elements of guard cell K+ inward rectifiers to stomatal physiology. In this context we have previously shown that a putative ATP-binding site in the amino acid sequence together with the requirement of KST1 for cytoplasmic ATP pointed to a tight coupling between channel activity and guard cell energy metabolism (12).
Here, we demonstrate that protonation and deprotonation in response to the H+ pumping activity and acid metabolism modulates the K+ uptake channel. Proton-induced increase in whole cell currents of KST1 is due to a shift in the half-maximum activation voltage rather than an increase in the single-channel conductance. To understand this mechanism of acid activation we studied the role of external histidine residues in the pH sensing of KST1.
MATERIALS AND METHODS
Generation of kst1 Mutants.
For mutants H160D, H160R, and H160A, the PflMI/BseRI segment of the kst1-coding region in pGEMHE (12) was replaced by PCR-generated fragments obtained with forward primer P1 (5′-CTGTGTGGACGAGTTCCAAATGG-3′) and reverse primers P2XXX (5′-AGCAATCTGAATCCAACTCCTCCGCTTTCTTTXXXACCCGTGAAG-3′, with XXX = GTC for aspartate, TCG for arginine, and GGC for alanine). To produce H271 and N274 mutants, a silent mutation creating a novel BglII site was first introduced into the kst1 coding region upstream of the H271 codon. This modification was achieved by exchanging the SphI fragment of the kst1 cDNA in the plasmid pKST1#8–1 (12) by a corresponding PCR fragment amplified with primers P3 (5′-CCATTCCAATCATTGATCCTCG-3′) and P4 (5′-CTCAGCATGCAGATCTCCATAACCG-3′). To obtain H271 mutants, the BglII/StyI fragment of the modified kst1 sequence was replaced by fragments generated via PCR using reverse primer P5 (5′-TTGCTTCGGAGGGAAGTATTCAGCTTC-3′) and forward primers P6XXX (5′-CGGTTATGGAGATCTGXXXGCTGAGAACTC-3′, with XXX = GAC for aspartate, CGA for arginine, and GCA for alanine). For N274 mutants forward primers P7XXX (5′-ATGGAGATCTGCATGCTGAGXXXTCTAGAGAGATGC-3′, with XXX = GAC for aspartate and GCA for alanine) and reverse primer P5 were employed. Mutated kst1 cDNAs were cloned as Asp718/BamHI fragments (blunt-ended; sense orientation) into the SmaI site of vector pGEMHE (19). All modifications were verified by DNA sequence analysis using the T7 Sequencing Kit from Pharmacia. Double mutants were derived from the corresponding single mutants by exchange of DNA fragments.
Patch-Clamp Recordings on Potato Guard Cell Protoplasts (in Vivo).
Patch-clamp studies on potato guard cell protoplasts were performed as described (12). The bath medium was adjusted to pH 8.5 and to pH 7.4 with 10 mM Hepes-Tris, to pH 6.5 and to pH 5.6 with 10 mM Mes-Tris, and to pH 4.5 with 10 mM citrate-Tris. Single-channel events recorded from excised outside–out patches with 100 mM K+ in the bath and 150 mM K+ in the pipette were filtered at 1 kHz.
Expression of kst1 and Mutants in Xenopus Oocytes and Voltage-Clamp Recordings (in Vitro).
Xenopus frogs were purchased from Nasco (Fort Atkinson, WI). Oocytes were isolated as described elsewhere (20) and injected with 1–10 ng of cRNA per oocyte using a General Valve (Fairfield, NJ) Picospritzer II microinjector. Forty-eight hours after injection whole-cell K+ currents were measured with a two-microelectrode voltage-clamp amplifier (Turbotec-01C; NPI Instruments, Tamm, Germany) using 0.5–2 MΩ pipettes filled with 3 M KCl (12). Solutions were composed of 30 mM KCl, 2 mM MgCl2, and 1 mM CaCl2. pH values were buffered with 10 mM Mes-Tris (pH 5.2–6.5) and 10 mM Tris-Mes (pH 7.0–9.2), respectively. All solutions were adjusted to 220 mOsm with d-sorbitol.
Biophysical Analyses.
Open probabilities (po) were deduced from a double-voltage step protocol. In two-electrode voltage-clamp measurements time- and voltage-dependent K+ currents were induced during the first 5 s activation pulse to hyperpolarizing voltages (V) between −50 mV and −170 mV in −10 mV increments.§ During the second pulse to a fixed voltage (VF = −70 mV) inward currents relaxed. The I0–V relationship, obtained from extrapolating the relaxation time course of the second pulse to t = 0 ms with an exponential function, is proportional to the open probability of the channel at the end of the activation pulse:
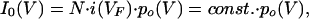 |
where N denotes the number of KST1 channels within the membrane, I0 the instantaneous tail current, VF the voltage of the following-pulse, and i(VF) the single channel current at VF (N⋅i(VF) = const.). Patch-clamp data analyses were performed based on the same equations and similar voltage protocols.
To obtain biophysical parameters that allow a comparative analysis of acid activation, we described the channel by a two state model (closed and open). Independent of its state the channel can be protonated or deprotonated. According to the law of mass action the distribution between protonated and deprotonated states can be described by
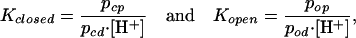 |
where pcp, pcd, pop, and pod denote the probabilities to find the channel in one of the different states (c = closed, o = open, p = protonated, and d = deprotonated), [H+] the proton concentration, and Kclosed and Kopen the reaction constants of the protonation reaction.
Using Boltzmann statistics as well as the equation pcp + pcd + pop + pod = 1 the measurable open probability popen = pop + pod derives to
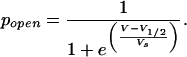 |
1 |
Here Vs denotes the slope factor, which is correlated to the gating charge, and V1/2 the pH-dependent half-activation voltage
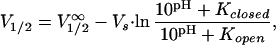 |
2 |
where V1/2∞ corresponds to the half-activation voltage of the completely deprotonated channel.
Measured open probabilities were fitted with Eq. 1 whereby the half-activation voltage V½ was determined for each pH value independently, whereas the slope factor Vs was adjusted for each cell.
Cloning of K+ Channel Sequences.
Additional K+ channel sequences were identified by reverse transcriptase–PCR experiments. For this purpose poly(A)+ RNA was isolated from different plant species and tissues using the RNeasy extraction kit (Qiagen, Chatsworth, CA) in conjunction with paramagnetic beads (Dynal, Oslo). Obtained mRNA was reverse transcribed using Superscript RT (GIBCO/BRL) and an oligo(dT) 16-mer primer according to the manufacturers instructions. The resulting 1st strand cDNA was used in PCR experiments as template for the amplification of K+ channel fragments. The primers used were designed on the basis of the plant K+ channel consensus sequences FFAIDI (forward primers) and MLFNLG (reverse primers). Whenever possible codon usage-based primers were designed. In other cases inositoles substituted for variable bases within certain codons. Due to the choice of primers a 600-bp PCR fragment could be expected which was extracted from the agarose gel (Jet-Pure; Genomed, Bad Oeynhausen) and cloned into the EcoRV site of the pZERO plasmid (Invitrogen). Cloned K+ channel fragments were verified by sequencing and sequences were deposited in GenBank database.
RESULTS
Protons Modulate the Voltage Dependence.
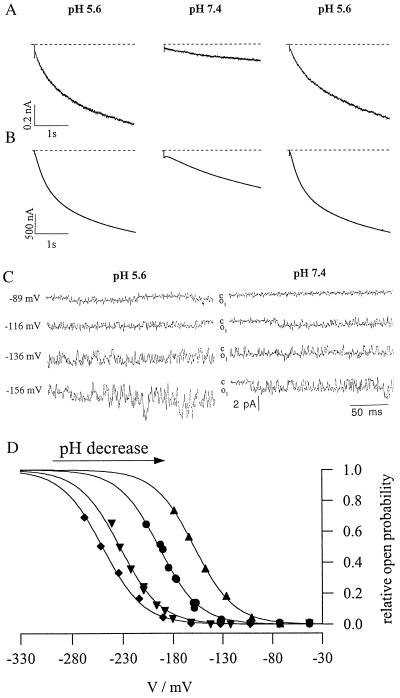
To elucidate the molecular mechanism of pH sensing, K+ uptake was studied after heterologous expression of kst1 in Xenopus oocytes (in vitro) and in in its natural environment in isolated guard cell protoplasts from Solanum tuberosum (in vivo). Using the whole-cell configuration of the patch-clamp technique (21), we recorded inward K+ currents in protoplasts in response to plasma membrane hyperpolarization. When changing the pH of the extracellular solution from 5.6 to 7.4, the currents of the inward rectifying K+ channel decreased in a reversible manner (Fig. 1A). These two pH values reflect apoplastic conditions around closed (pH 7) and open stomata (pH 5) (8, 9). To test whether protons directly act on the channel protein we heterologously expressed kst1 in Xenopus oocytes. Because the capability to activate upon acidification has been maintained in KST1 (Fig. 1B), the interaction with protons, like voltage and ATP activation (11, 12, 22), seems to reflect an intrinsic property of the K+ channel protein. While the single-channel conductance of the guard cell channel (7.4 ± 0.8 pS, n = 8) (P.D. and R.H., unpublished data) was pH-insensitive (Fig. 1C) like the parameter N⋅i(VF) obtained from experiments in oocytes (see Materials and Methods), the voltage-dependent activation of the K+ uptake channel in isolated protoplasts changed as a function of the extracellular pH (Fig. 1D). With decreasing pH the half-maximum-activation potential V½ shifted to less negative potentials.
Figure 1.
Acid activation of a guard cell inward rectifying K+ channel in vivo and in vitro. (A) In the whole-cell configuration guard cell protoplasts from Solanum tuberosum were clamped at −63 mV to elicit inward K+ currents during subsequent 3-s pulses to −180 mV. (B) Two-electrode voltage-clamp recordings of inward K+ currents through kst1 expressed in Xenopus oocytes. With the oocyte clamped at −20 mV KST1-specific inward K+ currents were induced by membrane hyperpolarization to −170 mV. In contrast to KAT1 an artificial acidification of the cytoplasm of oocytes using acetate did not affect the gating behavior of KST1 (data not shown). In both systems channel activity of the inward rectifier increased with the extracellular proton concentration (pH 7.4–5.6). (C) Open–closed transitions of single inward K+ channels in cell-free outside–out membrane patches from isolated protoplasts exposed to pH 5.6 and 7.4. Single channels were recorded between t = 100 and 300 ms during steps to voltages indicated from a holding potential of −46 mV. Note that open-channel amplitudes (o1) were identical for both proton concentrations. (D) pH- and voltage-dependent open probability of the K+ uptake channel from one representative guard cell protoplast. Data points were fitted by Boltzmann functions (po = N⋅{1 + exp[(V − V½)/Vs]}−1) and scaled to the value N obtained in pH 4.5. The activation threshold shifts to less negative potentials with a decrease in pH (♦, pH 7.4; ▾, pH 6.5; •, pH 5.6; ▴, pH 4.5).
The Role of Histidines in pH Sensing.
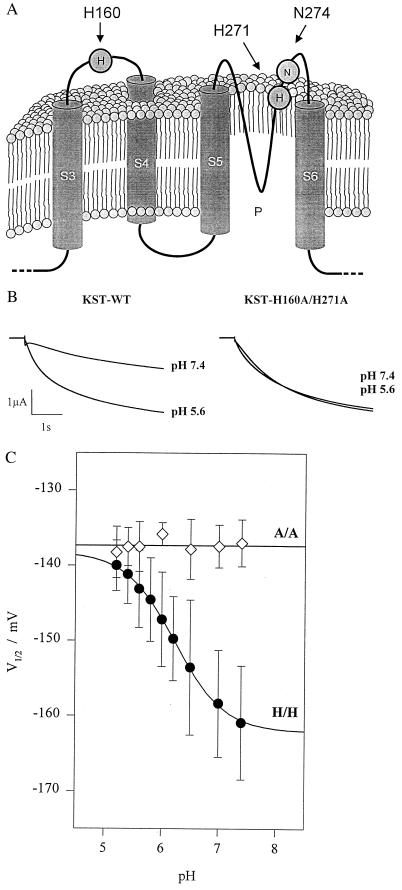
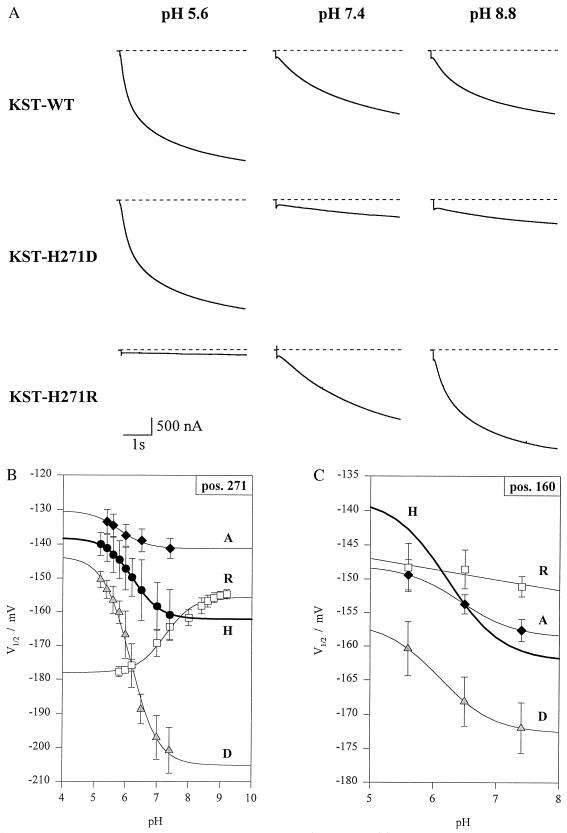
Histidines, cysteines, and glutamates are proposed to mediate the proton block of animal inward-rectifying K+ and cyclic nucleotide-gated channels (15, 23). Most strikingly KST1 contains no external cysteine, but two external histidines. One histidine is located within the linker between S3 and S4 (H160) and another in the outer pore (P) of the potato guard cell K+ channel (H271) (Fig. 2A). To determine whether these histidines represent key amino acids of the pH sensor we generated histidine mutants of KST1. When both extracellular histidines were replaced by alanines, the double mutant KST1–H160A/H271A completely lost its pH dependence (Fig. 2 B and C). To distinguish between the relative contribution of these two sites we initially focused on the role of the pore histidine 271. Single mutations at this position affected the pH dependence drastically. Substitution by aspartate (KST1–H271D) further increased the pH sensitivity, whereas replacement of histidine by arginine (KST1-H271R) even inverted the pH dependence (Fig. 3 A and B). Following the removal of the charge at position 271 (KST1–H271A), KST1 still exhibits a significant but less pronounced pH sensitivity (Fig. 3B). In the next step we analyzed H271-related mutations at position 160 (KST1–H160A, –H160D, –H160R). All these mutants independent of the charge at this site were less pH-sensitive, when compared with the wild type (Fig. 3C). Identical mutations in the pore asparagine 274 (Fig. 2A), however, did not alter the pH dependence (not shown). We therefore propose that both histidines in KST1 are required for the pH sensing mechanism.
Figure 2.
pH-dependence of wild-type KST1 and the histidine double mutant. (A) Cartoon of the predicted topology of segments S3 to S6, including the pore region P and the extracellular linker between S3 and S4 [in relation to the Shaker K+ channel structure (18)]. (B) Compared with wild-type (left traces) inward currents of the double mutant KST1-H160A/H271A were not affected by changes in the extracellular proton concentration (right traces). (C) In contrast to the wild-type (H/H) substitution of both histidines by alanine (A/A) resulted in a loss of pH-sensitivity of the half-maximum activation potential. Data points represent the mean ± SE of three to six measurements, and solid lines represents best fits according to Eq. 2.
Figure 3.
pH-sensitivity of wild-type KST1 and single mutants. (A Top) Wild-type inward currents in response to pH 5.6, 7.4, and 8.8 in the bath medium. (Middle) Pronounced reduction of K+ current in mutant KST1–H271D in response to an increase in extracellular pH from 5.6 to 7.4 and 8.8 with respect to wild-type KST1 and KST1–H271R. (Bottom) The mutant KST1–H271R converts the acid activation into an alkaline activation. Note the absence of inward K+ current at pH 5.6 compared with wild-type KST1 and KST1–H271D. (B) pH-sensitivity of the half-activation potential (V½) in wild-type KST1 (H) and the mutants KST1–H271R (R), –H271D (D), and –H271A (A) (n = 3–6). (C) Reduction in pH dependence of the mutants KST1–H160R (R), –H160A (A), and –H160D (D) compared with the wild-type channel (H) (n = 3–6). Solid lines represent best fits according to Eq. 2.
The Pore Histidine Together with Asparagine Represent Plant-Specific Residues.
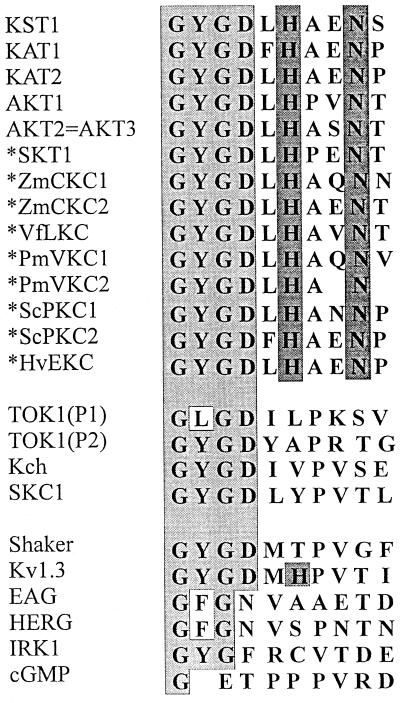
To decide whether pH sensing based on histidines may represent a guard cell-specific feature, we identified further K+ channel sequences from various plant species and cell types (Fig. 4). These new sequences (marked by an asterisk in Fig. 4) were aligned to known sequences of plants, yeast, bacteria, and animals. This comparison revealed that the histidine at position 271 together with asparagine N274 is highly conserved among plants, but is not restricted to the guard cell channels KST1 and KAT1. Kv1.3, a mammalian lymphocyte K+ channel, contains the pore histidine, too (ref. 24 and Fig. 4). However, the pH sensitivity of Kv1.3 does not depend on this histidine (25).
Figure 4.
Alignment of amino acids of the outer pore region C terminal to the GXG sequence from K+ channels of plants, yeast, bacteria, and animals. We cloned KAT- and AKT-related K+ channels (16, 17) (marked by an asterisk) from Solanum tuberosum epidermal fragments (SKT1), Zea mays coleoptiles (ZmCKC1 and -2), Vicia faba developing leafs (VfLKC), veins of Plantago major (PmVKC1 and -2), pollen of Secale cereale (ScPKC1 and -2), and Hordium vulgare epidermis (HvEKC). Note the conservation of a histidine (H271) close to an asparagine (N274) in plant K+ channels only. The GenBank accession numbers of the aligned channel sequences are as follows: KST1 (X79779X79779), KAT1 (M86990M86990), KAT2 (U25694U25694), AKT1 (X62907X62907), AKT2 and AKT3 appear to be identical (U40154U40154 and U44745U44745), SKT1 (X86021X86021), ZmCKC2 (Y07632Y07632), VfLKC1 (Y09749Y09749), ZmCKC1 (Y09747Y09747), PmVKC1 (Y09750Y09750), PmVKC2 (Y09751Y09751), ScPKC1 (Y09752Y09752), ScPKC2 (Y09753Y09753), HvEKC1 (Y09748Y09748), TOK1 (U28005U28005), Kch (L12044L12044), SKC1 (Z37969Z37969), Shaker (M17211M17211), Kv1.3 (M30312M30312), EAG (M61157M61157), HERG (U04270U04270), IRK1 (X73052X73052), cGMP (X51604X51604).
DISCUSSION
In this study we demonstrated that acid activation of the potato guard cell K+ uptake channel KST1 is due to a shift in the voltage dependence rather than an increase in the single-channel conductance (Fig. 1). This mechanism fundamentally differs from that found for cyclic nucleotide-gated and inward-rectifying K+ channels in animals. In the latter protons block the open channel and thereby reduce the single-channel conductance (15, 23). Consequently, we would expect a different molecular basis for pH dependence in plant K+ channels. In line with this hypothesis we identified a plant-specific histidine in the outer pore of K+ uptake channels in all plant species and tissues investigated (Fig. 4). Because mutations of this residue in KST1 drastically affected the pH sensitivity we conclude that H271 plays a central role in the pH sensing mechanism. The conservation of the pore histidine may implicate that acid activation represents a common regulation mechanism of plant K+ uptake channels. The histidine (H160) of the potato K+ channel which seems to be related to the pH dependence of KST1 too, was not found in other plant K+ uptake channels cloned so far. Variations at this position may therefore result in a species- and cell type-specific fine-tuning of the pH effect.
Because the pH-dependent shift of the half-activation voltage implicates interaction between pH sensing and gating, future detailed analyses will address the question about the molecular link between the pH and the voltage sensor.
Acknowledgments
We thank A. Basner and K. Zander as well as K. Neuwinger for technical assistance as well as Katrin Philippar and Peter Ache for supporting us with new K+ channel sequences. We gratefully acknowledge E. Liman for providing us with the pGEMHE vector, and O. Pongs (Hamburg) for helpful comments on the manuscript. B.M.-R. is a Junior Scientist of the Max-Planck Society. These investigations were funded by Deutsche Forschungsgemeinschaft grants to R.H.
Footnotes
References
- 1.Weisenseel M H, Jaffe L F. Planta. 1976;133:1–7. doi: 10.1007/BF00385998. [DOI] [PubMed] [Google Scholar]
- 2.Satter R L, Morse M J, Lee Y, Crain R C, Coté G G, Moran N. Bot Acta. 1988;101:205–213. [Google Scholar]
- 3.Cosgrove J. Plant Physiol. 1993;102:1–6. doi: 10.1104/pp.102.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raschke K. In: Encyclopedia of Plant Physiology: Physiology of Movements. Haupt W, Feinleib M E, editors. Berlin: Springer; 1979. pp. 383–441. [Google Scholar]
- 5.Shimazaki K, Iino M, Zeiger E. Nature (London) 1986;319:324–326. [Google Scholar]
- 6.Behl R, Raschke K. Planta. 1987;172:531–538. doi: 10.1007/BF00393871. [DOI] [PubMed] [Google Scholar]
- 7.Kochian L V, Lucas W J. Adv Bot Res. 1988;15:93–174. [Google Scholar]
- 8.Edwards M C, Smith J N, Bowling D J F. J Exp Bot. 1988;39:1541–1547. [Google Scholar]
- 9.Mühling K H, Plieth C, Hansen U-P, Sattelmacher B. J Exp Bot. 1995;46:377–382. [Google Scholar]
- 10.Blatt M R. J Gen Physiol. 1992;99:615–644. doi: 10.1085/jgp.99.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedrich R, Moran O, Conti F, Busch H, Becker D, Gambale F, Dreyer I, Küch A, Neuwinger K, Palme K. Eur Biophys J. 1995;24:107–115. doi: 10.1007/BF00211406. [DOI] [PubMed] [Google Scholar]
- 12.Müller-Röber B, Ellenberg J, Provart N, Willmitzer L, Busch H, Becker D, Dietrich P, Hoth S, Hedrich R. EMBO J. 1995;14:2409–2416. doi: 10.1002/j.1460-2075.1995.tb07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilan N, Schwartz A, Moran N. J Membr Biol. 1996;154:169–181. doi: 10.1007/s002329900142. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki M, Takahashi K, Ikeda M, Hayakawa H, Ogawa A, Kawaguchi Y, Sakai O. Nature (London) 1994;367:642–645. doi: 10.1038/367642a0. [DOI] [PubMed] [Google Scholar]
- 15.Coulter K L, Périer F, Radeke C M, Vandenberg C A. Neuron. 1995;15:1157–1168. doi: 10.1016/0896-6273(95)90103-5. [DOI] [PubMed] [Google Scholar]
- 16.Anderson J A, Huprikar S S, Kochian L V, Lucas W J, Gaber R F. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon J-M, Gaymard F, Grignon C. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- 18.Durell S R, Guy H R. Biophys J. 1992;62:238–250. doi: 10.1016/S0006-3495(92)81809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liman E R, Tytgat J, Hess P. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- 20.Stühmer W, Parekh A B. In: Single Channel Recording. Sakmann B, Neher E, editors. New York: Plenum; 1995. pp. 341–355. [Google Scholar]
- 21.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 22.Hoshi T. J Gen Physiol. 1995;105:309–328. doi: 10.1085/jgp.105.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Root M J, MacKinnon Science. 1994;265:1852–1856. doi: 10.1126/science.7522344. [DOI] [PubMed] [Google Scholar]
- 24.North R A. Handbook of Receptors and Channels: Ligand- and Voltage-Gated Ion Channels. Boca Raton, FL: CRC; 1995. [Google Scholar]
- 25.Kavanaugh M P, Varnum M D, Osborne P B, Christie M J, Busch A E, Adelman J P, North R A. J Biol Chem. 1991;266:7583–7587. [PubMed] [Google Scholar]