Abstract
Six highly temperature sensitive ion channels of the transient receptor potential (TRP) family have been implicated to mediate temperature sensation. These channels, expressed in sensory neurons innervating the skin or the skin itself, are active at specific temperatures ranging from noxious cold to burning heat. In addition to temperature sensation thermoTRPs are the receptors of a growing number of environmental chemicals (chemesthesis). Recent studies have provided some striking new insights into the molecular mechanism of thermal and chemical activation of these biological thermometers.
Introduction
We have evolved an acute peripheral thermo-sensory capability to maintain thermal homeostasis and to avoid potentially harmful stimuli. In vertebrates temperature sensation is mediated by specialized sensory neurons. These neurons, whose cell bodies are located in trigeminal and dorsal root ganglia (DRG), project long neurites to the skin where they detect thermal cues. Thermoceptive neurons are functionally heterogeneous: different subpopulations of neurons are tuned to detect different temperatures.
The molecular mechanism underlying temperature sensation has been the subject of intense study. Recently, several highly temperature-sensitive TRP channels (ThermoTRPs) have been identified as candidate temperature sensors [1,2]. Both cold-activated (TRPM8 and TRPA1) and heat activated (TRPV1-4) thermoTRPs with disparate activation thresholds have been characterized, fitting the functional heterogeneity in DRG neurons (Table 1). Surprisingly, TRPV3 and TRPV4 were shown to be most prominently expressed in epithelial cells (keratinocytes) rather than in DRG neurons [1,2]. In vivo studies of mice deficient in specific thermoTRPs have confirmed the proposed role of several of these ion channels in thermosensation, and have suggested a thermosensory role for keratinocytes, in addition to sensory neurons [3–8].
Table 1.
| ThermoTRP | Thermal activation | Chemical agonist | Sensory neuron/skin expression | Temperature phenotype of null mutant |
|---|---|---|---|---|
| TRPV1 | > 42°C | capsaicin, acidic pH, camphor, ethanol, resiniferatoxin, 2-APB piperine, eugenol, gingerol, VaTx1-3 (spider toxin) | sensory neuron | impaired thermal avoidance and hyperalgesia |
| TRPV2 | > 52°C | 2-APB camphor, menthol, thymol, carvacrol, eugenol, 2-APB | sensory neuron | not reported |
| TRPV3 | > 33°C | keratinocytes/sensory neuron? | impaired thermotaxis and thermal avoidance. | |
| TRPV4 | > 25–34°C | 4αPDD, bisandrographolide | keratinocytes/sensory neuron | impaired thermotaxis, thermal avoidance, and hyperalgesia |
| TRPM2* | > 35°C | H2O2, ADP-ribose, βNAD | not reported | not reported |
| TRPM4* | heat** | cytosolic Ca2+ | not reported | not reported |
| TRPM5* | heat** | cytosolic Ca2+ | not reported | not reported |
| TRPM8 | < 25°C | menthol, icilin, l-carvone eucalyptol, isopulegol, geraniol, linalool | sensory neuron | impaired cold sensation |
| TRPA1 | < 17°C | cinnamaldehyde, mustard oil, eugenol, icilin, allicin, acrolein methyl salicylate, gingerol, GsMTx-4 (spider toxin), etc. | sensory neuron |
TRPM2, TRPM4 and TRPM5 are temperature sensitive; however, evidence for their expression in DRG or skin is lacking [28,72]. The expression of TRPM5 in taste cells however suggests a potential explanation for the intriguing observation that temperature can affect taste perception [72].
Activity of TRPM4 and TRPM5 is increased by heating but thermal activation thresholds have not been determined.
In addition to temperature sensation, thermoTRPs mediate the pungent qualities of a number of natural sensory chemicals we might encounter in our environment. For example, capsaicin, the “hot” ingredient in chili peppers, causes a “burning” sensation by activating TRPV1, a thermoTRP that functions as a sensor for heat >42°C [9]. Other examples are shown in table 1. Indeed, it is now recognized that chemesthesis (defined as a chemical sense distinct from taste or smell) is mainly caused by thermoTRP activation.
This review focuses on recent insights into mechanisms underlying thermoTRP activation by thermal and chemesthetic stimuli. For more detailed discussion on the physiological role of thermoTRPs we refer to other recent reviews [1,2].
Structural features of thermoTRPs
To date 28 mammalian TRP channels are known [10,11]. Nine TRP channels have been shown to be activated or strongly modulated by distinct temperatures and six of these (TRPV1-4, TRPM8 and TRPA1) are thought to have a role in temperature sensation and chemesthesis (Figure 1, Table 1). TRP channels are part of the larger superfamily of voltage-gated like (VGL) ion channels and are generally assumed to be similar in global structure [12]. Thus TRP channels presumably form tetramers, with each subunit containing six transmembrane domains (S1–S6). The N- and C- termini are located on the intracellular side of the membrane, and the channel pore is formed by helices S5 and S6 as well as the S5–S6 linker of the four subunits. Similar to voltage gated channels, many TRP channels are voltage sensitive, albeit only weakly, and the voltage sensor region appears to involve the S4 transmembrane helix [13,14●●]. TRP channels have extended cytosolic N- and C- termini that contain a variety of structural features including coiled-coil domains, a TRP domain, and ankyrin repeat domains (Figure 1). These domains, while not present in every TRP channel, have been implicated in channel formation, regulation by cellular factors, etc [15●●–22].
Figure 1.
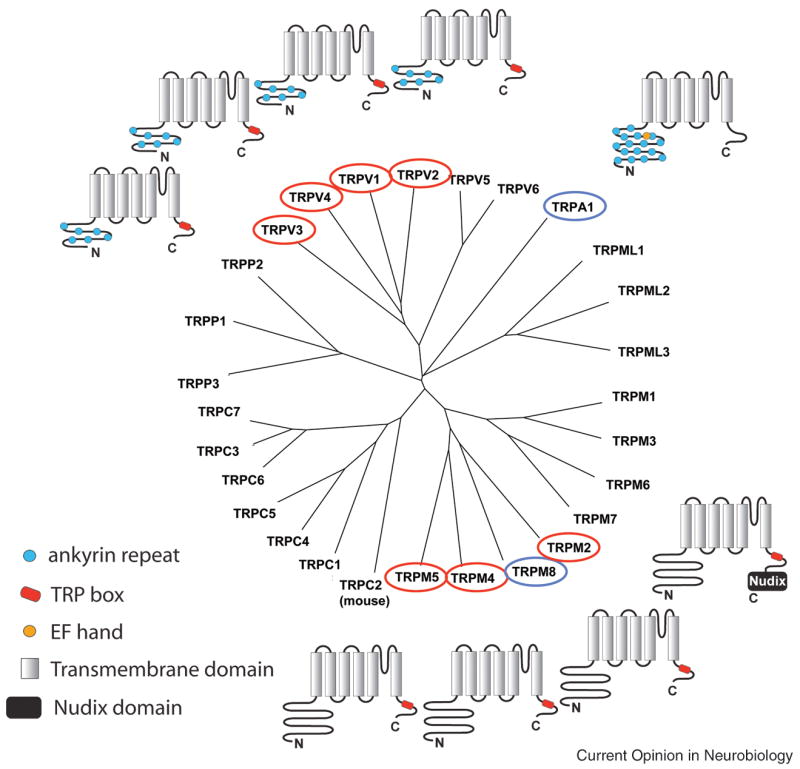
Phylogenetic analysis of TRP channels. ThermoTRPs are indicated by blue (cold activated) and red (heat activated) circles. Human TRP amino acid sequences were used for the analysis with the exception of TRPC2 for which the mouse channel was used. Topology models are shown including several characteristic domains (predicted using http://www.ebi.ac.uk/InterProScan and/or indicated in [17,19,24,55]). TRPM2, TRPM4 and TRPM5 are temperature sensitive; however, evidence for their expression in DRG or skin is lacking [28,72].
Mechanism of thermal activation
As might be expected, ThermoTRPs are steeply temperature dependent. A measure for the temperature dependence of a biological process is its 10-degree temperature coefficient (Q10). This is defined as the relative increase in the rate of a process over a 10°C temperature (T) interval: Q10=rate(T+10)/rate(T) [23]. While most enzymatic processes and ion channels have a Q10 of 2–4, thermoTRPs have Q10 values as high as 20, indicating their unique suitability to act as thermo-sensors [24]. What causes thermoTRPs to be so exquisitely temperature sensitive? Temperature activation of most thermoTRPs is retained in cell-free membranes, arguing for a membrane delimited mechanism independent of cytoplasmic processes. In contrast, activation of TRPV4 by warm temperatures is lost in cell free membranes suggesting that intracellular factors may play a role in some thermoTRPs [25].
A link with voltage sensing?
Further insight into the thermal sensitivity of thermoTRPs came from studies showing that TRPV1 and TRPM8 are voltage sensitive [26]. Membrane depolarization can activate the channels and temperature changes alter this voltage dependent activation. Analysis of channel kinetics led to a simple two-state model for channel activation in which the transitions between the closed and open state are voltage and temperature sensitive. According to this model temperature and voltage sensitivity are closely associated, with both stimuli acting on the same gating step. In agreement with this, mutations affecting the voltage sensitivity of TRPM8 also affect temperature sensitivity [14●●]. In addition, most thermoTRPs have been observed to be voltage sensitive [27]. However, an exception to the rule seems to be TRPM2. This channel is activated at warm temperatures (although it does not appear to play a role in temperature sensation). Interestingly, TRPM2 functions similarly at different voltages suggesting that temperature activation can occur independently of voltage activation [28].
An alternate model for thermal activation was put forward in another study [29]. While confirming the voltage sensitivity of TRPM8, the study suggests a multi-state kinetic model in which temperature and voltage can activate thermoTRPs independently of each other, but affect each other allosterically.
Molecular determinants of temperature sensing
Irrespective of which gating step temperature affects, one is left to wonder how temperature modulates gating on a molecular level. For instance, are specific hydrogen bonds or hydrophobic interactions in the protein disrupted or generated? And what molecular mechanism determines whether a TRP is cold or heat activated? Identification of structural elements in the channel involved in temperature sensing would be a first step towards such a molecular description. A tyrosine residue in the S3 domain of TRPV4 was found to be required for activation by both heat and 4αPDD, a TRPV4 agonist [30]. Given that TRPV4 heat sensitivity is absent in cell free membranes it was suggested that heat may cause intracellular production of a 4αPDD-like agonist. Recent studies on TRPM8 and TRPV1 chimeras led to a striking observation. Replacing the c-terminus of TRPV1 with that of TRPM8 results in a reversal of the temperature sensitivity, making the channel cold sensitive instead of heat sensitive. Vise versa, TRPM8 could be turned into a heat sensitive channel by replacing its c-terminus with that of TRPV1 [31●●]. Thus the c-terminus appears to play a role in temperature sensation; however, whether this region is part of an actual temperature sensor that can translate thermal energy into conformational changes is unclear at this point.
TRPA1, the odd one in the pack
TRPA1 was originally identified as a cold activated channel [32]. In heterologous expression, activation was observed at temperatures below 17°C, and TRPA1 was suggested as a candidate receptor for noxious cold temperatures. Cold activation of TRPA1 has been debated as some groups are unable to observe cold responses in TRPA1 expressing cells [1,2]. Recent evidence suggests that calcium may play an important role in cold activation of TRPA1. First, TRPA1 was shown to be activated by intracellular calcium in the absence of cold or chemical stimuli [33●,34]. This activation was shown to be mediated by a putative calcium binding domain (an EF-hand domain in the N-terminus). Second, mutations in the EF-hand domain resulted in the loss of both calcium and cold activation [33●]. Mustard oil responses were also affected in these mutants but it was concluded that the effect on cold was more profound. Since the authors observed a cold-evoked increase in intracellular calcium in non-transfected HEK293 cells they propose that cold triggers a release of calcium through an unknown mechanism, which in turn activates TRPA1 [33●]. Whether such a mechanism could occur in DRG neurons was not investigated. Therefore, indirect activation via calcium could represent a physiologically relevant mechanism of sensing cold through TRPA1. Ultimately the physiological role of TRPA1 as a cold sensor could come from in vivo studies. However two independent studies on TRPA1 deficient mice led to different conclusions on TRPA1’s role in cold sensing [5,35]. Thus the role of TRPA1 in thermosensation remains unclear and further studies will be needed.
Mechanism of chemical activation
Promiscuous thermoTRPs
ThermoTRP activation is thought to underlie the chemesthetic sensation of a large number of natural and synthetic chemicals [3,9,32,35–51](Table 1). The chemical structures of compounds that can activate thermoTRPs vary widely (Figure 2). Although certain TRP channels appear to be selective for specific compounds there is also considerable cross reactivity. Menthol for instance activates TRPM8, providing a molecular logic for the cooling sensation this compound is known to elicit [38,39]. More recently menthol was shown to also activate TRPV3 and inhibit TRPA1, perhaps accounting for the claims that menthol can act as an analgesic in pain rubs [45]. Another example is camphor which activates TRPV3 and TRPV1, but blocks TRPA1 [42]. This cross reactivity suggests conserved structural and functional properties among different thermoTRP channels.
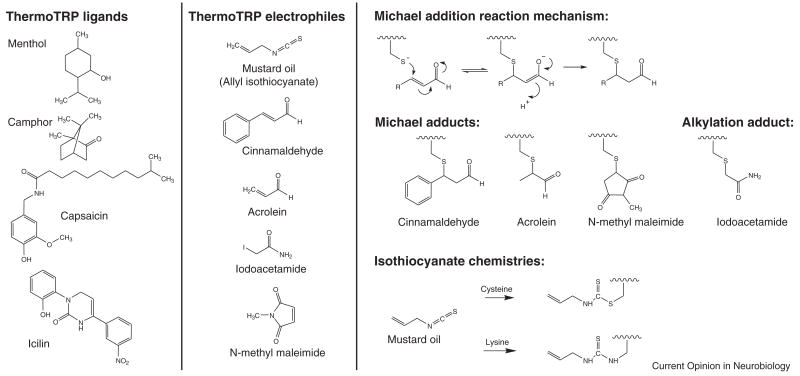
Figure 2.
Left panels: Chemical structures of TRP agonists. Agonists that behave as traditional ligands (left), and also those that covalently modify amino acids (right). Right panel: Electrophilic Michael addition acceptors, such as alpha beta unsaturated aldehydes found in cinnamaldehyde, acrolein, and N-methyl maleimide can form adducts with cysteine residues in proteins using the reaction scheme shown. Below, are the structures of the cysteine adducts formed by the three electrophiles. Alkylating agents such as iodoacetamide may also form cysteine adducts in proteins, producing the structure shown. Isothiocyanate compounds such as allyl isothiocyanate can form adducts with both cysteine and lysine residues in proteins
Structural determinants of chemical sensing
What is known about the structural elements involved in thermoTRP activation by chemicals? Recent studies have provided evidence for an important role of the region spanning transmembrane segments S2 to S4 in the activation of different thermoTRPs by structurally diverse hydrophobic compounds. First identified as (part of) the capsaicin binding site in TRPV1, recent evidence implicates this region in binding of menthol and icilin in TRPM8 [14●●,15●●,52,53]. The S2–S4 region includes the proposed voltage sensor [14●●]. Thus it appears that direct modulation of the voltage sensor is a plausible mechanism for a number of agonists. However, there is evidence that the mechanism of activation by these agonists may be more complicated. Notably, mutations in the C-terminal TRP domain of TRPM8 were found to dramatically reduce the efficacy of menthol (rather than the affinity) while the mutant channels appeared normal with respect to cold and voltage sensitivity as well as with their sensitivity to the signaling lipid phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2, see below) [15●●]. This suggests that this domain influences events downstream of initial menthol binding, and that activation by menthol involves mechanisms distinct and separable from activation by cold, voltage or PI(4,5)P2. A model was proposed in which the TRP domain acts as a gating domain that couples menthol binding to the conformational changes required for channel opening [15●●]. Although the S2–S4 region may include part of the binding pocket for a number of agonists, other thermoTRP agonists such as low pH for TRPV1, or cinnamaldehyde and mustard oil for TRPA1 (see below) are thought to act via different mechanisms [54●●,55●●,56]
Activation by stable modification
While many sensory compounds are ligands that bind to their receptors transiently, other, more reactive compounds can activate thermoTRPs by covalent modification of the channel itself [54●●,55●●]. This novel mechanism of activation is an unexpected property of TRP channels that may be important for sensing noxious, cell-damaging agents.
Pungent natural compounds such as those found in mustard and cinnamon (allyl isothiocyanate and cinnamaldehyde) and environmental irritants (acrolein and pentenal) have been shown to activate TRPA1 channels in vitro [35,37,41]. One commonality among these pungent compounds and irritants is that they are electrophiles, capable of covalently binding to cysteine residues; and in case of isothiocyanates, also to lysine residues (Figure 2). Furthermore, cysteine reactive compounds such as iodoacetamide (IA) and N-methyl maleimide (NMM), previously unrecognized as TRPA1 agonists, were shown to activate the channel [54●●,55●●]. These reactive compounds bind nonspecifically to many proteins within the cell, likely at every solvent-accessible free sulfhydryl. In addition, they were found to bind to several cysteine residues within TRPA1 [55●●]. Reactive compounds activate the channel from the cytoplasmic side of the membrane and cysteine residues within the N-terminus have been identified which, when mutated individually, disrupt activation by electrophiles. Two groups have each identified a set of three cysteine residues in human or mouse TRPA1 which are required for normal channel function, although only one residue is in common among the two studies [54●●,55●●]. This discrepancy may be due to inter-species differences in channel activity, conformation, or gating. Future studies are required to elucidate the mechanism by which covalent modification of TRPA1 can translate into gating of the channel.
Sensing reactive compounds is important for an organism to avoid potentially harmful environments. TRPA1 is uniquely tuned to respond to such compounds, regardless of structure in order to signal pain. An intriguing problem is posed by this unique mode of activation of TRPA1: are such modifications reversible within a cell? In heterologously expressed TRPA1, some agonists remain bound to the channel for over an hour; however we all know that the pain produced by reactive compounds like cinnamon and mustard does not persist interminably (at least at low doses!) [55●●]. Perhaps there are mechanisms within trigeminal and dorsal root neurons to remove the covalent modification or to quickly recycle modified channels. The channel itself may desensitize through calcium- or voltage- dependent mechanisms, preventing pain signaling despite continued agonist binding. These are important questions which will need to be addressed in future studies to understand the mechanisms of pain sensation.
Electrophiles found in reactive oxygen and nitrogen species (ROS and RNS) are also able to bind directly to cysteine residues in proteins. Hydrogen peroxide, SNAP (nitric oxide donor), and pyridyldisulfides (cysteine reactive reagents) were shown to activate TRPC5 channels in vitro [57]. Other TRP channels have also been shown to be activated by changes in the redox environment of the cell [58,59]. This indicates that TRP channels are sensors for both internal and external stimuli.
Desensitization and sensitization
Swimmers may appreciate that the initial freezing sensation upon entering cold water passes after a coupe of minutes. Indeed desensitization is an integral part of thermosensation warranting a discussion. Although the mechanisms underlying thermal desensitization may be complex, modulation of thermoTRPs may explain at least part of the in vivo phenomenon. Activation of most thermoTRPs gradually decreases over time even though the stimulus (either thermal or chemical) is maintained. TRPM8 and TRPV1 are most thoroughly studied with respect to their desensitization properties. In both cases desensitization to chemical stimulation (capsaicin or menthol) is found to depend on extracellular calcium, while desensitization to temperature activation is independent of extracellular calcium suggesting different mechanisms [9,39]. Desensitization of TRPM8 to menthol stimulation is proposed to involve the signaling lipid PI(4,5)P2, which is thought to bind to the channel at the TRP domain [20●●,60●]. TRPM8 activity requires the presence of PI(4,5)P2 and desensitization was proposed to result from calcium influx during TRPM8 activity, leading to the activation of a calcium-sensitive PLC and subsequent depletion of PI(4,5)P2 [20●●]. It should be noted that an alternative model for TRPM8 desensitization involving a calcium sensitive PKC mediated process has been proposed [61]. Desensitization of TRPV1 to capsaicin has been found to depend on a number of intracellular components including PKA and calmodulin [62,63]. Recent evidence is consistent with a role for PI(4,5)P2 depletion in desensitization of TRPV1, similar to what has been proposed for TRPM8 [64●,65●]. These findings are in apparent contradiction with studies claiming that PI(4,5)P2 depletion results in sensitization rather than desensitization of TRPV1 [66,67]. Further research will be required to elucidate the role of PI(4,5)P2 on TRPV1 modulation.
In contrast to desensitization under normal conditions, injury or inflammation often causes a state of sensitization called thermal hyperalgesia (hypersensitivity to noxious heat) or allodynia (nociceptive response to innocuous heat). Sensitization of TRPV1 signaling by inflammatory mediators (for instance bradykinin, ATP, NGF) is thought to underlie this phenomenon. Indeed, mice deficient in TRPV1 do not develop thermal hyperalgesia [4,7]. Several mechanisms have been proposed to contribute to TRPV1 sensitization by various inflammatory mediators. These include a direct phosphorylation of TRPV1 by PKC and PKA; an increase in plasma membrane targeting of TRPV1; and the release from PI(4,5)P2 mediated inhibition. For further details on the mechanisms of TRPV1 sensitization see [68,69].
Many questions on thermoTRP (de)sensitization remain. For instance, very little is known on the mechanisms underlying thermal desensitization; the difference in desensitization rates for different TRPV1 agonists; or the unusual sensitization observed for TRPV3 upon repeated chemical and thermal activation [42,70,71]. Future research is expected to provide further insight into thermoTRP sensitization and desensitization.
Conclusion
The strong temperature sensitivity of thermoTRPs as well as their modulation by structurally diverse compounds makes these ion channels fascinating targets for mechanistic studies. Important progress has been made with regard to understanding thermal activation, and future studies will undoubtedly be focused on gaining structural insight in thermal activation. As for chemical activation, it appears a number of structurally diverse hydrophobic compounds such as capsaicin and menthol may act through a conserved mechanism, by binding close to the voltage sensing domain. In addition, covalent modification has now been implicated in the activation of TRP channels. This unique mode of activation raises many questions that are expected to be addressed in the near future. Given that some thermoTRPs are sensors for painful stimuli, advances in understanding their activation mechanisms are expected to lead to novel strategies for pain management.
Acknowledgments
This work was supported by NIH grants (NS046303, NS049104, DE016927), and by the Novartis Research Foundation. M.B. is supported by a postdoctoral fellowship from the American Heart Association. L.M. is supported by a Ruth Kirschstein predoctoral fellowship from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as
● of special interest
●● of outstanding interest
- 1.Dhaka A, Viswanath V, Patapoutian A. TRP Ion Channels and Temperature Sensation. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 2.Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 3.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 4.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 5.Kwan KY, Allchorne AJ, Vollrath MA, Christensen A, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50 doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 6.Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 8.Dhaka A, Murray AN, Mathur J, Earley T, Petrus M, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007 doi: 10.1016/j.neuron.2007.1002.1024. [DOI] [PubMed] [Google Scholar]
- 9.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 10.Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- 11.Montell C. The TRP superfamily of cation channels. Sci STKE 2005. 2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 12.Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE 2004. 2004:re15. doi: 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]
- 13.Tombola F, Pathak MM, Isacoff EY. How does voltage open an ion channel? Annu Rev Cell Dev Biol. 2006;22:23–52. doi: 10.1146/annurev.cellbio.21.020404.145837. [DOI] [PubMed] [Google Scholar]
- 14.Voets T, Owsianik G, Janssens A, Talavera K, Nilius B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol. 2007;3:174–182. doi: 10.1038/nchembio862. ●● This study suggests charged residues in the S4 and S4–5 linker of TRPM8 form the voltage sensor. Mutations in this region also affected menthol affinity. Mutation induced changes in voltage sensitivity were paralleled by changes in thermal sensitivity in agreement with the proposed model in which thermal and voltage activation act on the same gating step. [DOI] [PubMed] [Google Scholar]
- 15.Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci. 2006;9:493–500. doi: 10.1038/nn1665. ●● The authors take a novel high-throughput approach to identify structural elements involved in TRPM8 activation by menthol. The study identifies two distinct sites on the channel required for menthol sensitivity. They propose the S2 region is involved in menthol binding while the c-terminal TRP domain is required to couple menthol binding to channel opening. This study suggests a common mechanism of activation for structurally diverse compounds such as capsaicin and menthol. [DOI] [PubMed] [Google Scholar]
- 16.Erler I, Al-Ansary DM, Wissenbach U, Wagner TF, Flockerzi V, Niemeyer BA. Trafficking and assembly of the cold-sensitive TRPM8 channel. J Biol Chem. 2006;281:38396–38404. doi: 10.1074/jbc.M607756200. [DOI] [PubMed] [Google Scholar]
- 17.Jin X, Touhey J, Gaudet R. Structure of the N-terminal ankyrin repeat domain of the TRPV2 ion channel. J Biol Chem. 2006;281:25006–25010. doi: 10.1074/jbc.C600153200. [DOI] [PubMed] [Google Scholar]
- 18.Lepage PK, Boulay G. Molecular determinants of TRP channel assembly. Biochem Soc Trans. 2007;35:81–83. doi: 10.1042/BST0350081. [DOI] [PubMed] [Google Scholar]
- 19.McCleverty CJ, Koesema E, Patapoutian A, Lesley SA, Kreusch A. Crystal structure of the human TRPV2 channel ankyrin repeat domain. Protein Sci. 2006 doi: 10.1110/ps.062357206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. ●● Similar to [60] this study demonstrates PI(4,5)P2 is an essential requirement for TRPM8 function and can activate the channel. Desensitization to chemical activation is shown to be mediated by PI(4,5)P2 depletion which is suggested to be caused by a calcium sensitive PLC. Charged residues in the TRP domain are implicated as PI(4,5)P2 binding sites. [DOI] [PubMed] [Google Scholar]
- 21.Tsuruda PR, Julius D, Minor DL., Jr Coiled coils direct assembly of a cold-activated TRP channel. Neuron. 2006;51:201–212. doi: 10.1016/j.neuron.2006.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owsianik G, D’Hoedt D, Voets T, Nilius B. Structure-function relationship of the TRP channel superfamily. Rev Physiol Biochem Pharmacol. 2006;156:61–90. [PubMed] [Google Scholar]
- 23.Hille B. Ion channels of excitable membranes. 3. Sunderland, Mass: Sinauer; 2001. [Google Scholar]
- 24.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 26.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 27.Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. Gating of TRP channels: a voltage connection? J Physiol. 2005;567:35–44. doi: 10.1113/jphysiol.2005.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. Embo J. 2006;25:1804–1815. doi: 10.1038/sj.emboj.7601083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brauchi S, Orio P, Latorre R. Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc Natl Acad Sci U S A. 2004;101:15494–15499. doi: 10.1073/pnas.0406773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci. 2006;26:4835–4840. doi: 10.1523/JNEUROSCI.5080-05.2006. ●● Using domain swap experiments the authors show that TRPV1 can be converted from a heat into a cold sensing channel by replacing the c-terminal domain with that of TRPM8. In the reciprocal experiment TRPM8 was changed into a heat activated channel. This data is suggested to support a model for thermal activation in which temperature and voltage affect the channel independently. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 33.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca(2+) Nat Neurosci. 2007 doi: 10.1038/nn1843. ● This study proposes cold activation may result from the release of intracellular calcium. [DOI] [PubMed] [Google Scholar]
- 34.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007 doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 35.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious Cold Ion Channel TRPA1 Is Activated by Pungent Compounds and Bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 38.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 39.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 40.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Blair NT, Clapham DE. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci. 2005;25:8924–8937. doi: 10.1523/JNEUROSCI.2574-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith PL, Maloney KN, Pothen RG, Clardy J, Clapham DE. Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J Biol Chem. 2006;281:29897–29904. doi: 10.1074/jbc.M605394200. [DOI] [PubMed] [Google Scholar]
- 44.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: Promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, et al. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- 47.Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, Basbaum AI, King D, Julius D. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444:208–212. doi: 10.1038/nature05285. [DOI] [PubMed] [Google Scholar]
- 48.Hill K, Schaefer M. TRPA1 is differentially modulated by the amphipathic molecules trinitrophenol and chlorpromazine. J Biol Chem. 2007;282:7145–7153. doi: 10.1074/jbc.M609600200. [DOI] [PubMed] [Google Scholar]
- 49.McNamara FN, Randall A, Gunthorpe MJ. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1) Br J Pharmacol. 2005;144:781–790. doi: 10.1038/sj.bjp.0706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci. 2004;24:5177–5182. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- 52.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 53.Chuang HH, Neuhausser WM, Julius D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron. 2004;43:859–869. doi: 10.1016/j.neuron.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 54.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. ●● This study proposes that TRPA1 is activated by electrophilic compounds covalently binding to cysteine and perhaps lysine residues in the channel. Using site-directed mutagenesis, three cysteine residues and a lysine residue are shown to be important for channel activation by these compounds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. ●● Hypothesizing that covalent binding is a mechanism for TRPA1 activation, the authors demonstrate that cysteine reactive reagents bind directly to cysteine residues in TRPA1 using click chemistry and mass spectrometry. This group also identifies three cysteine residues which are required for normal channel activation by cysteine reactive compounds. [DOI] [PubMed] [Google Scholar]
- 56.Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 58.Massullo P, Sumoza-Toledo A, Bhagat H, Partida-Sanchez S. TRPM channels, calcium and redox sensors during innate immune responses. Semin Cell Dev Biol. 2006;17:654–666. doi: 10.1016/j.semcdb.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- 60.Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:1674–1681. doi: 10.1523/JNEUROSCI.3632-04.2005. ● These authors report that PI(4,5)P2 is an essential requirement for TRPM8 function. See also [20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abe J, Hosokawa H, Sawada Y, Matsumura K, Kobayashi S. Ca2+-dependent PKC activation mediates menthol-induced desensitization of transient receptor potential M8. Neurosci Lett. 2006;397:140–144. doi: 10.1016/j.neulet.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 63.Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci U S A. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. ● These studies suggest that depletion of PI(4,5)P2 occurs concomitantly with TRPV1 activation and that and that recovery of TRPV1 activity after desensitization requires PI(4,5)P2 resynthesis. Together with [65] this suggests a novel role for PI(4,5)P2, in which it is required for TRPV1 activity rather than inhibition [66,67]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. ●These authors show that sequestering PI(4,5)P2 by means of polylysine inhibits TRPV1 and application of PI(4,5)P2 to inside-out excised patches dramatically potentiates TRPV1. This agrees with [64] but appears at odds with the initially proposed inhibitory role of PI(4,5)P2 [66,67] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 67.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X, McNaughton PA. Why pain gets worse: the mechanism of heat hyperalgesia. J Gen Physiol. 2006;128:491–493. doi: 10.1085/jgp.200609676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 70.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 71.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 72.Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]