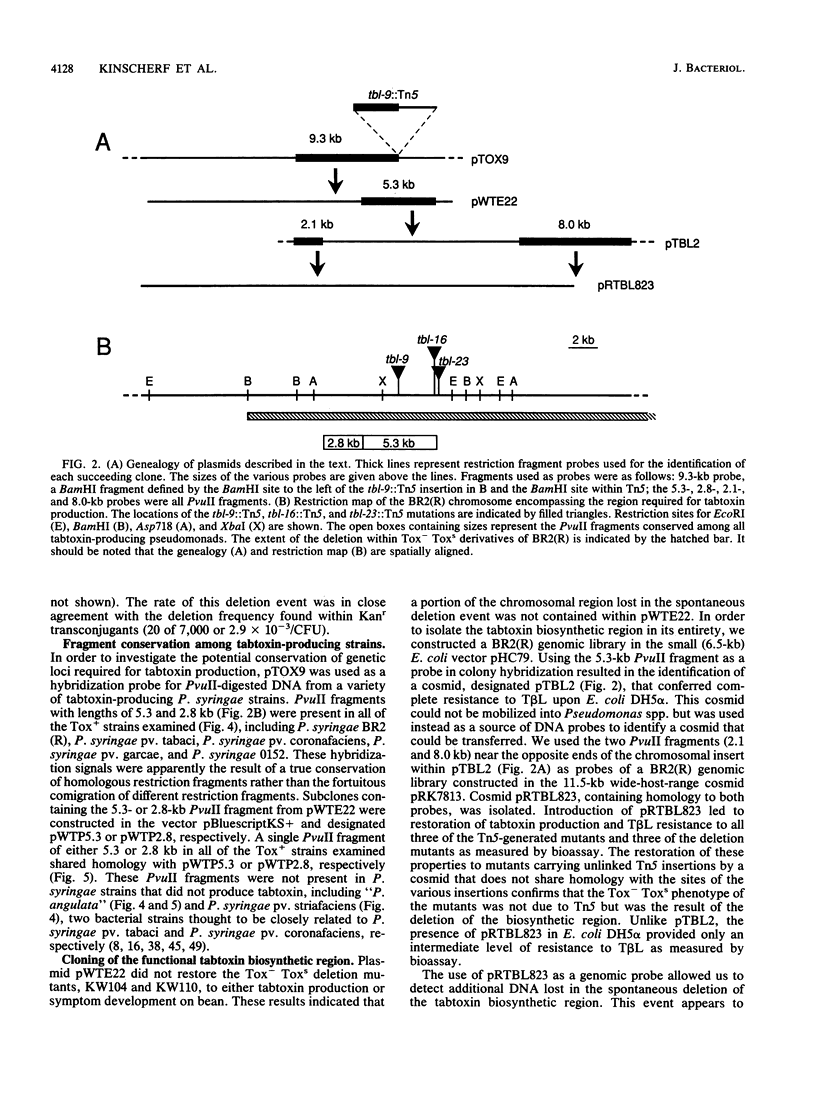
Abstract
Pseudomonas syringae BR2, a causal agent of bean wildfire, was subjected to Tn5 mutagenesis in an effort to isolate mutants unable to produce the beta-lactam antibiotic tabtoxin. Three of the tabtoxin-minus (Tox-) mutants generated appeared to have physically linked Tn5 insertions and retained their resistance to the active toxin form, tabtoxnine-beta-lactam (T beta L). The wild-type DNA corresponding to the mutated region was cloned and found to restore the Tn5 mutants to toxin production. The use of cloned DNA from the region as hybridization probes revealed that the region is highly conserved among tabtoxin-producing pathovars of P. syringae and that the region deletes at a relatively high frequency (10(-3)/CFU) in BR2. The Tox- deletion mutants also lost resistance to tabtoxinine-beta-lactam. A cosmid designated pRTBL823 restored toxin production and resistance to BR2 deletion mutants. This cosmid also converted the tabtoxin-naive P. syringae epiphyte Cit7 to toxin production and resistance, indicating that pRTBL823 contains a complete set of biosynthetic and resistance genes. Tox- derivatives of BR2 did not produce disease symptoms on bean. Clones that restored toxin production to both insertion and deletion mutants also restored the ability to cause disease. However, tabtoxin-producing Cit7 derivatives remained nonpathogenic on bean and tobacco, suggesting that tabtoxin production alone is not sufficient to cause disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzai H., Yoneyama K., Yamaguchi I. The nucleotide sequence of tabtoxin resistance gene (ttr) of Pseudomonas syringae pv. tabaci. Nucleic Acids Res. 1990 Apr 11;18(7):1890–1890. doi: 10.1093/nar/18.7.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch A., Häusler A., Hütter R. Genome rearrangement and genetic instability in Streptomyces spp. J Bacteriol. 1990 Aug;172(8):4138–4142. doi: 10.1128/jb.172.8.4138-4142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner D. P., Sykes R. B. Structure activity relationships among the monobactams. J Antimicrob Chemother. 1984 Oct;14(4):313–327. doi: 10.1093/jac/14.4.313. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J. Indicator technique for antimetabolic toxin production by phytopathogenic species of pseudomonas. Appl Environ Microbiol. 1980 Jan;39(1):25–29. doi: 10.1128/aem.39.1.25-29.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Gutterson N. An efficient mobilizable cosmid vector, pRK7813, and its use in a rapid method for marker exchange in Pseudomonas fluorescens strain HV37a. Gene. 1987;61(3):299–306. doi: 10.1016/0378-1119(87)90193-4. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Knight T. J., Durbin R. D., Langston-Unkefer P. J. Role of glutamine synthetase adenylylation in the self-protection of Pseudomonas syringae subsp. "tabaci" from its toxin, tabtoxinine-beta-lactam. J Bacteriol. 1986 Apr;166(1):224–229. doi: 10.1128/jb.166.1.224-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T. J., Durbin R. D., Langston-Unkefer P. J. Self-protection of Pseudomonas syringae pv. "tabaci" from its toxin, tabtoxinine-beta-lactam. J Bacteriol. 1987 May;169(5):1954–1959. doi: 10.1128/jb.169.5.1954-1959.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orser C., Staskawicz B. J., Panopoulos N. J., Dahlbeck D., Lindow S. E. Cloning and expression of bacterial ice nucleation genes in Escherichia coli. J Bacteriol. 1985 Oct;164(1):359–366. doi: 10.1128/jb.164.1.359-366.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Transposon Tn5 specifies streptomycin resistance in Rhizobium spp. J Bacteriol. 1984 May;158(2):580–589. doi: 10.1128/jb.158.2.580-589.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden S. L., Durbin R. D. Glutamine synthetase inhibition: possible mode of action of wildfire toxin from Pseudomonas tabaci. Nature. 1968 Jul 27;219(5152):379–380. doi: 10.1038/219379a0. [DOI] [PubMed] [Google Scholar]
- Staskawicz B. J., Dahlbeck D., Keen N. T. Cloned avirulence gene of Pseudomonas syringae pv. glycinea determines race-specific incompatibility on Glycine max (L.) Merr. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6024–6028. doi: 10.1073/pnas.81.19.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz B., Dahlbeck D., Keen N., Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987 Dec;169(12):5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Koster W. H., Bonner D. P. The new monobactams: chemistry and biology. J Clin Pharmacol. 1988 Feb;28(2):113–119. doi: 10.1002/j.1552-4604.1988.tb05734.x. [DOI] [PubMed] [Google Scholar]
- Thomas M. D., Langston-Unkefer P. J., Uchytil T. F., Durbin R. D. Inhibition of Glutamine Synthetase from Pea by Tabtoxinine-beta-lactam. Plant Physiol. 1983 Apr;71(4):912–915. doi: 10.1104/pp.71.4.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLLEY D. W., PRINGLE R. B., BRAUN A. C. Isolation of the phytopathogenic toxin of Pseudomonas tabaci, an antagonist of methionine. J Biol Chem. 1952 May;197(1):409–417. [PubMed] [Google Scholar]
- Zieg J., Kushner S. R. Analysis of genetic recombination between two partially deleted lactose operons of Escherichia coli K-12. J Bacteriol. 1977 Jul;131(1):123–132. doi: 10.1128/jb.131.1.123-132.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]