Summary
Background
COX-2 is overexpressed in some cancers, including prostate cancer; however, little is known about the effect of COX-2 overexpression on outcome in radiation-treated patients with prostate cancer. We aimed to study COX-2 overexpression and outcome in a well-defined cohort of men who received treatment with short-term androgen deprivation (STAD) plus radiotherapy or long-term androgen deprivation (LTAD) plus radiotherapy.
Methods
Men with prostate cancer who had participated in the Radiation Therapy Oncology Group (RTOG) 92-02 trial and for whom sufficient diagnostic tissue was available for immunohistochemical staining and image analysis of COX-2 expression were enrolled in this study. Patients in the 92-02 trial had been randomly assigned to treatment with STAD plus radiotherapy or LTAD plus radiotherapy. Multivariate analyses by Cox proportional hazards models were done to assess whether associations existed between COX-2 staining intensity and the RTOG 92-02 primary endpoints of biochemical failure (assessed by the American Society for Therapeutic Radiology and Oncology [ASTRO] and Phoenix criteria), local failure, distant metastasis, cause-specific mortality, overall mortality, and any failure.
Findings
586 patients with sufficient diagnostic tissue for immunohistochemical staining and image analysis of COX-2 expression were included in this study. In the multivariate analyses, the intensity of COX-2 staining as a continuous covariate was an independent predictor of distant metastasis (hazard ratio [HR] 1.181 [95% CI 1.077–1.295], p=0.0004); biochemical failure by two definitions (ASTRO HR 1.073 [1.018–1.131], p=0.008; Phoenix HR 1.073 [1.014–1.134], p=0.014); and any failure (HR 1.068 [1.015–1.124], p=0.011). The higher the expression of COX-2, the greater the chance of failure. As a dichotomous covariate, COX-2 overexpression seemed to be most discriminating of outcome for those who received STAD compared with those who received LTAD.
Interpretation
To our knowledge, this is the first study to establish an association of COX-2 expression with outcome in patients with prostate cancer who have had radiotherapy. Increasing COX-2 expression was significantly associated with biochemical failure, distant metastasis, and any failure. COX-2 inhibitors might improve patient response to radiotherapy in those treated with or without androgen deprivation. Our findings suggest that LTAD might overcome the effects of COX-2 overexpression. Therefore, COX-2 expression might be useful in selecting patients who need LTAD.
Introduction
The cyclooxygenase-2 (COX-2) enzyme, one of three isozymes, converts arachidonic acid to prostaglandins and other eicosanoids.1–3 Apart from its well-characterised role in inflammation, COX-2 is overexpressed in some human cancers, including prostate cancer;4–8 although, one study noted COX-2 expression in benign prostate tissue and not in tumour tissue.9 A population-based case–control study showed that sequence variations in the COX-2 gene affect the risk of developing prostate cancer,10 linking inflammation with prostate carcinogenesis.
With the advent of selective COX-2 inhibitors, in-vitro and in-vivo studies that use these drugs have highlighted the effect of COX-2 on angiogenesis11,12 and tumorigenesis.13 Additionally, COX-2 overexpression is linked to chemotherapy and radiation treatment resistance in patients with prostate and other tumours.14–18 The effects of selective COX-2 inhibitors have been shown to be independent of androgen-responsiveness in prostate cancer cell lines.15,16,19,20 Therefore, COX-2 is a potentially useful tumour marker for predicting poor outcome in patients with prostate cancer.
We aimed to study the association between COX-2 expression and outcome in men with prostate cancer who had participated in the Radiation Therapy Oncology Group (RTOG) 92-02 trial, and who were treated with radiotherapy and short-term or long-term androgen deprivation (STAD or LTAD). The RTOG 92-02 trial was a phase III randomised study involving men with locally advanced (T2c–T4) prostate cancer and prostate-specific androgen (PSA) concentrations less than 150 ng/mL.21
Methods
Patients and procedures
Details of the RTOG 92-02 trial protocol have been described previously.21,22 Briefly, the RTOG 92-02 trial was a randomised trial studying long-term neoadjuvant concurrent and adjuvant androgen deprivation (28 months goserelin) versus short-term neoadjuvant and concurrent androgen deprivation (4 months goserelin and flutamide) with external-beam radiotherapy (65–70 Gy to the prostate and 44–50 Gy to the pelvic lymph nodes) in patients with locally advanced prostate cancer (T2c–T4). The findings of the RTOG 92-02 trial supported the use of LTAD with radiotherapy rather than STAD with radiotherapy for patients with T2c–T4 stage cancer. A further exploratory subset analysis of patients with Gleason scores 8–10 showed LTAD also provided a survival advantage compared with STAD in this subgroup.
Tissue samples were collected from patients before informed consent for specimen collection was needed for this type of analysis. However, the data were de-identified and the study was approved by an internal review board at the Fox Chase Cancer Center, Philadelphia, PA, USA.
Immunohistochemical staining
Paraffin-embedded, formalin-fixed pretreatment diagnostic tissue cut onto slides was deparaffinised and rehydrated. Antigen retrieval was done by heating the slides in citrate buffer in a pressure cooker for 50 min. Incubation was done in an autostainer (DakoCytomation, Glostrup, Denmark) with an antibody directed against COX-2 (catalogue number 804-112-C050, 1:200 dilution for 1 h; Alexis Biochemicals, Lausen, Switzerland).11 A biotinylated secondary antibody (Dako LSAB 2 Kit, Dako, Glostrup, Denmark) was overlaid for 10 min, followed by streptavidin for 10 min, with rinses of Tris buffer (at pH 7.6) between stainings. Diaminobenzidine chromagen was then applied for 5 min. The slides were counterstained with haematoxylin (Dako, Carpinteria, CA, USA), dehydrated, and coverslipped. A known colon adeno-carcinoma tissue sample was used as a positive control with each batch. Negative controls (without COX-2 antibody) were used on initial antibody testing, but were not used with each staining batch.
The intensity of COX-2 cytoplasmic staining was scored by use of an automated imaging system (ACIS II, Clarient Inc, San Juan Capistrano, CA, USA).23 A best-colour threshold setting (“p53_D_histo.app”) chosen from a range of predefined thresholds provided on the system was used for all slides analysed, and a freehand instrument was used to outline at least six regions of interest. The colour threshold consisted of three parameters: hue, colour, and luminosity; these settings have been optimised by the manufacturer by use of a range of tissue-specific antibody stains. An experienced pathologist (MEHH) did the initial threshold studies and analysed a training set of slides. These slides were from about 40 random patients and were digitally imaged by use of ACIS scan applications, each with a different predefined threshold. The threshold instrument provided a brown or blue overlay, of which pixels were deemed positive or negative, respectively. The application with the best overlap of pixels on the tissue samples was chosen, and all subsequent slides were imaged by use of that application. The remaining slides were then analysed by LYK. The tumour group with the strongest intensity was chosen, regardless of the tumour grade. Any staining above background was deemed positive. Consistent moderate-to-dark intensity staining was noted in tumour tissue from all patients. Adjacent benign prostate epithelium acted as internal controls; the cytoplasmic staining intensity of the epithelial cells was scored randomly to ensure consistent staining throughout the study.
Definition of endpoints
The endpoints in this study were: biochemical failure, local failure, distant metastasis, cause-specific mortality, overall mortality, and any failure. Distant metastasis, cause-specific mortality, and overall mortality were protocol-defined. The failure event for overall mortality was defined as death due to any cause. The failure event for cause-specific mortality was death certified as due to prostate cancer, death due to treatment complications, death from unknown causes with active malignancy (clinical disease relapse), or from another cancer with documented bone metastases attributed to prostate cancer before the appearance of the second independent cancer. Local failure was defined as clinical evidence of local recurrence (by methods including physical examination, pathology, CT scan, MRI, ultrasonography, endorectal MRI, cystoscopy, and proctoscopy) or persistent disease (tumour regrowth by 25% or stable disease beyond 18 months). Distant metastasis was defined by clinical evidence of distant disease by any method. Time to the endpoints was measured from date of randomisation to date of failure event or date of last follow-up. Two definitions were considered in defining biochemical failure: the American Society of Therapeutic Radiology and Oncology (ASTRO) consensus definition (three consecutive increases in PSA concentrations or initiation of salvage treatment)24—the definition used in the updated RTOG protocol 92-02 report;21 and the current standard definition, the Phoenix definition (PSA >nadir+2 ng/mL after treatment or initiation of salvage treatment).25 Time to biochemical failure defined by ASTRO was from date of randomisation to the midpoint between date of last nadir and date of first increase in PSA concentration beyond the nadir. The patient was censored at date of last PSA measurement if the PSA concentration was declining or at the nadir. Time to biochemical failure as defined by Phoenix was from date of randomisation to date of failure event. Any failure was defined as a first event from ASTRO biochemical failure, local failure, distant metastasis, or cause-specific mortality.
Statistical analyses
The study cohort was characterised by pre-treatment covariates, which were dichotomised as follows according to trial stratification, unless stated otherwise: age (<70 years vs ≥70 years, and median age), Gleason score (2–6 vs 7 vs 8–10), initial PSA (iPSA) (≤30 vs >30 ng/mL), tumour stage (T2 vs T3 or T4; the T3 and T4 groups were combined due to small numbers), and assigned treatment (STAD plus radiotherapy vs LTAD plus radiotherapy). All outcome data were frozen on Sept 29, 2005. COX-2 staining intensity was tested as continuous and categorical variables. As a categorical variable, COX-2 staining intensity was dichotomised in three separate analyses at the 25th, 50th, and 75th percentiles. Cox proportional hazards models were used to identify relations between COX-2 staining intensity and the endpoints. We assumed that no interactions occurred between the covariates, and a statistical significance level of 0.05 was used with χ² test statistics. The Kaplan-Meier method was used to calculate actuarial estimates of overall mortality and comparisons were done with the log-rank test. The cumulative incidence method was used to estimate biochemical failure, distant metastasis, local failure, cause-specific mortality, and any failures, and comparisons were done with Gray’s test to consider competing risks. Competing risks for cause-specific mortality were death not meeting one of the following criteria: death certified as due to prostate cancer, death due to treatment complications, death from unknown causes with active malignancy (clinical disease relapse), or from another cancer with documented bone metastases attributed to prostate cancer before the appearance of the second independent cancer. The competing risk for local failure, distant metastasis, and biochemical failure was death without failure events. Patients who received androgen deprivation (salvage hormone treatment) before clinical failure was identified were not censored, but were assessed for subsequent failure events because salvage hormone treatment was not an original trial endpoint. In the assessment of local failure, if distant metastasis was the first of these two events, the patients were assessed for both failures. We also checked for interactions between COX-2 staining intensity and androgen deprivation duration (treatment group) by use of χ² test statistics. SAS software (version 9.1) was used for all statistical analyses and a significance level of 0.05 was used for all tests.
Role of the funding source
The sponsors of the study had no role in the design of the study; collection, analysis, or interpretation of the data; or in the writing of this report. LYK, KB, AP, MEHH, and APD had access to the raw data. The corresponding author had full access to all of the data and final responsibility to submit for publication.
Results
586 patients (out of a parent cohort of 1521 patients, ie, 39%) with sufficient tissue and suitable COX-2 staining were included in this study; 17 patients who had intensely high stromal and background staining and two patients who had no staining were excluded. 50 patients (9%) had tumour specimens obtained from transurethral resections and 536 (91%) had needle-core biopsies.
Based on pretreatment characteristics, no statistically significant differences were noted between patients with and without COX-2 scores (tables 1 and 2); however, the distribution by assigned treatment was significantly different (p=0.012), therefore, the cohort of patients who had COX-2 data were representative of the parent cohort. We did not record any statistically significant differences between the group with and the group without COX-2 data for any of the primary endpoints tested. 270 patients were treated with STAD plus radiotherapy and 316 patients received LTAD plus radiotherapy. The median age of patients was 70 years (258 patients were aged <70 years and 328 patients were aged ≥70 years, range 43–88). 212 patients had Gleason score of 2–6, 182 patients had Gleason score of 7, and 149 patients had Gleason score of 8–10 (43 patients had unknown Gleason score). iPSA was 30 ng/mL or under for 389 patients. 268 patients had stage T2 disease. The median follow-up for the surviving patients with data on COX-2 expression was 107 months (range 2.0–155.3). 251 of 278 surviving patients (90%) had more than 60 months of follow-up (median 123.4, range 60.3–155.3).
Table 1.
Characteristics of patients
| With COX-2 intensity score (n=586) | Missing COX-2 intensity score (n=935) | p* | |
|---|---|---|---|
| Age | |||
|
| |||
| Median, years (range) | 70 (43–88) | 70 (43–88) | .. |
| <70, n (%) | 258 (44) | 423 (45) | 0.64 |
| ≥70, n (%) | 328 (56) | 512 (55) | .. |
| Gleason score, n (%) | |||
| Unknown or missing | 43 (7) | 57 (6) | 0.33† |
| 2–6 | 212 (36) | 370 (40) | 0.37‡ |
| 7 | 182 (31) | 296 (32) | .. |
| 8–10 | 149 (25) | 212 (23) | .. |
| Clinical stage, n (%) | |||
| T2 | 268 (46) | 424 (45) | 0.88 |
| T3 or T4 | 318 (54) | 511 (55) | .. |
| PSA, ng/mL | |||
| Median (range) | 20.8 (0.4–219.7) | 19.5 (0.1–250.0) | |
| ≤30 | 389 (66) | 632 (68) | 0.62 |
| >30 | 197 (34) | 303 (32) | .. |
| Assigned treatment | |||
| STAD+radiotherapy | 270 (46) | 493 (53) | 0.012 |
| LTAD+radiotherapy | 316 (54) | 442 (47) | .. |
p values were derived from χ² statistics.
p value is the comparison of data for missing COX-2 staining intensity score vs 2–6 vs 7–10.
p value is the comparison of data for missing COX-2 staining intensity score vs 2–6 vs 7 vs 8–10. Unless otherwise stated, data are n and %.
Table 2.
Distribution of patients by COX-2 staining intensity score
| COX-2 staining intensity score ≤ 134* (n=304) | COX-2 staining intensity score >134* (n=282) | p† | |
|---|---|---|---|
| Age | |||
|
| |||
| Median, years (range) | 70 (43–88) | 71 (49–88) | .. |
| <70 | 138 (45) | 120 (43) | 0.49 |
| ≥70 | 166 (55) | 162 (57) | .. |
| Gleason score, n (%) | |||
| Unknown or missing, n (%) | 19 (6) | 24 (9) | 0.49‡ |
| 2–6 | 108 (36) | 104 (37) | 0.50§ |
| 7 | 93 (31) | 89 (32) | .. |
| 8–10 | 84 (28) | 65 (23) | .. |
| Clinical stage, n (%) | |||
| T2 | 144 (47) | 124 (44) | 0.41 |
| T3 or T4 | 160 (53) | 158 (56) | .. |
| PSA, ng/mL | |||
| Median, (range) | 19.5 (0.8–149.0) | 23.2 (0.4–219.7) | .. |
| ≤30 | 208 (68) | 181 (64) | 0.28 |
| >30 | 96 (32) | 101 (36) | .. |
| Assigned treatment | |||
| STAD+radiotherapy | 145 (48) | 125 (44) | 0.41 |
| LTAD+radiotherapy | 159 (52) | 157 (56) | .. |
COX-2 intensity score (in arbitrary units) dichotomised at the median.
p values were derived from χ² statistics.
p value is the comparison of data for missing COX-2 intensity score vs 2–6 vs 7–10.
p value is the comparison of data for missing COX-2 intensity score vs 2–6 vs 7 vs 8–10. Unless otherwise stated, data are n and %.
In univariate analysis (table 3), COX-2 staining intensity as a continuous variable was significantly associated with distant metastasis (HR 1.12 [95% CI 1.04–1.22], p=0.006), biochemical failure by both definitions (ASTRO: HR 1.07 [1.02–1.12], p=0.007; Phoenix: HR 1.06 [1.00–1.12], p=0.033), and any failure (HR 1.06 [1.02–1.11], p=0.010). The higher the intensity of COX-2 staining, the greater the risk of failure for each of these endpoints. In the multivariate analyses (table 4), controlling for treatment group, age, Gleason score, tumour, and iPSA concentration, COX-2 as a continuous covariate was an independent predictor of distant metastasis (HR 1.181 [1.077–1.295], p=0.0004), biochemical failure by both definitions (ASTRO: HR 1.073 [1.018–1.131], p=0.008; Phoenix: HR 1.073 [1.014–1.134], p=0.014), and any failure (HR 1.068 [1.015–1.124], p=0.011). Gleason score of 8–10 was also associated with distant metastasis (HR 3.773 [2.263–6.289], p<0.0001) and biochemical failure defined by Phoenix (HR 1.526 [1.138–2.047], p=0.005). All other factors in the multivariate analyses were included as dichotomous covariates by use of the median (age) or protocol-defined stratification (PSA, Gleason score, and stage) cut-off points. All the assumptions necessary for COX modelling were met.
Table 3.
Univariate analysis of COX-2 staining intensity score as a continuous variable
| n | Failure, n | HR* (95% CI) | p† | |
|---|---|---|---|---|
| Overall mortality | 586 | 308 | 1.02 (0.97–1.07) | 0.51 |
| Cause-specific mortality | 586 | 91 | 1.03 (0.94–1.14) | 0.48 |
| Distant metastasis | 586 | 113 | 1.12 (1.04–1.22) | 0.006 |
| Local failure | 586 | 68 | 1.07 (0.96–1.19) | 0.23 |
| Biochemical failure—ASTRO | 586 | 350 | 1.07 (1.02–1.12) | 0.007 |
| Biochemical failure—Phoenix | 586 | 300 | 1.06 (1.00–1.12) | 0.033 |
| Any failure‡ | 586 | 371 | 1.06 (1.02–1.11) | 0.010 |
HR are for a change of 10 units in the COX-2 intensity score.
p value from χ² test by use of Cox proportional hazards model.
Failures included cause-specific mortality, distant metastasis, local failure, and ASTRO-defined biochemical failure.
Table 4.
Multivariate analysis of COX-2 staining intensity score as a continuous variable
| Covariate | Group | Patients, n | Failures, n | HR (95% CI) | p* |
|---|---|---|---|---|---|
| Distant metastasis (total failures=100) | |||||
|
| |||||
| COX-2 intensity | Continuous | 543 | 100 | 1.181 (1.077–1.295) | 0.0004 |
| Treatment group | LTAD+radiotherapy | 291 | 41 | 0.658 (0.438–0.987) | 0.043 |
| Age, years | ≥70 | 308 | 43 | 0.705 (0.470–1.059) | 0.09 |
| PSA, ng/mL | >30 | 177 | 44 | 1.359 (0.908–2.032) | 0.14 |
| Gleason score† | 7 | 182 | 26 | 1.154 (0.653–2.038) | 0.62 |
| 8–10 | 149 | 51 | 3.773 (2.263–6.289) | <0.0001 | |
| Clinical stage | T3 or T4 | 287 | 65 | 1.590 (1.049–2.409) | 0.029 |
|
| |||||
| Biochemical failure—ASTRO (total failures=317) | |||||
|
| |||||
| COX-2 intensity | Continuous | 543 | 317 | 1.073 (1.018–1.131) | 0.008 |
| Treatment group | LTAD+radiotherapy | 291 | 143 | 0.468 (0.374–0.585) | <0.0001 |
| Age, years | ≥70 | 308 | 158 | 0.683 (0.548–0.853) | 0.0008 |
| PSA, ng/mL | >30 | 177 | 122 | 1.405 (1.118–1.766) | 0.004 |
| Gleason score† | 7 | 182 | 105 | 0.935 (0.717–1.221) | 0.62 |
| 8–10 | 149 | 92 | 1.066 (0.807–1.409) | 0.65 | |
| Clinical stage | T3 or T4 | 287 | 174 | 1.161 (0.927–1.455) | 0.19 |
|
| |||||
| Biochemical failure—Phoenix (total failures=278) | |||||
|
| |||||
| COX-2 intensity | Continuous | 543 | 278 | 1.073 (1.014–1.134) | 0.014 |
| Treatment group | LTAD+radiotherapy | 291 | 120 | 0.491 (0.387–0.624) | <0.0001 |
| Age, years | ≥70 | 308 | 136 | 0.703 (0.554–0.891) | 0.004 |
| PSA, ng/mL | >30 | 177 | 109 | 1.466 (1.150–1.870) | 0.002 |
| Gleason score† | 7 | 182 | 91 | 1.033 (0.774–1.378) | 0.83 |
| 8–10 | 149 | 88 | 1.526 (1.138–2.047) | 0.005 | |
| Clinical stage | T3 or T4 | 287 | 151 | 1.129 (0.889–1.433) | 0.32 |
|
| |||||
| Any failure‡ (total failures=338) | |||||
|
| |||||
| COX-2 intensity | Continuous | 543 | 338 | 1.068 (1.015–1.124) | 0.011 |
| Treatment group | LTAD+radiotherapy | 291 | 156 | 0.484 (0.390–0.601) | <0.0001 |
| Age, years | ≥70 | 308 | 175 | 0.761 (0.614–0.944) | 0.013 |
| PSA, ng/mL | >30 | 177 | 127 | 1.373 (1.099–1.716) | 0.005 |
| Gleason score† | 7 | 182 | 110 | 0.920 (0.710–1.192) | 0.52 |
| 8–10 | 149 | 100 | 1.102 (0.842–1.441) | 0.48 | |
| Clinical stage | T3 or T4 | 287 | 184 | 1.131 (0.910–1.407) | 0.27 |
p values are from χ² test by use of Cox proportional hazards model.
43 patients without Gleason score were not included.
Failures included cause-specific mortality, distant metastasis, local failure, and biochemical failure—ASTRO.
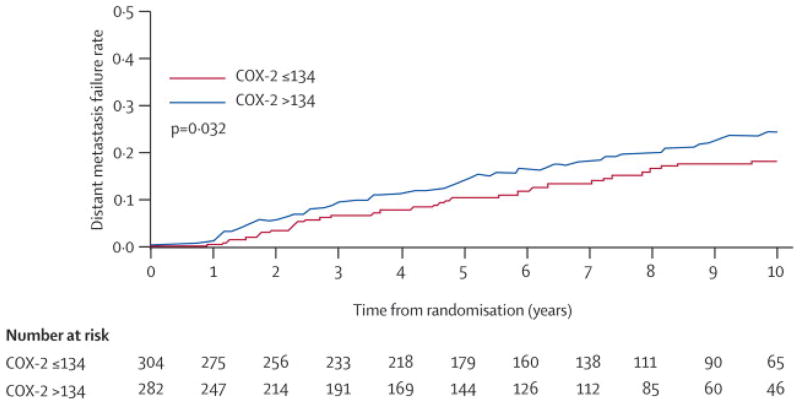
The COX-2 intensities were then dichotomised by use of the median (134 arbitrary units), and the 25th (119 arbitrary units) and 75th percentiles (150 arbitrary units) to establish whether a dichotomised COX-2 staining intensity by each cut-off point was predictive of outcome. The 25th and 75th percentile cut-off points were not significant. However, the dichotomised COX-2 staining intensity by the median of 134 arbitrary units (range 69–214) yielded a significant difference for distant metastasis. Table 5 shows the univariate analyses. We noted a significant association between COX-2 staining intensity and distant metastasis (HR 1.500 [1.035–2.173], p=0.032). The corresponding failure curve for distant metastasis is shown in figure 1. The 5-year distant metastasis occurrence was 10.6% (7.0–14.2) for an intensity score of 134 arbitrary units or fewer versus 14.1% (9.9–18.3) for an intensity score of over 134 arbitrary units. The 8-year distant metastasis occurrence was 16.4% (12.0–20.9) for an intensity score of 134 arbitrary units or fewer versus 19.9% (14.9–24.8) for an intensity score of over 134 arbitrary units. These occurrences were estimated by cumulative incidence with death without distant metastasis as a competing risk. In the multivariate analyses shown in table 6, dichotomised COX-2 staining intensity remained significantly associated with distant metastasis (HR 1.590 [1.070–2.363], p=0.022) when adjusted for other covariates. Gleason scores of 8–10 and distant metastasis were associated significantly (HR 3.667 [2.221–6.057], p<0.0001). We did not note a significant association between COX-2 expression as a dichotomous covariate and biochemical failure defined by ASTRO or Phoenix (data not shown). The association of COX-2 expression as a continuous covariate with distant metastasis and biochemical failure was stronger; this finding was not surprising, given that the continuous approach is a more efficient and powerful use of the data.
Table 5.
Univariate analysis of COX-2 intensity score as a dichotomous variable
| COX-2 cut-off point | n | Failures | HR (95% CI) | p* | |
|---|---|---|---|---|---|
| Overall mortality | |||||
|
| |||||
| Median | ≤134 | 304 | 150 | 1.069 (0.853–1.339) | 0.56 |
| >134 | 282 | 158 | |||
|
| |||||
| 25t.h percentile | ≤119 | 153 | 76 | 1.019 (0.785–1.322) | 0.89 |
| >119 | 433 | 232 | |||
|
| |||||
| 75th percentile | ≤150 | 446 | 229 | 1.008 (0.779–1.305) | 0.95 |
| >150 | 140 | 79 | |||
|
| |||||
| Distant metastasis | |||||
|
| |||||
| Median | ≤134 | 304 | 51 | 1.500 (1.035–2.173) | 0.03 |
| >134 | 282 | 62 | |||
|
| |||||
| 25th percentile | ≤119 | 153 | 26 | 1.321 (0.852–2.049) | 0.21 |
| >119 | 433 | 87 | |||
|
| |||||
| 75th percentile | ≤150 | 446 | 80 | 1.467 (0.977–2.201) | 0.06 |
| >150 | 140 | 33 | |||
|
| |||||
| Local failure | |||||
|
| |||||
| Median | ≤134 | 304 | 32 | 1.236 (0.765–1.997) | 0.39 |
| >134 | 282 | 36 | |||
|
| |||||
| 25th percentile | ≤119 | 153 | 19 | 0.923 (0.542–1.570) | 0.77 |
| >119 | 433 | 49 | |||
|
| |||||
| 75th percentile | ≤150 | 446 | 47 | 1.467 (0.877–2.455) | 0.14 |
| >150 | 140 | 21 | |||
|
| |||||
| Biochemical failure—ASTRO | |||||
|
| |||||
| Median | ≤134 | 304 | 177 | 1.109 (0.899–1.368) | 0.33 |
| >134 | 282 | 173 | |||
| 25th percentile | ≤119 | 153 | 81 | 1.271 (0.991–1.630) | 0.06 |
| >119 | 433 | 269 | |||
|
| |||||
| 75th percentile | ≤150 | 446 | 259 | 1.309 (1.031–1.663) | 0.03 |
| >150 | 140 | 91 | |||
|
| |||||
| Biochemical failure—Phoenix | |||||
|
| |||||
| Median | ≤134 | 304 | 145 | 1.238 (0.987–1.553) | 0.06 |
| >134 | 282 | 155 | |||
| 25th percentile | ≤119 | 153 | 71 | 1.165 (0.893–1.521) | 0.26 |
| >119 | 433 | 229 | |||
|
| |||||
| 75th percentile | ≤150 | 446 | 223 | 1.198 (0.925–1.553) | 0.17 |
| >150 | 140 | 77 | |||
|
| |||||
| Any failure† | |||||
|
| |||||
| Median | ≤134 | 304 | 190 | 1.057 (0.862–1.296) | 0.59 |
| >134 | 282 | 181 | |||
| 25th percentile | ≤119 | 153 | 85 | 1.272 (0.998–1.621) | 0.05 |
| >119 | 433 | 286 | |||
| 75th percentile | ≤150 | 446 | 276 | 1.277 (1.011–1.612) | 0.04 |
| >150 | 140 | 95 | |||
p values are from χ² test by use of Cox proportional hazards model.
Failures included cause-specific mortality, distant metastasis, local failure, and biochemical failure defined by ASTRO.
Figure 1.
Failure curve of distant metastasis by dichotomised COX-2 staining intensity score (median cut-off point)
Table 6.
Multivariate analysis of COX-2 staining intensity score as a dichotomous variable
| Covariate | Group | n | Failures | HR (95% CI) | p* |
|---|---|---|---|---|---|
| Distant metastasis (total failures=100) | |||||
|
| |||||
| COX-2 intensity | >134 | 258 | 54 | 1.590 (1.070–2.363) | 0.022 |
| Treatment group | LTAD+radiotherapy | 291 | 41 | 0.605 (0.406–0.903) | 0.014 |
| Gleason score† | 7 | 182 | 26 | 1.167 (0.663–2.053) | 0.59 |
| 8–10 | 149 | 51 | 3.667 (2.221–6.057) | <0.0001 | |
| Clinical stage | T3 or T4 | 287 | 65 | 1.657 (1.095–2.509) | 0.017 |
p values are from χ² test by use of Cox proportional hazards model.
43 patients without Gleason score were not included.
For patients who received salvage androgen deprivation and, therefore, who had a delay in failure events, we studied the distribution of salvage androgen deprivation administration before the diagnosis of distant metastasis for patients with high and low (median cut-off point) COX-2 intensity scores. Of the 184 of 586 patients (31%) who had salvage androgen deprivation administered before the diagnosis of distant metastasis, 99 patients had high COX-2 expression and 85 patients had low COX-2 expression. We did not note a significant difference in the distribution of COX-2 expression by the use of salvage androgen deprivation before the diagnosis of distant metastasis (p=0.3020). Salvage androgen deprivation was given before the diagnosis of biochemical failure in 170 patients; 168 biochemical failures were recorded by Phoenix and 169 biochemical failures were recorded by ASTRO. We did not note a significant difference in the timepoint when the patients failed biochemically by ASTRO or Phoenix criteria in relation to when they received salvage androgen deprivation (data not shown). Furthermore, we did not record a significant association between COX-2 expression (median cut-off point) and salvage androgen deprivation given before the diagnosis of biochemical failure by use of either definition (data not shown).
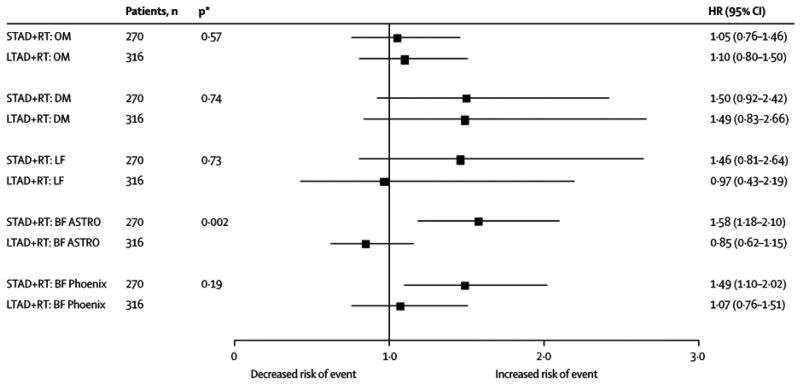
We also studied whether the association between dichotomised COX-2 expression and patient outcome was affected by length of androgen deprivation (assigned protocol treatment; table 7). An association was noted between dichotomised COX-2 staining intensity and treatment group for the ASTRO biochemical failure endpoint (χ² test statistics=10.1, p=0.002), but not for biochemical failure defined by Phoenix (χ² test statistics=1.7, p=0.19). Figure 2 shows the HR by the dichotomised COX-2 intensities (by use of the median cut-off point). COX-2 expression was an important determinant of biochemical failure for patients treated with STAD plus radiotherapy, but not for those treated with LTAD plus radiotherapy.
Table 7.
Test for an interaction between COX-2 staining intensity and length of androgen deprivation
| Covariate | Group | HR (95% CI) | p* |
|---|---|---|---|
| Overall mortality | |||
|
| |||
| COX-2 intensity | >134 | 1.006 (0.717–1.413) | 0.97 |
| Treatment group | LTAD+radiotherapy | 0.849 (0.610–1.182) | 0.33 |
| Interaction between COX-2 and treatment | 1.146 (0.720–1.823) | 0.57 | |
|
| |||
| Distant metastasis | |||
|
| |||
| COX-2 intensity | >134 | 1.566 (0.937–2.619) | 0.09 |
| Treatment group | LTAD+radiotherapy | 0.603 (0.337–1.081) | 0.09 |
| Interaction between COX-2 and treatment | 0.875 (0.393–1.947) | 0.74 | |
|
| |||
| Local failure | |||
|
| |||
| COX-2 intensity | >134 | 1.309 (0.690–2.484) | 0.41 |
| Treatment group | LTAD+radiotherapy | 0.568 (0.274–1.179) | 0.13 |
| Interaction between COX-2 and treatment | 0.836 (0.300–2.324) | 0.73 | |
|
| |||
| Biochemical failure—ASTRO | |||
|
| |||
| COX-2 intensity | >134 | 1.629 (1.209–2.195) | 0.001 |
| Treatment group | LTAD+radiotherapy | 0.644 (0.473–0.876) | 0.005 |
| Interaction between COX-2 and treatment | 0.486 (0.312–0.759) | 0.002 | |
|
| |||
| Biochemical failure—Phoenix | |||
|
| |||
| COX-2 intensity | >134 | 1.442 (1.055–1.970) | 0.022 |
| Treatment group | LTAD+radiotherapy | 0.556 (0.397–0.779) | 0.0006 |
| Interaction between COX-2 and treatment | 0.728 (0.453–1.171) | 0.19 | |
p values are from χ² test by use of Cox proportional hazards model.
Figure 2. Forest plot of HR by dichotomised COX-2 staining intensity score (median cut-off point).
RT=radiotherapy. OM=overall mortality. DM=distant metastasis. LF=local failure. BF=biochemical failure. *p value is from the χ² test of the interation between COX-2 staining intensity and treatment. HR are plotted on x-axis.
Discussion
In this study, COX-2 staining intensity as a continuous covariate was associated significantly with distant metastasis, biochemical failure by ASTRO and Phoenix definitions, and any failure in multivariate analyses. When COX-2 was dichotomised around the median, these associations were weaker; a significant association was noted only with distant metastasis.
COX-2 inhibition has been associated with enhanced responses of cancer cells to irradiation.17,18,26–28 COX-2 overexpression in patients who have undergone prostatectomy has been shown to be associated with high tumour grade7,29–31 and biochemical failure.32,33 Although COX-2 inhibitors are being used in the treatment of patients with recurrent prostate cancer in a phase II trial,34 and in newly diagnosed patients or patients with recurrent disease after local treatment in a phase III trial (STAMPEDE trial, ISRCTN78818544, Medical Research Council, UK), to our knowledge, no other studies have been published on the predictive usefulness of COX-2 expression in patients with prostate cancer who have been treated with radiotherapy.
We did not note a statistically significant association between COX-2 expression and local failure. In men treated with radiotherapy, local persistence of disease is not assessed systematically by routine prostate biopsies and imaging tests are not helpful. The use of PSA as a determinant of failure has led to earlier diagnosis of recurrence, typically before local progression is diagnosed by digital rectal exam. Therefore, local failure is not as reliable an endpoint after radiotherapy to treat prostate cancer as it is for other malignancies that can be assessed with serial imaging. COX-2 overexpression has been shown to be associated with local responses of cervical, oesophageal, and rectal cancer to radiotherapy with or without chemotherapy.35–37 Since local failure leads to distant failure in patients with prostate cancer,38–41 COX-2 overexpression might lead to local persistence of disease and, consequently, a greater incidence of distant metastasis. Alternatively, COX-2 overexpression might be a risk factor for early metastasis, which might be supported by the finding that COX-2 overexpression is associated with angiogenesis.42,43
Miyamoto and colleagues44 proposed that androgen deprivation activates the AKT, COX-2, and matrix metalloprotease-9 (MMP-9) pathways, promoting the transition from androgen sensitivity to resistance. By combining androgen deprivation with inhibitors of these pathways, including COX-2 inhibitors, the androgen-responsive state might be prolonged. COX-2 inhibitors have been shown to induce apoptosis in prostate-cancer cell lines regardless of their androgen-receptor status.45,46 Furthermore, COX-2 inhibitors are known radiosensitisers of different cancer types.17,47 By use of in-vitro and in-vivo methods, Wen and co-workers15 showed radiosensitisation of the androgen-resistant prostate-cancer cell line, DU145, by the COX-2 inhibitor, NS398. Immunoblot studies confirmed the down-regulation of COX-2 concentrations. In a phase II clinical trial of patients with prostate cancer who had recurrent disease after prostatectomy or radiotherapy, Pruthi and colleagues34 highlighted the effectiveness of the selective COX-2 inhibitor, celecoxib, in decreasing patients’ PSA concentrations, thereby possibly delaying the need for androgen treatment. The results of this current study lend support to an association between COX-2 expression and response to androgen deprivation treatment.
As a dichotomous variable around the median COX-2 staining intensity score, COX-2 was a relatively weak correlate of patient outcome in our study. However, our data indicate that the length of androgen deprivation when combined with radiotherapy has a large effect on the association between COX-2 overexpression and outcome. We noted a significant increase in biochemical failure in those who overexpressed COX-2 and who were treated with STAD plus radiotherapy; this association of COX-2 to outcome was not noted in patients who were treated with LTAD plus radiotherapy. Since we did not record a difference in the time from randomisation to the start of radiotherapy for either treatment group (STAD group: mean 62.0 days, median 60 days [range 15–150]; LTAD group: mean 61.3 days, median 60 days [range 7–181]), the increased number of failures was not due to a variation in the length of pre-radiotherapy androgen deprivation. The results suggest that LTAD might overcome the effects of COX-2 overexpression. Woodward and co-workers48 postulated that the additive effect of androgen deprivation on radiotherapy in the treatment of prostate cancer arises from inhibition of vascular endothelial growth factor (VEGF) by androgen deprivation, followed by endothelial cell death in immature tumour vessels. This increases oxygenation to the tumour population, resulting in radiosensitisation. We propose that by adding a COX-2 inhibitor, the anti-angiogenic effects of COX-2 and androgen deprivation might be compounded, causing additional radiosensitisation.
Quantitative immunohistochemical studies that use image analysis are proving more useful in the analysis of proteins expressed abnormally in tumours, including prostate cancer. However, such studies have shortcomings, including some of the data that have been described in this report. For example, although an estimate of positively stained benign epithelial cells was used as an internal control and an estimate of positively stained colon adenocarcinoma was used as an external control in this study, the quantification of staining intensity by image analysis used in our study might be less precise than suggested by the data reported here. Comparisons of COX-2 expression in tissue from an analogous cohort of men treated with radiotherapy for prostate cancer and the establishment of controls that could be used between laboratories would strengthen the findings. Also, findings noted in the multivariate analyses suggest that age is a significant covariate. The data indicate that increasing age is associated with a decreased number of trial events. This is probably due to the finding that a higher number of older patients, i.e., those aged 70 years or over, died before registering an event (167 older vs 57 younger patients in the distant metastasis analysis, 102 older vs 38 younger patients in the ASTRO biochemical failure analysis, and 110 older vs 37 younger patients in the Phoenix biochemical failure analysis; data not shown). Therefore, younger patients had more opportunity to register an event.
In conclusion, COX-2 expression—used as a continuous variable—was significantly associated with biochemical failure, distant metastasis, and any failure for men with high-risk prostate cancer who were treated with androgen deprivation plus radiotherapy. Although the median cut-off point of COX-2 staining intensity was significant for distant metastasis, this parameter overall was a weak discriminator of outcome. The potential for treatment stratification was more apparent when the patients were subdivided by the protocol randomisation in the RTOG 92-02 trial, i.e., long-term versus short-term androgen deprivation. The data suggest two areas where COX-2 might affect the treatment of patients with prostate cancer. First, COX-2 overexpression seemed to be most discriminating of outcome for patients who received STAD (ie, an increased risk of events was noted). Therefore, COX-2 expression might be useful for selecting patients who require LTAD to overcome this increased risk of events. Second, COX-2 inhibitors might potentially modify responses to radiotherapy with or without androgen deprivation, possibly by enhancing response to radiotherapy and allowing for STAD in those with COX-2 overexpression. Confirmation of the results in an independent cohort of patients with intermediate-risk to high-risk prostate cancer is planned.
Acknowledgments
This study was supported, in part, by grants CA-006927, CA-101984-01, CA-109556, CA-21661, and CA-32115 from the National Cancer Institute, and a grant from the Pennsylvania Department of Health (AP).
Footnotes
Contributors
APD, LYK, AP, and MEHH planned and implemented the COX-2 analysis, and wrote the report. KB did the statistical analyses. VMV, SAR, MAR, HMS, GEH, and WUS designed and contributed to the original RTOG 92-02 protocol. DJG did the central pathological review. All authors reviewed the data and provided input on the analyses and report preparation.
Conflicts of interest
The authors declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 2.Dubois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–73. [PubMed] [Google Scholar]
- 3.Chandrasekharan NV, Dai H, Roos KL, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926–31. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73–78. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura R, Sano H, Masuda C, et al. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer. 2000;89:589–96. [PubMed] [Google Scholar]
- 6.Kirschenbaum A, Klausner AP, Lee R, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology. 2000;56:671–76. doi: 10.1016/s0090-4295(00)00674-9. [DOI] [PubMed] [Google Scholar]
- 7.Madaan S, Abel PD, Chaudhary KS, et al. Cytoplasmic induction and over-expression of cyclooxygenase-2 in human prostate cancer: implications for prevention and treatment. BJU Int. 2000;86:736–41. doi: 10.1046/j.1464-410x.2000.00867.x. [DOI] [PubMed] [Google Scholar]
- 8.Rubio J, Ramos D, Lopez-Guerrero JA, et al. Immunohistochemical expression of Ki-67 antigen, COX-2 and Bax/Bcl-2 in prostate cancer; prognostic value in biopsies and radical prostatectomy specimens. Eur Urol. 2005;48:745–51. doi: 10.1016/j.eururo.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Zha S, Gage WR, Sauvageot J, et al. Cyclooxygenase-2 is up-regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res. 2001;61:8617–23. [PubMed] [Google Scholar]
- 10.Shahedi K, Lindstrom S, Zheng SL, et al. Genetic variation in the COX-2 gene and the association with prostate cancer risk. Int J Cancer. 2006;119:668–72. doi: 10.1002/ijc.21864. [DOI] [PubMed] [Google Scholar]
- 11.Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–11. [PubMed] [Google Scholar]
- 12.Kirschenbaum A, Liu X, Yao S, Levine AC. The role of cyclooxygenase-2 in prostate cancer. Urology. 2001;58(2 suppl 1):127–31. doi: 10.1016/s0090-4295(01)01255-9. [DOI] [PubMed] [Google Scholar]
- 13.Liu CH, Chang SH, Narko K, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–69. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 14.Dandekar DS, Lokeshwar BL. Inhibition of cyclooxygenase (COX)-2 expression by Tet-inducible COX-2 antisense cDNA in hormone-refractory prostate cancer significantly slows tumor growth and improves efficacy of chemotherapeutic drugs. Clin Cancer Res. 2004;10:8037–47. doi: 10.1158/1078-0432.CCR-04-1208. [DOI] [PubMed] [Google Scholar]
- 15.Wen B, Deutsch E, Eschwege P, et al. Cyclooxygenase-2 inhibitor NS398 enhances antitumor effect of irradiation on hormone refractory human prostate carcinoma cells. J Urol. 2003;170:2036–39. doi: 10.1097/01.ju.0000092239.98832.52. [DOI] [PubMed] [Google Scholar]
- 16.Palayoor ST, Arayankalayil MJ, Shoaibi A, Coleman CN. Radiation sensitivity of human carcinoma cells transfected with small interfering RNA targeted against cyclooxygenase-2. Clin Cancer Res. 2005;11:6980–86. doi: 10.1158/1078-0432.CCR-05-0326. [DOI] [PubMed] [Google Scholar]
- 17.Nakata E, Mason KA, Hunter N, et al. Potentiation of tumor response to radiation or chemoradiation by selective cyclooxygenase-2 enzyme inhibitors. Int J Radiat Oncol Biol Phys. 2004;58:369–75. doi: 10.1016/j.ijrobp.2003.09.061. [DOI] [PubMed] [Google Scholar]
- 18.Raju U, Ariga H, Dittmann K, Nakata E, Ang KK, Milas L. Inhibition of DNA repair as a mechanism of enhanced radioresponse of head and neck carcinoma cells by a selective cyclooxygenase-2 inhibitor, celeCOXib. Int J Radiat Oncol Biol Phys. 2005;63:520–28. doi: 10.1016/j.ijrobp.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Kamijo T, Sato T, Nagatomi Y, Kitamura T. Induction of apoptosis by cyclooxygenase-2 inhibitors in prostate cancer cell lines. Int J Urol. 2001;8:S35–39. doi: 10.1046/j.1442-2042.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 20.Shigemura K, Shirakawa T, Wada Y, Kamidono S, Fujisawa M, Gotoh A. Antitumor effects of etodolac, a selective cyclooxygenase-II inhibitor, against human prostate cancer cell lines in vitro and in vivo. Urology. 2005;66:1239–44. doi: 10.1016/j.urology.2005.06.076. [DOI] [PubMed] [Google Scholar]
- 21.Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21:3972–78. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Pollack A, DeSilvio M, Khor LY, et al. Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy plus androgen deprivation: Radiation Therapy Oncology Group Trial 92-02. J Clin Oncol. 2004;22:2133–40. doi: 10.1200/JCO.2004.09.150. [DOI] [PubMed] [Google Scholar]
- 23.Faith DA, Isaacs WB, Morgan JD, et al. Trefoil factor 3 overexpression in prostatic carcinoma: prognostic importance using tissue microarrays. Prostate. 2004;61:215–27. doi: 10.1002/pros.20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox JD, Gallagher MJ, Hammond EH, Kaplan RS, Schellhammer PF. Consensus statements on radiation therapy of prostate cancer: guidelines for prostate re-biopsy after radiation and for radiation therapy with rising prostate-specific antigen levels after radical prostatectomy. American Society for Therapeutic Radiology and Oncology Consensus Panel. J Clin Oncol. 1999;17:1155. doi: 10.1200/JCO.1999.17.4.1155. [DOI] [PubMed] [Google Scholar]
- 25.Roach M, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Kishi K, Petersen S, Petersen C, et al. Preferential enhancement of tumor radioresponse by a cyclooxygenase-2 inhibitor. Cancer Res. 2000;60:1326–31. [PubMed] [Google Scholar]
- 27.Liao Z, Milas L. COX-2 and its inhibition as a molecular target in the prevention and treatment of lung cancer. Expert Rev Anticancer Ther. 2004;4:543–60. doi: 10.1586/14737140.4.4.543. [DOI] [PubMed] [Google Scholar]
- 28.Rich TA, Shepard R. COX-2 inhibitors as radiation sensitizers for upper GI tract cancers: esophagus, stomach, and pancreas. Am J Clin Oncol. 2003;26:S110–13. doi: 10.1097/01.COC.0000074148.37768.3E. [DOI] [PubMed] [Google Scholar]
- 29.Shappell SB, Manning S, Boeglin WE, et al. Alterations in lipoxygenase and cyclooxygenase-2 catalytic activity and mRNA expression in prostate carcinoma. Neoplasia. 2001;3:287–303. doi: 10.1038/sj.neo.7900166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee LM, Pan CC, Cheng CJ, Chi CW, Liu TY. Expression of cyclooxygenase-2 in prostate adenocarcinoma and benign prostatic hyperplasia. Anticancer Res. 2001;21:1291–94. [PubMed] [Google Scholar]
- 31.Dassesse T, de Leval X, de Leval L, Pirotte B, Castronovo V, Waltregny D. Activation of the thromboxane A2 pathway in human prostate cancer correlates with tumor Gleason score and pathologic stage. Eur Urol. 2006;50:1021–31. doi: 10.1016/j.eururo.2006.01.036. discussion 1031. [DOI] [PubMed] [Google Scholar]
- 32.Di Lorenzo G, De Placido S, Autorino R, et al. Expression of biomarkers modulating prostate cancer progression: implications in the treatment of the disease. Prostate Cancer Prostatic Dis. 2005;8:54–59. doi: 10.1038/sj.pcan.4500768. [DOI] [PubMed] [Google Scholar]
- 33.Cohen BL, Gomez P, Omori Y, et al. Cyclooxygenase-2 (COX-2) expression is an independent predictor of prostate cancer recurrence. Int J Cancer. 2006;119:1082–87. doi: 10.1002/ijc.21749. [DOI] [PubMed] [Google Scholar]
- 34.Pruthi RS, Derksen JE, Moore D, et al. Phase II trial of celeCOXib in prostate-specific antigen recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. Clin Cancer Res. 2006;12:2172–77. doi: 10.1158/1078-0432.CCR-05-2067. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa H, Ohno T, Kato S, et al. Cyclooxygenase-2 impairs treatment effects of radiotherapy for cervical cancer by inhibition of radiation-induced apoptosis. Int J Radiat Oncol Biol Phys. 2006;66:1347–55. doi: 10.1016/j.ijrobp.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Xi H, Baldus SE, Warnecke-Eberz U, et al. High cyclooxygenase-2 expression following neoadjuvant radiochemotherapy is associated with minor histopathologic response and poor prognosis in esophageal cancer. Clin Cancer Res. 2005;11:8341–47. doi: 10.1158/1078-0432.CCR-04-2373. [DOI] [PubMed] [Google Scholar]
- 37.Smith FM, Reynolds JV, Kay EW, et al. COX-2 overexpression in pretreatment biopsies predicts response of rectal cancers to neoadjuvant radiochemotherapy. Int J Radiat Oncol Biol Phys. 2006;64:466–72. doi: 10.1016/j.ijrobp.2005.07.961. [DOI] [PubMed] [Google Scholar]
- 38.Kuban DA, el-Mahdi AM, Schellhammer PF. Prognosis in patients with local recurrence after definitive irradiation for prostatic carcinoma. Cancer. 1989;63:2421–25. doi: 10.1002/1097-0142(19890615)63:12<2421::aid-cncr2820631208>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Zagars GK, von Eschenbach AC, Ayala AG, Schultheiss TE, Sherman NE. The influence of local control on metastatic dissemination of prostate cancer treated by external beam megavoltage radiation therapy. Cancer. 1991;68:2370–77. doi: 10.1002/1097-0142(19911201)68:11<2370::aid-cncr2820681107>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 40.Fuks Z, Leibel SA, Wallner KE, et al. The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with 125I implantation. Int J Radiat Oncol Biol Phys. 1991;21:537–47. doi: 10.1016/0360-3016(91)90668-t. [DOI] [PubMed] [Google Scholar]
- 41.Coen JJ, Zietman AL, Thakral H, Shipley WU. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002;20:3199–205. doi: 10.1200/JCO.2002.01.086. [DOI] [PubMed] [Google Scholar]
- 42.Liu XH, Kirschenbaum A, Yao S, et al. Upregulation of vascular endothelial growth factor by cobalt chloride-simulated hypoxia is mediated by persistent induction of cyclooxygenase-2 in a metastatic human prostate cancer cell line. Clin Exp Metastasis. 1999;17:687–94. doi: 10.1023/a:1006728119549. [DOI] [PubMed] [Google Scholar]
- 43.Fujita H, Koshida K, Keller ET, et al. Cyclooxygenase-2 promotes prostate cancer progression. Prostate. 2002;53:232–40. doi: 10.1002/pros.10152. [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto H, Altuwaijri S, Cai Y, Messing EM, Chang C. Inhibition of the Akt, cyclooxygenase-2, and matrix metalloproteinase-9 pathways in combination with androgen deprivation therapy: potential therapeutic approaches for prostate cancer. Mol Carcinog. 2005;44:1–10. doi: 10.1002/mc.20121. [DOI] [PubMed] [Google Scholar]
- 45.Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–49. [PubMed] [Google Scholar]
- 46.Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- 47.Davis TW, Hunter N, Trifan OC, Milas L, Masferrer JL. COX-2 inhibitors as radiosensitizing agents for cancer therapy. Am J Clin Oncol. 2003;26:S58–61. doi: 10.1097/01.COC.0000074158.59269.9F. [DOI] [PubMed] [Google Scholar]
- 48.Woodward WA, Wachsberger P, Burd R, Dicker AP. Effects of androgen suppression and radiation on prostate cancer suggest a role for angiogenesis blockade. Prostate Cancer Prostatic Dis. 2005;8:127–32. doi: 10.1038/sj.pcan.4500779. [DOI] [PubMed] [Google Scholar]