Abstract
Background
Activating somatic mutations in epidermal growth factor receptor (EGFR) confer unique biologic features to non-small cell lung cancer (NSCLC) cells, but the transcriptional mediators of EGFR in this subgroup of NSCLC have not been fully elucidated.
Methodology/Principal Findings
Here we used genetic and pharmacologic approaches to elucidate the transcriptomes of NSCLC cell lines. We transcriptionally profiled a panel of EGFR-mutant and -wild-type NSCLC cell lines cultured in the presence or absence of an EGFR tyrosine kinase inhibitor. Hierarchical analysis revealed that the cell lines segregated on the basis of EGFR mutational status (mutant versus wild-type), and expression signatures were identified by supervised analysis that distinguished the cell lines based on mutational status (wild-type versus mutant) and type of mutation (L858R versus Δ746-750). Using an EGFR mutation-specific expression signature as a probe, we mined the gene expression profiles of two independent cohorts of NSCLC patients and found the signature in a subset. EGFR tyrosine kinase inhibitor treatment regulated the expression of multiple genes, and pharmacologic inhibition of the protein products of two of them (PTGS2 and EphA2) inhibited anchorage-independent growth in EGFR-mutant NSCLC cells.
Conclusions/Significance
We have elucidated genes not previously associated with EGFR-mutant NSCLC, two of which enhanced the clonogenicity of these cells, distinguishing these mediators from others previously shown to maintain cell survival. These findings have potential clinical relevance given the availability of pharmacologic tools to inhibit the protein products of these genes.
Introduction
Treatment with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) leads to rapid and sustained tumor shrinkage in a subset of patients with non–small cell lung cancer (NSCLC) [1]–[3]. The tumor cells in these patients have somatic mutations in the EGFR kinase domain that constitutively activate EGFR [1]–[3]. The activating mutations identified thus far cluster in the region that encodes the kinase domain (exons 18–21) and are most frequently either Δ746–750 deletion or L858R point mutations [1]–[3]. Mouse models constructed to investigate the oncogenicity of mutant EGFR develop invasive lung adenocarcinomas that regress after treatment with EGFR TKIs [4;5]. Immortalized human bronchial epithelial cells acquire malignant properties after transfection with mutant EGFR [6]. Treatment with EGFR TKIs induces apoptosis of these EGFR-transfected cells and NSCLC cells with somatic mutations in EGFR [7;8]. Thus, evidence from human, murine, and cellular models indicates that mutant EGFR is oncogenic and confers dependence on EGFR for NSCLC cell survival.
The potency of mutant EGFR as an oncogene is supported by evidence that its biochemical properties differ sharply from those of wild-type EGFR. The EGFR kinase domain is constitutively activated by the somatic mutations, and it displays enhanced binding and sensitivity to EGFR TKIs [9]–[11]. EGFR-mutant NSCLC cells typically express high levels of EGFR and its dimeric partners ErbB2 and ErbB3 and multiple ErbB ligands, all of which potentiate EGFR-dependent signaling [8]. Multiple signaling pathways are constitutively activated in these cells, some of which have been shown to promote cell survival. For example, EGFR forms a heterodimeric complex with ErbB3, which binds to and directly activates phosphatidylinositol 3-kinase and maintains cell survival through AKT-dependent mechanisms [12]. Other pro-survival signals are mediated through Src family kinases, which are constitutively activated in EGFR-mutant NSCLC cells [13;14].
In contrast to the advances made in elucidating mediators of cell survival, less progress has been made in understanding the mechanisms by which mutant EGFR confers other neoplastic properties, such as the ability to migrate, invade, proliferate in an anchorage-independent manner, and promote angiogenesis. Here we sought to identify those mediators by interrogating the transcriptomes of a panel of NSCLC cell lines that have been characterized for the presence of EGFR mutations. We found a transcriptional profile of EGFR-mutant NSCLC cells that included genes not previously been known to be EGFR-dependent. Although the range of cellular functions attributed to these genes is broad, many of them are linked through known or predicted protein interaction networks. In conclusion, the transcriptional profile identified in EGFR-mutant NSCLC cells has informed us about biologic processes and potential therapeutic targets that could be effective in patients with this disease.
Results
Transcriptional Analysis of NSCLC Cell Lines
We used a panel of eight NSCLC cell lines (Table 1) that had been characterized for the presence or absence of somatic EGFR mutations (five cell lines with such mutations and three without) and Ras mutations (two cell lines with such mutations and six without). Of the five EGFR-mutant cell lines, three had exon 19 deletion mutations (Δ746–750) (HCC827, HCC2279, H4006), one had an exon 21 point mutation (L858R) (H3255), and one had L858R in combination with the T790M gatekeeper mutation that confers resistance to EGFR TKIs (H1975). Of the three EGFR-wild-type cell lines, one had a somatic mutation in N-ras (H1299), and one had a K-ras mutation (H460). RNA was extracted and prepared from cells after they had been cultured for 2 h at 70% confluence in the presence or absence of the EGFR TKI gefitinib (1.0 µM). This duration of TKI treatment was chosen to identify the earliest transcriptional events induced by EGFR inhibition and to minimize the detection of gene expression changes due to apoptosis. The RNA was subjected to Affymetrix gene expression profiling, and the profiles were examined for differences in gene expression at baseline and after TKI treatment.
Table 1. Characteristics of NSCLC Cell Lines.
| Cell Line | EGFR | Gefitinib IC50 | Ras |
| H1299 | WT | 38.0 µM | N-RasQ61K |
| H1819 | WT | 4.7 µM | WT |
| H460 | WT | 8.0 µM | K-RasQ61H |
| H1975 | L858R, T790M | 1.9 µM | WT |
| HCC2279 | Δ746–750 | 5.0 µM | WT |
| H3255 | L858R | 9.0 nM | WT |
| H4006 | Δ746–750 | 30.0 nM | WT |
| HCC827 | Δ746–750 | 16.0 nM | WT |
Abbreviations: WT, wild-type; IC50 , 50% inhibitory concentration Gefitinib IC50 values have been reported (Fujimoto et al., 2005).
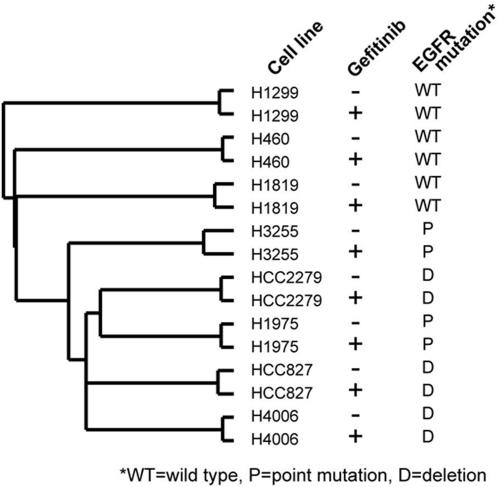
We first examined EGFR mutational status as a classifier of the transcriptional profiles. Hierarchical analysis revealed clustering of the cell lines based on mutational status; the EGFR-mutant cells lines clearly segregated from the -wild-type cell lines (Fig. 1). However, there was no clear separation between the two types of mutations (L858R versus Δ746–750) (Fig. 1). By supervised analysis using specific criteria (at least a 2.0-fold increase or a 50% decrease in EGFR-mutant cell lines relative to that of wild-type, P<0.05), 194 unique genes were identified that distinguished the EGFR-mutant cell lines from the –wild-type cell lines. These genes are listed in File S1 and illustrated in a clustered heat map in Fig. 2A . We examined 29 of the 194 genes by quantitative PCR and validated that 19 (68%) of them were differentially expressed between EGFR-mutant and –wild-type cell lines (File S2).
Figure 1. Hierarchical analysis of gene expression profiles in eight NSCLC cell lines.
Dendrogram illustrating the relatedness of expression profiles from cell lines treated with (+) or without (−) gefitinib. The presence or absence of EGFR somatic mutations and the type of mutations, including L858R point mutation (P) or Δ746–750 deletion mutation (D), are indicated. WT, wild-type.
Figure 2. Identification of a mutant EGFR gene expression profile.
(A) A 194-gene signature that distinguishes EGFR-wild-type (WT) from -mutant NSCLC cell lines (treated with [+] or without [−] gefitinib) is aligned with the expression profiles from (B) the Michigan cohort (86 patients) and the Harvard cohort (84 patients) and (D) MCF-7 cells transfected with the indicated genes. A list of the genes that overlapped in all three data sets is indicated on the far right. (C) Numbers of patients in the Michigan and Harvard cohorts manifesting the mutant EGFR expression patterns (P<0.05, Pearson's correlation), along with the number manifesting a randomly generated pattern (SD based on 100 simulations). (E) Venn diagram illustrating the overlap between signatures from the EGFR-mutant NSCLC cells and EGFR-transfected MCF-7 cells.
Identification of the Mutant EGFR Signature in Cohorts of Patients with NSCLC
We next queried publicly available gene expression databases of tumor biopsy samples derived from two independent cohorts of patients with NSCLC from the United States (15, 16) to determine whether a subset of tumors expressed this genetic signature. Of the 194 genes, 102 (53%) were represented on the profiling platform used in the Harvard cohort, and 65 (34%) were represented in the Michigan cohort. Based on a heat map representation of their gene expression patterns, the human lung tumors were heterogeneous in their expression patterns; however, a subset of tumors (9 of 84 [11%] in the Harvard cohort and 10 of 86 [12%] in the Michigan cohort) exhibited an expression pattern similar to that of the EGFR-mutant NSCLC gene signature (Fig. 2B ).
To determine whether the similarities observed by heat map analysis reached statistical significance, parameters were created that defined similarity as a positive Pearson's correlation of P<0.05 (two-sided) between the mutant EGFR signature pattern and the gene expression values of the tumor, taking into account both the over- and under-expressed genes in the signature. By this definition, tumors manifesting this signature would recapitulate the patterns of over-and under-expression observed in the EGFR-mutant cell lines. The incidence of tumors manifesting the signature was statistically significant (P<0.01 for each cohort) based on simulations using 100 randomly selected gene signatures generated from each of the cohorts (Fig. 2C).
Although the EGFR mutational status of tumors from these patient cohorts has, to our knowledge, not been reported, K-ras (codons 12, 13, or 61) is known to be mutated in 29% and 45% of the tumors from the Harvard and Michigan cohorts, respectively [15;16]. Somatic mutations in EGFR and K-ras are mutually exclusive in NSCLC [17], which has led investigators to hypothesize that these events are genetically redundant and that a change in both genes does not confer a further advantage when these events occur together in the same cell [17]. We postulated that, if these somatic events are genetically redundant, then having the K-ras mutation will confer the mutant EGFR transcriptional profile. Consistent with this idea, we noted that K-ras mutational status correlated, albeit weakly, with the mutant EGFR gene signature (Fig. 2B, P = 0.07 and P = 0.04 in the Harvard and Michigan cohorts, respectively, by Wilcoxon rank-sum tests of the cohort profiles ordered by average expression of genes that were increased in EGFR-mutant cell lines).
Mutant EGFR Signature Enriched with EGFR-Dependent Genes
To investigate whether any of the genes in the 194-gene signature were regulated in an EGFR-dependent manner, we queried a publicly available database of MCF-7 breast cancer cells that had been stably transfected with constitutively active kinases (Raf1, MEK1, ErbB2) or with wild-type EGFR, which was activated by short-term EGF treatment [18]. We investigated the overlap between the gene signatures from EGFR-transfected MCF-7 cells and EGFR-mutant NSCLC cells. Of the 194 genes in the mutant EGFR signature, 139 (72%) were represented on the profiling platform for the MCF-7 cell transfectants (11079 genes in all were represented in both the MCF-7 and NSCLC datasets). Analysis of these 139 genes revealed that subsets of the genes that were increased in MCF-7 cells because of MEK, Raf1, or EGFR transfection overlapped with those in the mutant EGFR expression signature in NSCLC cells (Fig. 2D ).
Of the 119 genes that were both represented in the MCF-7 dataset and increased in EGFR-mutant NSCLC cells, 44 (31%) were increased with P<0.05 in EGFR-transfected MCF-7 cells, which represented a highly significant overlap (P<1E–12, one-sided Fisher's exact test, Fig. 2E ). By chance, 14 of the 119 genes would be expected to overlap, indicating that the amount of overlap we observed exceeded what would be expected if the EGFR-transfected MCF-7 cells and EGFR-mutant NSCLC cells nothing biologically in common with each other. When we used a more stringent cut point of P<0.01 instead of P<0.05 to define genes that were increased in EGFR-transfected MCF-7 cells (576 genes in all), 28 overlapped with the mutant EGFR NSCLC signature (chance expected of seven genes, P<1E–10, one-sided Fisher's exact test). We concluded that, based on its commonalities with the signature from EGFR-transfected MCF-7 cells, the signature from EGFR-mutant NSCLC cells was enriched for genes transcriptionally up-regulated by EGFR.
Identification of PTGS2 as a gene required for anchorage-independent growth
The genes that were differentially expressed in EGFR-mutant NSCLC cell lines fell into a broad range of functional classes (categorized in the Gene Ontology Molecular Functions, www.geneontology.org) (Files S3 and S4). The categories with highest representation were signal transduction, metabolism, immune response, ion transport, and cell cycle and proliferation. Genes in these categories that were highly expressed included those encoding ErbB ligands (TGFA, AREG, and EREG), cyclooxygenase-2 (PTGS2), a ligand for the CX3CR1 chemokine receptor (CX3CL1), intracellular and transmembrane kinases (TRIB2, MET, MYLK, STK39, ACVR1, TAOK3, and IFIH1), protein phosphatases (PTPN22, DUSP10, PPAP2B, PTPRR, and PTPRE), a lipid phosphatase (SGPP2), adhesion molecules (CEACAM6, ITGAV, PCDH7, and THBS1), and calcium ion-binding proteins (S100A14 and S100A16).
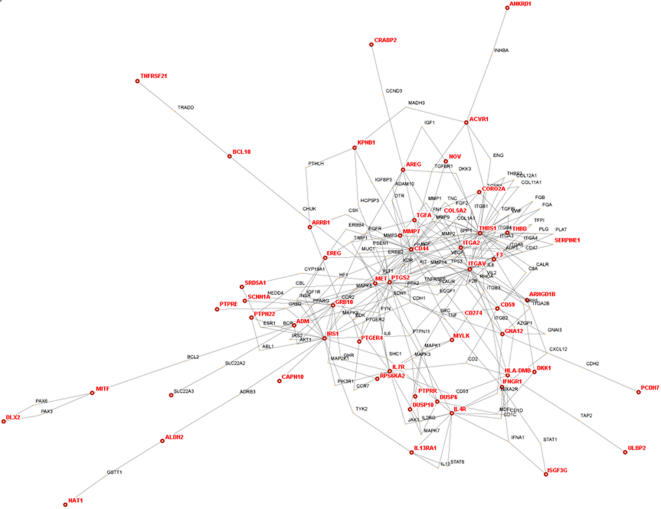
To examine whether these differentially expressed genes, which have disparate biologic functions, were part of one or more signal transduction networks, we analyzed their positions within known or predicted global protein interaction networks (interactomes) using the HiMAP software program (http://www.himap.org/index.jsp). Interactomes identified by this approach are organized into a series of modular structures characterized by centrally-located nodes (called hubs) that have multiple connections with other proteins [19]. Although this approach is purely exploratory and carries no statistical weight, findings in yeast show that centrality in a protein interactome predicts a protein's biological importance [20].
Of the 194 differentially expressed genes, 118 had been annotated in Gene Ontology, of which 102 were included in the HiMAP software program (19). Of those 102 genes, 52 mapped within a single network (Fig. 3). The hubs in the network with the highest numbers of links (≥ 10) included CD44, MET, IRS1, GRB10, ITGA2, PTGS2, and THBS1, all of which were highly expressed in EGFR-mutant NSCLC cells, indicating that some of the differentially expressed genes were at central positions of this signaling network.
Figure 3. Interactome of genes in the mutant EGFR expression signature.
Theoretical protein-protein physical and functional interaction map (interactome) was drawn using HiMAP software. Genes from the signature are indicated in red.
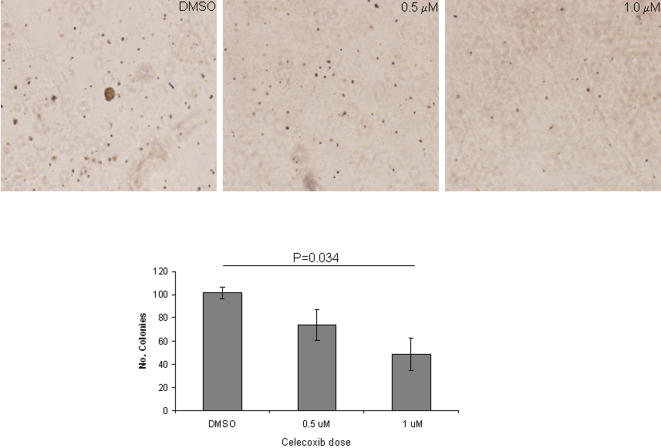
In light of the centrality of the PTGS2 gene in the interactome network and the known importance of its gene product cyclooxygenase-2 (COX-2) in the survival and metastasis of cancer cells and its potential clinical impact given the availability of pharmacologic tools to inhibit its enzymatic function [21], we sought to investigate its role in EGFR-mutant NSCLC cells. We examined whether the cyclooxygenase-2 inhibitor celecoxib abrogated the ability of these cells to proliferate in monolayer cultures and to form colonies in soft agar. Using celecoxib at low doses (0.5 µM and 1.0 µM) to minimize nonspecific effects, colony formation in soft agar decreased in a dose-dependent manner (Fig. 4), whereas proliferation in monolayers did not change (data not shown).
Figure 4. Cyclooxygenase-2 inhibition decreases NSCLC anchorage-independent growth.
Representative images of colonies of NSCLC cell lines (upper panels) were quantified (lower panels) after growing them in soft agar in the presence or absence of celecoxib. Results are the means of at least three independent experiments.
Cell Lines with EGFR Deletions and Point Mutations Have Distinct Expression Profiles
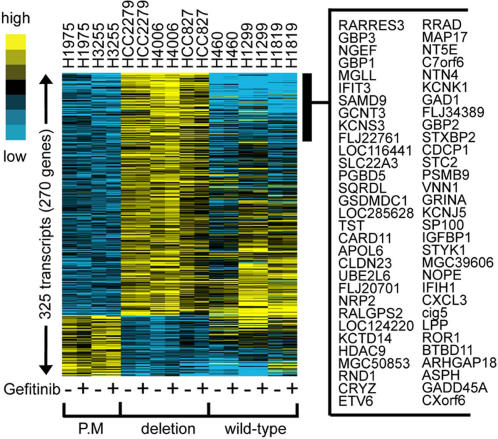
Among patients with EGFR-mutant NSCLC, differences in survival duration and responsiveness to EGFR TKI treatment have been observed depending on the type of EGFR somatic mutation (exon 21 point mutations versus exon 19 deletions) [22;23], suggesting that these two types of somatic mutations confer distinct biologic properties to NSCLC cells. Supporting this possibility is evidence that these two types of somatic mutations confer distinct biochemical properties to EGFR [9]–[11]. Although hierarchical clustering did not segregate the two types of mutations into two distinct subgroups (Fig. 1), we hypothesized that supervised analysis would reveal transcriptional differences between the two types of mutations. Indeed, clear transcriptional differences were observed. Using specific criteria (P<0.01, at least two-fold change), we identified a 270-gene signature in EGFR-mutant cell lines (which was not present in EGFR-wild-type cell lines) that distinguished the two types of mutations. These genes are listed in File S5 and illustrated in a clustered heat map in Fig. 5. We examined 7 of the 270 genes by quantitative PCR and validated that 4 (57%) were differentially expressed between cell lines with L858R mutations versus those with Δ746–750 mutations (File S6). The 270-gene signature was analyzed for enrichment in specific gene functions as defined by the Gene Ontology Signature Database. L858R-mutant cells were enriched for genes in the cyclic AMP-dependent protein kinase- , protein phosphatase-2ε-, Ras-family member RAB3D-, and phospholipase-Cβ1-dependent pathways, whereas the Δ746–750 mutants were enriched for genes in the CXC chemokine ligands (2 and 3)-, integrin α6-, guanylate binding proteins (1, 2, and 3)-, and interleukin-7 and 10-dependent pathways (File S7), demonstrating that the gene sets activated by the two mutation types are functionally distinct.
Figure 5. Expression signature that distinguishes two types of EGFR mutations.
A 194-gene signature present in EGFR-mutant but not EGFR–wild-type cell lines distinguishes L858R (P.M. [point mutation]) from Δ746–750 (deletion). Cells were treated with (+) or without (−) gefitinib. A partial list of the genes that are differentially expressed in the two groups is indicated on the right.
Genes Regulated by EGFR TKI Treatment
To identify gene expression changes that preceded, and possibly contributed to, the biologic effects of EGFR TKI treatment on EGFR-mutant NSCLC cells, the cells were subjected to short-term treatment with gefitinib. As a negative control in this experiment, we used the TKI-resistant H1975 cells, which have a T790M mutation that blocks binding to the EGFR TKI [17]. Using specific criteria (P<0.05), we found that 54 genes were regulated by TKI exclusively in the TKI-sensitive cell lines (HCC827, H3255, and H4006) (File S8), none of which have, to our knowledge, been reported to be EGFR-dependent genes. Among these genes, we examined 14 by quantitative PCR and validated that 10 (71%) were differentially regulated in TKI-sensitive and resistant cells (File S9). Genes that increased in response to TKI included, among others, cell cycle regulators (CCNG2, CDKN1B, ID2, and KNTC2) and a ligand for EphA2 (EFNA1). Genes that decreased include the EphA2 receptor tyrosine kinase, cytokines (LIF, CCL20, and IL17B), transcription factors (FOXD1 and POU1F1), and protein phosphatases (DUSP4 and DUSP6).
Reciprocal Regulation of EphA2 and EFNA1 is EGFR-Dependent
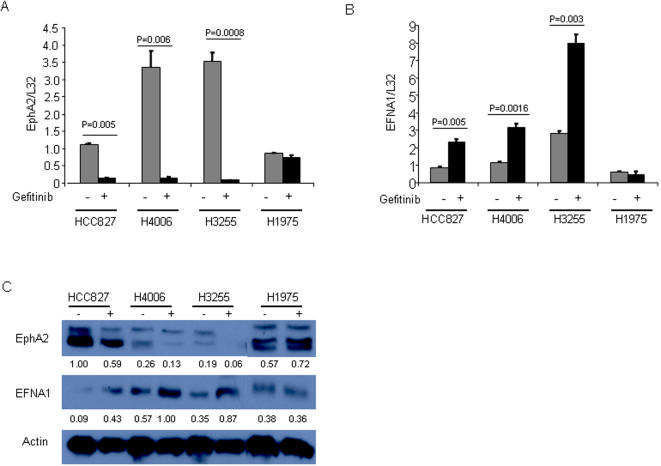
In light of the importance of the EphA axis in tumorigenesis [24;25], we further investigated the effects of TKI treatment on the expression of EphA2, other EphA family members, and their ligand EFNA1. Quantitative PCR and western analysis confirmed that gefitinib reciprocally regulated the expression of EphA2 and its ligand EFNA1 in TKI-sensitive cell lines (HCC827, H4006, and H3255) but not in the TKI-resistant H1975 cells (Fig. 6A–C ). EphA1, EphA5, and EphA6 did not change with gefitinib treatment (Fig. 7A, E, and F ); EphA4 decreased in only a subset of TKI-sensitive cells (H4006 but not HCC827) (Fig. 7C ); and EphA3 was inhibited in an EGFR-independent manner (indicated by the TKI-induced suppression in H1975 cells) (Fig. 7B ). To more fully evaluate whether the suppression of EphA2 expression was EGFR-mediated, we examined the effect of another EGFR TKI, erlotinib, and found that it too inhibited EphA2 expression in TKI-sensitive cells, to an extent similar to that of gefitinib (Fig. 7F ).
Figure 6. Reciprocal regulation of EphA2 and EFNA1 in NSCLC cell lines.
Quantitative PCR analysis (A, B) and western blotting (C) of EphA2 (A, C) and EFNA1 (B, C) in cell lines treated for 6 h with (+) or without (−) gefitinib. Quantitative PCR results represent the means of at least three independent experiments and were normalized based on expression of the housekeeping gene L32. The numbers under the bands are the results of densitometric analysis after normalizing for loading differences based on actin.
Figure 7. EGFR TKI-induced changes in expression of EphA family members.
Cells were treated for 6 h with vehicle, 1 µM gefitinib (A–E), or 1 µM erlotinib (F). RNA was extracted and subjected to quantitative PCR. Results represent the means of at least three independent experiments and were normalized based on L32 expression.
EphA2 Activation is Required for Anchorage-Independent Growth
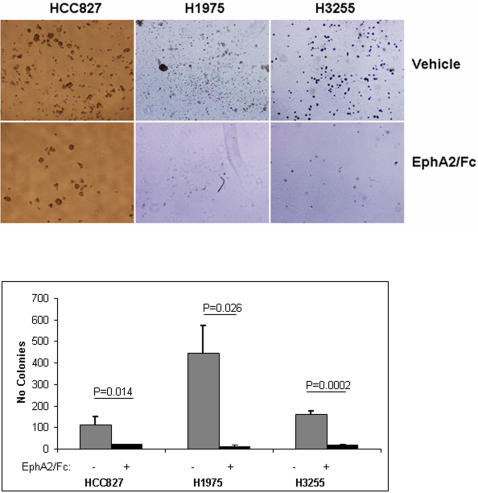
We postulated that EphA2 signaling maintains neoplastic features of NSCLC cells and tested this hypothesis by treating HCC827 cells with EphA2-Fc, a recombinant peptide containing the EphA2 extracellular domain fused to the Fc fragment of IgG, which prevents the interaction of ephrin A ligands with endogenous EphA, effectively blocking EphA activation [26]. Relative to that of controls, EphA2-Fc-treated cells exhibited decreased colony formation in soft agar (Fig. 8) whereas their proliferation in monolayer cultures did not change (data not shown), indicating that EphA was required for the anchorage-independent proliferation of these cells.
Figure 8. EphA2-Fc inhibits NSCLC anchorage-independent growth.
Representative images of colonies of NSCLC cell lines (top) with quantification of colonies (bottom) after growth in soft agar in the presence or absence of EphA2-Fc. Results are the means of at least three independent experiments.
Discussion
Here we report that NSCLC cells with somatic EGFR mutations have a unique transcriptional profile and that cell lines with the two most common types of EGFR mutations have clear transcriptional differences. By mining gene expression databases using a mutant EGFR-specific signature as a probe, we found that many of the genes in this expression signature were EGFR-dependent, converged into common networks on the basis of known or predicted protein interactomes, and were expressed in tumors from a subset of patients with NSCLC. Two genes were elucidated, EphA2 and PTGS2, that promoted the clonogenicity of EGFR-mutant NSCLC cells, which are of particular interest from a clinical standpoint because they can be inhibited pharmacologically.
Genes within the mutant EGFR gene expression signature encode proteins with a diverse set of cellular functions. The influence of EGFR on this signature was demonstrated by its overlap with that of EGFR-transfected MCF-7 cells and the presence of known EGFR transcriptional targets, including PTGS2, the ErbB ligands EREG and AREG, and Met, a receptor tyrosine kinase that was recently reported to be activated in EGFR-mutant NSCLC cells and to promote TKI resistance in these cells [8;27–29]. Here we showed that the gene product of PTGS2, cyclooxygenase-2, has an important role, promoting anchorage-independent growth. This signature contained many genes not previously known to be highly expressed in EGFR-mutant NSCLC, including LY96 and CX3CL1, which have known immunomodulatory functions. LY96 encodes MD2, an accessory molecule required for the activation of toll-like receptor-4, which promotes cell survival and induces the secretion of immunosuppressive molecules that promote tumor evasion from immune surveillance [30]. CX3CL1 encodes a secreted protein called fractalkine that recruits CX3CR1-expressing natural killer and T lymphocytes to the tumor microenvironment, thereby promoting natural killer-dependent antitumor responses in vivo [31]. On the other hand, fractalkine has also demonstrated pro-metastatic properties based on evidence that it promotes tumor cell migration and enhances adhesion of tumor cells to endothelial cells [32;33].
To identify genes regulated in an EGFR-dependent manner, we treated EGFR-mutant NSCLC cells with gefitinib. Two of the genes identified by this approach, EphA2 and its ligand EFNA1, were regulated in a reciprocal fashion. Potentially mediating this effect of gefitinib, mitogen-activated protein kinase, a downstream effector of EGFR, inhibits EFNA1 expression, thereby relieving the EFNA1-induced suppression of EphA2 expression [25]. Moreover, we found that EphA2/EFNA1 interactions were required for the anchorage-independent growth of HCC827 cells, which corroborates findings from a previous study demonstrating that v-ErbB-dependent cellular transformation is attenuated by EphA2 ligand-binding [25]. Other genes we found to be regulated by gefitinib in TKI-sensitive cells include CCNG2, CDKN1B, ID2, and KNTC2, which are components of cell cycle regulatory pathways. Given that their expression changed before any biochemical evidence of proliferative arrest or apoptosis, these genes might be part of an anti-proliferative signaling program activated by gefitinib. Lastly, two of the genes that decreased in abundance with gefitinib treatment (CEACAM-6 and DUSP6) were also highly expressed in EGFR-mutant cells, suggesting that these genes are potentially important EGFR transcriptional targets in these cells.
In summary, we have identified a transcriptome in NSCLC cells that elucidates mutant EGFR-induced gene expression changes and provides a transcriptional basis for the biologic differences observed in NSCLC with the two most commonly occurring types of EGFR mutations. Further analysis of these genes may inform us about biologic processes that can be used to identify intracellular targets of potential therapeutic benefit for patients with this disease.
Materials and Methods
Reagents
Gefitinib (Astra Zeneca Pharmaceuticals, Wilmington, DE) and erlotinib (OSI Pharmaceuticals, Melville, NY) were gifts. We purchased a recombinant murine EphA2-Fc chimera (R&D Systems, Minneapolis, MN), polyclonal antibodies derived in rabbits against EphA2 and EFNA1 (Santa Cruz Biotechnologies, Santa Cruz, CA), a horseradish peroxidase–linked anti-mouse and ant-rabbit secondary antibodies (Cell Signaling Biotechnology, Beverly, MA), and an antibody against β-actin (Sigma-Aldrich, St. Louis, MO).
Cell Lines
The NSCLC cell lines used in this study were purchased from the American Type Culture Collection (Manassas, VA) and were grown in 5% CO2 at 37°C in RPMI 1640 medium with high glucose (4.5 g/L; GIBCO-BRL, MD), supplemented with 10% fetal bovine serum (Hyclone, Logan, Utah).
Gene Expression Profiling
RNAs were isolated by using the RNeasy Mini Kit (Qiagen, Valencia, CA) and hybridized to Affimetrix U133+2.0 gene expression chips at the M.D. Anderson Cancer Center Microarray Core Facility (supported in part by grant CA #16672). dChip (http://www.dchip.org) (2005 version) was used to extract expression values for each probe set. Control probe sets and probe sets with suffixes “_s_at” and “_x_at” on their ID were excluded (because these probe sets may target more than one unique sequence), leaving 39,114 of 54,675 original probe sets for further analysis.
Determination of differentially expressed genes
Two-sample t tests (using log-transformed data) were used to determine significant differences in gene expression between EGFR-mutant and -wild-type NSCLC cell lines and between EGFR-transfected and parental MCF-7 cells (P values were two-sided). For the analysis of gefitinib treatment effects, a paired difference was calculated as the log2(gefitinib-treated/vehicle-treated).
GO enrichment analysis
Functional gene groups as defined by Gene Ontology (GO) annotation (http://www.geneontology.org) were evaluated for enrichment within our own experimentally-derived gene sets. Essentially as described in [34], one-sided Fisher's exact tests were performed to assess whether or not genes in each GO functional group were over-represented in our gene set (the 16,172 genes represented by the 39,114 probe sets on the array were used as the reference population). Gene Ontology annotations were obtained from the NCBI's annotation file gene2go (ftp://ftp.ncbi.nlm.nih.gov/gene/DATA). In addition to one-sided Fisher's exact P-values, Q-values were computed to account for multiple term testing, using the method by Storey et al. [35].
HiMAP interactome analysis
Gene lists were imported into the HiMAP program (http://www.himap.org/index.jsp) for protein-protein interaction network analysis. HiMAP (19) includes both experimentally-validated protein-protein interactions (as cataloged in the Human Protein Reference Database, or HPRD [www.hprd.org]), and predicted protein-protein interactions based on a probabilistic model integrating multiple factors, including interactome data from the Database of Interacting Proteins [36], protein domain data, genome-wide expression data, and functional annotation data from GO.
Analysis of the EGFR mutation gene signature in additional profile datasets
Expression values were visualized as color maps using the Cluster and Java TreeView programs [37; 38]. Expression values in the human lung adenocarcinoma datasets were transformed to standard deviations from the tumor mean. The set of profiles analyzed in the Harvard dataset were the same set as was analyzed in Beer et al. (15). For correlating the human NSCLC tumor profiles with the mutant EGFR gene signature (Figure 2C), +1 and -1 were used to represent each of the genes up and down, respectively, in the signature. The Pearson's correlation coefficient was computed based on the comparison of the mutant EGFR signature pattern to that of each human NSCLC tumor profile. In 100 separate simulation tests, a randomly generated gene signature (with the same number of up and down genes as there was in the mutant EGFR signature) was generated, and the Pearson's correlation was computed based on the comparison of this random signature to each human NSCLC tumor. In no single test did the number of tumors with significant positive correlations (p<0.05, two-sided) to the random pattern exceed the number that correlated positively with the mutant EGFR signature.
The Entrez Gene identifier was used for mapping genes from the NSCLC cell line dataset to those from the human NSCLC tumor and MCF-7 profile datasets. Where a gene was represented several times on a given platform, an appropriate rule was used to select the “best” gene probe in a manner not biased towards detecting patterns of concordance between datasets (for human lung tumor datasets, the probe with the greatest variation; for MCF-7 datasets, the probe with the greatest difference in either direction by t test between EGFR and control). As an alternative approach to exclude potential bias, overlap between the NSCLC and MCF-7 data sets was examined using probes randomly selected from the NSCLC expression arrays, which did not qualitatively affect the results reported.
Quantitative PCR
The level of mRNA for each gene was measured with SYBR-Green–based real-time PCR. The primers used for real-time PCR were designed by using Primer Express (Applied Biosystems, Foster City, CA). The primer sequences used are listed in File S10. Each cDNA sample (7 µl) was amplified by using SYBR Green PCR Master Mix according to the manufacturer's instructions. The PCR products and their dissociation curves were detected with the 7500 Fast Real-Time PCR System (Applied Biosystems). The level of the housekeeping gene Ribosomal gene Rpl32 (L32) in each sample was used as an internal control.
Western Blotting
Cells were lysed with M-PER Mammalian Protein Extraction Reagent (Pierce, Rockford, IL). Lysates were cleared by centrifugation and protein concentrations were quantified with 1X Quick Start Bradford Dye Reagent (Bio-Rad Laboratories, Hercules, CA) so that equal amounts of protein (40 µg) could be resolved on 10% SDS-polyacrylamide gels. After transfer to membranes, samples were processed and visualized with ECL Western Blotting Reagents (Amersham Biosciences, Piscataway, NJ). All of the Western blotting data shown in this study are representative of at least three independent experiments.
Anchorage-Independent Growth Assay
A bottom layer of agar was prepared in 60-mm wells by using 3 ml of 1% low melting temperature agarose in normal growth medium. Next, 3 ml of 0.5% low melting temperature agarose in normal growth medium containing 1×105 cells was added on top of the solidified bottom layer. Every 3 days, 5 µg of EphA2-Fc dissolved in normal growth medium was added to each plate. Colonies showing anchorage-independent growth were counted 10–30 days later.
Cell Viability Assay
Cell viability was measured with CCK-8 (Dojindo Molecular Technologies, Gaithersburg, MD) according to the manufacturer's protocol, in 96-well plates (seeding density, 5,000 cells per well) at 3 days after treatment with 5 µg/ml of EphA2-Fc or 0.5 µM and 1.0 µM of celecoxib.
Supporting Information
Comparison of Gene Expression in EGFR-mutant and -wild-type NSCLC Cells. Genes listed were increased or decreased in EGFR-mutant NSCLC cell lines (n = 4) relative to that of EGFR wild-type cell lines (n = 3).
(0.06 MB XLS)
Quantitative PCR Analysis of Selected Genes that were Differentially Expressed Based on Expression Profiling of EGFR-mutant and -wild-type NSCLC Cell Lines. Results normalized based on L32 ribosomal RNA expression.
(0.07 MB DOC)
Mutant EGFR expression profile includes genes with diverse functions. Differentially expressed genes were grouped based on their Gene Ontology functions and represented in a pie chart to illustrate their relative abundance.
(0.07 MB TIF)
Mutant EGFR expression profile includes genes with diverse functions. Differentially expressed genes are listed according to their Gene Ontology functions.
(0.43 MB XLS)
Comparison of gene expression in EGFR L858R versus del746-750 NSCLC Cells. Genes listed were increased or decreased in EGFR L858R NSCLC cell lines (n = 2) relative to that of EGFR del746-750 cell lines (n = 3).
(0.08 MB XLS)
Quantitative PCR analysis of selected genes that were differentially expressed in EGFR L858R and δ746-750 NSCLC Cell Lines. Results normalized based on L32 ribosomal RNA expression.
(0.08 MB DOC)
Gene Ontology terms enriched in NSCLC cell lines with EGFR L858R and del746-750.
(0.50 MB XLS)
Comparison of gefitinib-induced gene expression changes in TKI-sensitive and -resistant EGFR-mutant NSCLC cell lines. The fold difference was calculated by log2[Gefitinib-induced change in expression in sensitive cells (HCC827, H3255, H4006)/resistant cells (H1975)].
(0.03 MB XLS)
Quantitative PCR analysis of selected genes that were regulated by gefitinib treatment in TKI-sensitive NSCLC cells
(0.09 MB DOC)
Primers used for quantitative PCR
(0.03 MB XLS)
Acknowledgments
We thank Fang Liu for technical assistance with the expression analysis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Cancer Institute grants P50 CA70907, P30 CA125123, and R01 CA117965.
References
- 1.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, Doherty J, Politi K, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc.Natl.Acad.Sci.U.S.A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, et al. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 2006;20:1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji H, Li D, Chen L, Shimamura T, Kobayashi S, McNamara K, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Sato M, Vaughan MB, Girard L, Peyton M, Lee W, et al. Multiple oncogenic changes (K-RAS(V12), p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells. Cancer Res. 2006;66:2116–2128. doi: 10.1158/0008-5472.CAN-05-2521. [DOI] [PubMed] [Google Scholar]
- 7.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto N, Wislez M, Zhang J, Iwanaga K, Dackor J, et al. High expression of ErbB family members and their ligands in lung adenocarcinomas that are sensitive to inhibition of epidermal growth factor receptor. Cancer Res. 2005;65:11478–11485. doi: 10.1158/0008-5472.CAN-05-1977. [DOI] [PubMed] [Google Scholar]
- 9.Carey KD, Garton AJ, Romero MS, Kahler J, Thomson S, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res. 2006;66:8163–8171. doi: 10.1158/0008-5472.CAN-06-0453. [DOI] [PubMed] [Google Scholar]
- 10.Greulich H, Chen TH, Feng W, Janne PA, Alvarez JV, et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS.Med. 2005;2:e313. doi: 10.1371/journal.pmed.0020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelman JA, Janne PA, Mermel C, Pearlberg J, Mukohara T, et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc.Natl.Acad.Sci.U.S.A. 2005;102:3788–3793. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song L, Morris M, Bagui T, Lee FY, Jove R, et al. Dasatinib (BMS-354825) selectively induces apoptosis in lung cancer cells dependent on epidermal growth factor receptor signaling for survival. Cancer Res. 2006;66:5542–5548. doi: 10.1158/0008-5472.CAN-05-4620. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Kalyankrishna S, Wislez M, Thilaganathan N, Saigal B, et al. SRC-family kinases are activated in non-small cell lung cancer and promote the survival of epidermal growth factor receptor-dependent cell lines. Am J Pathol. 2007;170:366–376. doi: 10.2353/ajpath.2007.060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beer DG, Kardia SL, Huang CC, Giordano TJ, Levin AM, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat.Med. 2002;8:816–824. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc.Natl.Acad.Sci.U.S.A. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 18.Creighton CJ, Hilger AM, Murthy S, Rae JM, Chinnaiyan AM, et al. Activation of mitogen-activated protein kinase in estrogen receptor alpha-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor alpha-negative human breast tumors. Cancer Res. 2006;66:3903–3911. doi: 10.1158/0008-5472.CAN-05-4363. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes DR, Tomlins SA, Varambally S, Mahavisno V, Barrette T, et al. Probabilistic model of the human protein-protein interaction network. Nature.Biotechnol. 2005;23:951–959. doi: 10.1038/nbt1103. [DOI] [PubMed] [Google Scholar]
- 20.Jeong H, Mason SP, Barabasi AL, Oltvai ZN. Lethality and centrality in protein networks Nature. 2001;411:41–42. doi: 10.1038/35075138. [DOI] [PubMed] [Google Scholar]
- 21.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 22.Riely GJ, Pao W, Pham D, Li AR, Rizvi N, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 23.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl.Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 24.Walker-Daniels J, Hess AR, Hendrix MJ, Kinch MS. Differential regulation of EphA2 in normal and malignant cells. Am J Pathol. 2003;162:1037–1042. doi: 10.1016/S0002-9440(10)63899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macrae M, Neve RM, Rodriguez-Viciana P, Haqq C, Yeh J, et al. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell. 2005;8:111–118. doi: 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Brantley DM, Cheng N, Thompson EJ, Lin Q, Brekken RA, et al. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene. 2002;21:7011–7026. doi: 10.1038/sj.onc.1205679. [DOI] [PubMed] [Google Scholar]
- 27.Liu M, Yang SC, Sharma S, Luo J, Cui X, et al. EGFR Signaling is Required for TGF-{beta}1-mediated COX-2 Induction in Human Bronchial Epithelial Cells. Am J Respir Cell Mol Biol. 2007 doi: 10.1165/rcmb.2007-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu R, Abramson AL, Shikowitz MJ, Dannenberg AJ, Steinberg BM. Epidermal growth factor-induced cyclooxygenase-2 expression is mediated through phosphatidylinositol-3 kinase, not mitogen-activated protein/extracellular signal-regulated kinase kinase, in recurrent respiratory papillomas. Clin Cancer Res. 2005;11:6155–6161. doi: 10.1158/1078-0432.CCR-04-2664. [DOI] [PubMed] [Google Scholar]
- 29.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi M, Saitoh S, Tanimura N, Takahashi K, Kawasaki K, et al. Regulatory roles for MD-2 and TLR4 in ligand-induced receptor clustering. J Immunol. 2006;176:6211–6218. doi: 10.4049/jimmunol.176.10.6211. [DOI] [PubMed] [Google Scholar]
- 31.Lavergne E, Combadiere B, Bonduelle O, Iga M, Gao JL, et al. Fractalkine mediates natural killer-dependent antitumor responses in vivo. Cancer Res. 2003;63:7468–7474. [PubMed] [Google Scholar]
- 32.Fujimoto K, Imaizumi T, Yoshida H, Takanashi S, Okumura K, et al. Interferon-gamma stimulates fractalkine expression in human bronchial epithelial cells and regulates mononuclear cell adherence. Am J Respir Cell Mol Bio. 2001;25:233–238. doi: 10.1165/ajrcmb.25.2.4275. [DOI] [PubMed] [Google Scholar]
- 33.Shulby SA, Dolloff NG, Stearns ME, Meucci O, Fatatis A. CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Cancer Res. 2004;64:4693–4698. doi: 10.1158/0008-5472.CAN-03-3437. [DOI] [PubMed] [Google Scholar]
- 34.Creighton C, Kuick R, Misek DE, Rickman DS, Brichory FM, et al. Profiling of pathway-specific changes in gene expression following growth of human cancer cell lines transplanted into mice. Genome Biol. 2003;4:R46. doi: 10.1186/gb-2003-4-7-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–5. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salwinski L, Miller CS, Smith AJ, Pettit FK, Bowie JU, et al. The database of interacting proteins:2004 update. Nucleic Acids Res. 2004;32:D449–51. doi: 10.1093/nar/gkh086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc.Natl Acad.Sci.U.S.A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saldanha AJ. Java Treeview–extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of Gene Expression in EGFR-mutant and -wild-type NSCLC Cells. Genes listed were increased or decreased in EGFR-mutant NSCLC cell lines (n = 4) relative to that of EGFR wild-type cell lines (n = 3).
(0.06 MB XLS)
Quantitative PCR Analysis of Selected Genes that were Differentially Expressed Based on Expression Profiling of EGFR-mutant and -wild-type NSCLC Cell Lines. Results normalized based on L32 ribosomal RNA expression.
(0.07 MB DOC)
Mutant EGFR expression profile includes genes with diverse functions. Differentially expressed genes were grouped based on their Gene Ontology functions and represented in a pie chart to illustrate their relative abundance.
(0.07 MB TIF)
Mutant EGFR expression profile includes genes with diverse functions. Differentially expressed genes are listed according to their Gene Ontology functions.
(0.43 MB XLS)
Comparison of gene expression in EGFR L858R versus del746-750 NSCLC Cells. Genes listed were increased or decreased in EGFR L858R NSCLC cell lines (n = 2) relative to that of EGFR del746-750 cell lines (n = 3).
(0.08 MB XLS)
Quantitative PCR analysis of selected genes that were differentially expressed in EGFR L858R and δ746-750 NSCLC Cell Lines. Results normalized based on L32 ribosomal RNA expression.
(0.08 MB DOC)
Gene Ontology terms enriched in NSCLC cell lines with EGFR L858R and del746-750.
(0.50 MB XLS)
Comparison of gefitinib-induced gene expression changes in TKI-sensitive and -resistant EGFR-mutant NSCLC cell lines. The fold difference was calculated by log2[Gefitinib-induced change in expression in sensitive cells (HCC827, H3255, H4006)/resistant cells (H1975)].
(0.03 MB XLS)
Quantitative PCR analysis of selected genes that were regulated by gefitinib treatment in TKI-sensitive NSCLC cells
(0.09 MB DOC)
Primers used for quantitative PCR
(0.03 MB XLS)