Abstract
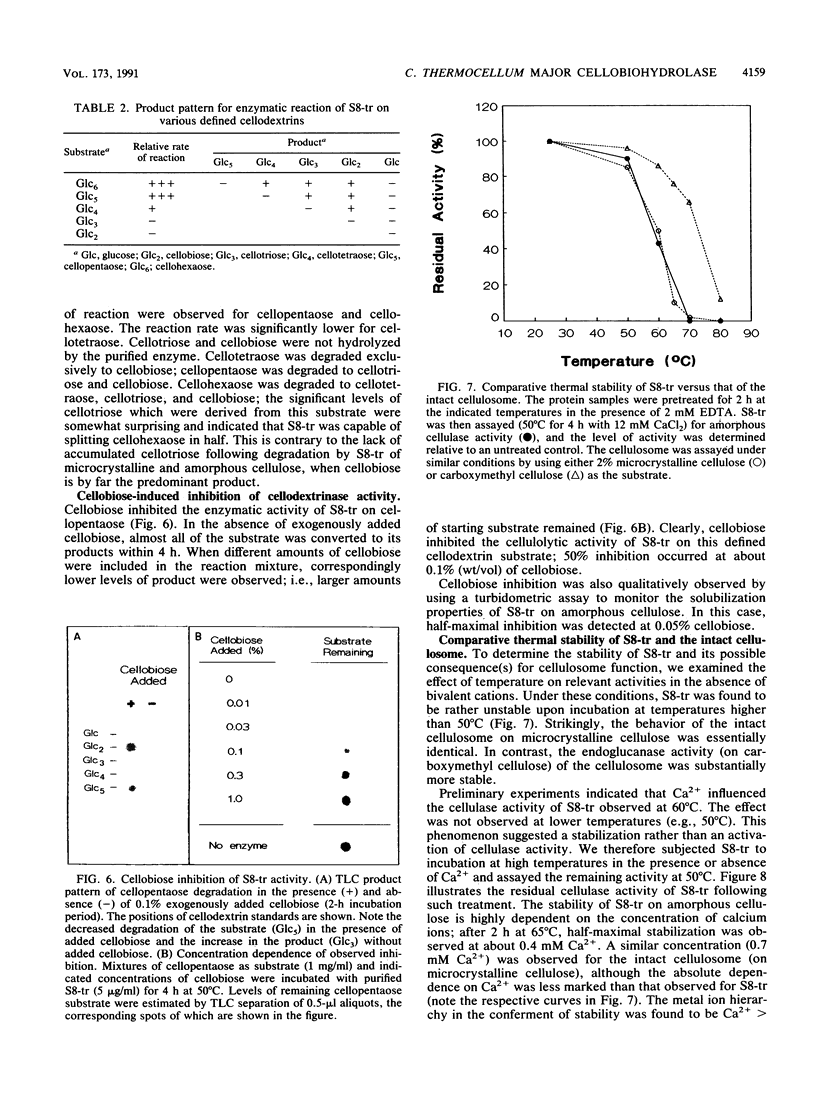
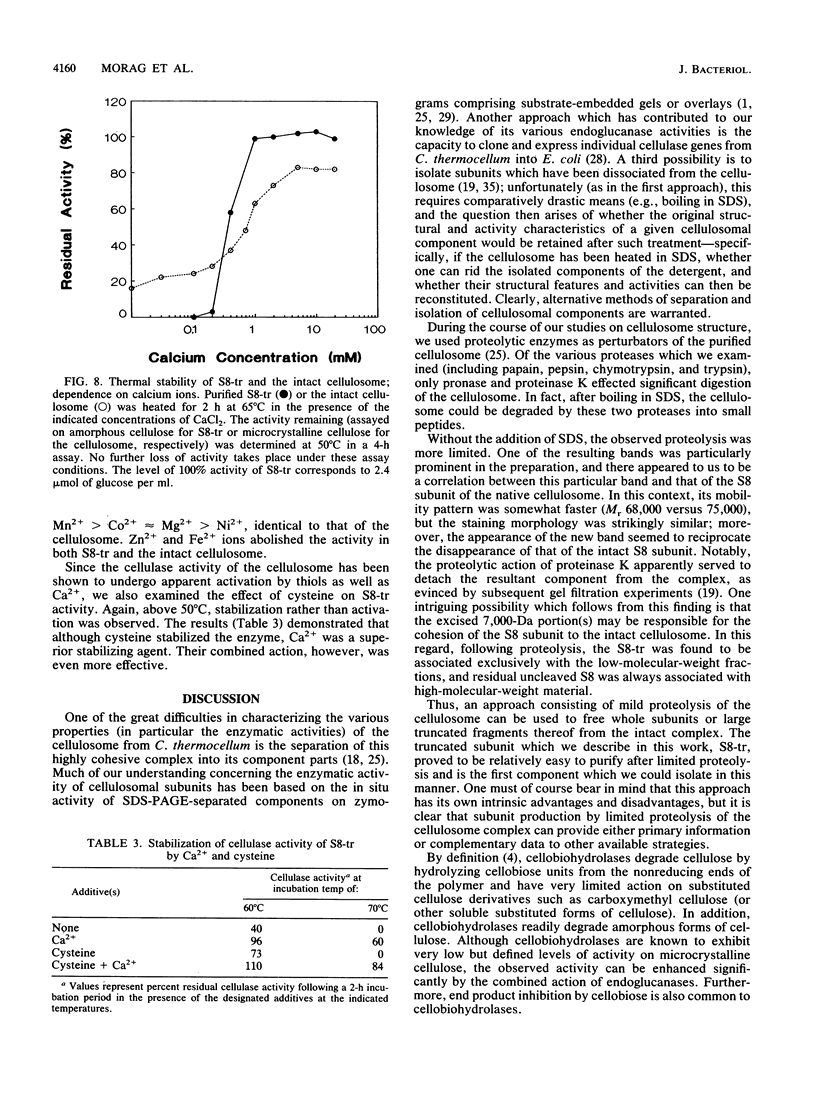
In the anaerobic, thermophilic, cellulolytic bacterium Clostridium thermocellum, efficient solubilization of the insoluble cellulose substrate is accomplished largely through the action of a cellulose-binding multienzyme complex, the cellulosome. A major cellobiohydrolase activity from the cellulosome has been traced to its Mr 75,000 S8 subunit, and an active fragment of this subunit was prepared by a novel procedure involving limited proteolytic cleavage. The truncated Mr 68,000 fragment, termed S8-tr, was purified by gel filtration and high-performance ion-exchange chromatography. The purified protein adsorbed weakly to amorphous cellulose, and its enzymatic action yielded cellobiose as the major end product from both amorphous and crystalline cellulose preparations. The high ratio of exo- to endo-beta-glucanase activities was supported by viscosimetric measurements. The use of model substrates showed that the smallest cellodextrin to be degraded was cellotetraose, but cellopentaose was degraded at a much greater rate. Cellobiose dramatically inhibited the cellulolytic activities. In the absence of calcium or other bivalent metal ions, both the truncated cellobiohydrolase activity of S8-tr and the true cellulase activity of the parent cellulosome were relatively unstable at temperatures above 50 degrees C. Cysteine further enhanced the stabilizing effect of calcium. This is the first report of a defined cellobiohydrolase in C. thermocellum. Its association with the cellulosome and the correspondence of several of their major distinctive properties suggest that this cellobiohydrolase plays a key role in the solubilization of cellulose by the intact cellulosomal complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisaria V. S., Mishra S. Regulatory aspects of cellulase biosynthesis and secretion. Crit Rev Biotechnol. 1989;9(2):61–103. doi: 10.3109/07388558909040616. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Béguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983 Jun;131(2):333–336. doi: 10.1016/0003-2697(83)90178-1. [DOI] [PubMed] [Google Scholar]
- Béguin P. Molecular biology of cellulose degradation. Annu Rev Microbiol. 1990;44:219–248. doi: 10.1146/annurev.mi.44.100190.001251. [DOI] [PubMed] [Google Scholar]
- Chauvaux S., Beguin P., Aubert J. P., Bhat K. M., Gow L. A., Wood T. M., Bairoch A. Calcium-binding affinity and calcium-enhanced activity of Clostridium thermocellum endoglucanase D. Biochem J. 1990 Jan 1;265(1):261–265. doi: 10.1042/bj2650261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Ganju R. K., Murthy S. K., Vithayathil P. J. Purification and characterization of two cellobiohydrolases from Chaetomium thermophile var. coprophile. Biochim Biophys Acta. 1989 Dec 8;993(2-3):266–274. doi: 10.1016/0304-4165(89)90175-x. [DOI] [PubMed] [Google Scholar]
- Gardner R. M., Doerner K. C., White B. A. Purification and characterization of an exo-beta-1,4-glucanase from Ruminococcus flavefaciens FD-1. J Bacteriol. 1987 Oct;169(10):4581–4588. doi: 10.1128/jb.169.10.4581-4588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes N. R., Langsford M. L., Kilburn D. G., Miller R. C., Jr, Warren R. A. Mode of action and substrate specificities of cellulases from cloned bacterial genes. J Biol Chem. 1984 Aug 25;259(16):10455–10459. [PubMed] [Google Scholar]
- Johnson E. A., Sakajoh M., Halliwell G., Madia A., Demain A. L. Saccharification of Complex Cellulosic Substrates by the Cellulase System from Clostridium thermocellum. Appl Environ Microbiol. 1982 May;43(5):1125–1132. doi: 10.1128/aem.43.5.1125-1132.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katamoto H., Yoneda N., Shimada Y. Effects of isoprothiolane and phytosterol on adipocyte metabolism and fatty acid composition of serum and tissue lipids in rats. J Vet Med Sci. 1991 Oct;53(5):905–910. doi: 10.1292/jvms.53.905. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamed R., Setter E., Bayer E. A. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J Bacteriol. 1983 Nov;156(2):828–836. doi: 10.1128/jb.156.2.828-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer F., Coughlan M. P., Mori Y., Ljungdahl L. G. Macromolecular Organization of the Cellulolytic Enzyme Complex of Clostridium thermocellum as Revealed by Electron Microscopy. Appl Environ Microbiol. 1987 Dec;53(12):2785–2792. doi: 10.1128/aem.53.12.2785-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morag E., Bayer E. A., Lamed R. Relationship of cellulosomal and noncellulosomal xylanases of Clostridium thermocellum to cellulose-degrading enzymes. J Bacteriol. 1990 Oct;172(10):6098–6105. doi: 10.1128/jb.172.10.6098-6105.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J. D., Bayer R. The limits of the ledger in public health promotion. Hastings Cent Rep. 1985 Dec;15(6):37–41. [PubMed] [Google Scholar]