Abstract
The tripeptide GSH is important in maintenance of renal redox status and defense against reactive electrophiles and oxidants. Previous studies showed that GSH is transported across the basolateral plasma membrane (BLM) into the renal proximal tubule by both sodium-coupled and sodium-independent pathways. Substrate specificity and inhibitor studies suggested the function of several carriers, including organic anion transporter 3 (Oat3). To test the hypothesis that rat Oat 3 can function in renal GSH transport, the cDNA for rat Oat3 was expressed as a His6-tagged protein in E. coli, purified from inclusion bodies and by Ni2+-affinity chromatography, and reconstituted into proteoliposomes. cDNA-expressed and reconstituted Oat3 transported both GSH and p-aminohippurate (PAH) in exchange for 2-oxoglutarate (2-OG) and 2-OG and PAH in exchange for GSH, and PAH uptake was inhibited by both probenecid and furosemide, consistent with function of Oat3. mRNA expression of Oat3 and several other potential carriers was detected by semiquantitative RT-PCR in rat kidney cortex but was absent from NRK-52E cells, a rat proximal tubular cell line. Basolateral uptake of GSH in NRK-52E cells showed little PAH- or 2-OG-stimulated uptake. We conclude that Oat3 can function in GSH uptake and that NRK-52E cells possess a low background rate of GSH uptake, making these cells a good model for overexpression of specific, putative GSH carriers.
Keywords: Glutathione, Transport, Rat Kidney, Basolateral Plasma Membrane, Organic Anion Transporters, mRNA Expression
1. Introduction
Selective tissue localization of membrane carriers for GSH and interorgan translocation are major determinants of cellular GSH status [1,2]. Whereas hepatocytes are the primary source of plasma and other pools of extracellular GSH, possessing carriers on the sinusoidal and canalicular plasma membranes that mediate efflux of GSH [3,4], the kidneys are relatively unique in possessing plasma membrane transport systems for both uptake and efflux of GSH [1,2,5]. Moreover, the kidneys possess the highest activity of any tissue of γ-glutamyltransferase (GGT) [6], which, along with dipeptidase activity, degrade GSH to its constituent amino acids in the tubular lumen. The constituent amino acids are reabsorbed into the proximal tubular (PT) cell by transport across the brush-border membrane (BBM), where they can serve as precursors for protein or for resynthesis of GSH. Hence, renal PT cells can obtain GSH from both the extracellular space, via transport across the basolateral membrane (BLM), or by intracellular synthesis from its precursors. The physiological function of BLM uptake of GSH is not completely understood, but the transport processes may be used pharmacologically to provide renal PT cells with exogenous GSH to protect against oxidant injury [7].
Observations in isolated, perfused kidneys from the rat and rabbit in the late-1970’s through the mid-1980’s [8–14] provided evidence that either endogenous or administered GSH was extracted from the plasma by both a basolateral and a luminal mechanism. While the latter clearly involves glomerular filtration, degradation by BBM enzymes, and uptake of the constituent amino acids by renal PT cells [1,9], there was controversy over whether a basolateral transport mechanism for the intact tripeptide of GSH actually existed or if the apparent basolateral extraction of plasma GSH was due to degradation of GSH by extraluminal GGT, renal cellular uptake of the constituent amino acids, and intracellular resynthesis of GSH [11,15,16]. This controversy was ultimately resolved by studies by Lash and Jones [17,18] and subsequent studies by Jones and colleagues [7]: These studies provided a biochemical description of both Na+-dependent and Na+-independent transport processes for uptake of GSH in isolated BLM vesicles [17,18] and isolated rat kidney cells [7]. GSH S-conjugates are also transported across the BLM, at least in part, by the same process(es) that transport(s) GSH [19].
The process of GSH uptake across the BLM comprises at least two components, a Na+-independent and a Na+-dependent pathway. Measurement of the stoichiometry of the Na+-dependent process showed coupling of GSH uptake to at least two Na+ ions with the net transfer across the membrane of at least one positive charge [18]. The Na+-coupled transport process in the intact BLM is thus electrogenic and membrane potential-sensitive. GSH uptake is also inhibited by organic anions, such as p-aminohippurate (PAH) and probenecid [18]. More recent studies of ours [20] showed that besides PAH, which is transported across the renal BLM primarily by the organic anion transporter 1 (Oat1; Slc22a6) but also by the organic anion transporter 3 (Oat3; Slc22a8), transport of GSH across the BLM is also inhibited by dimethylsuccinate (DMS), which implicates the sodium-dicarboxylate 2 (NaC2; formerly SDCT2 or NaDC-3; Slc13a3) exchanger and/or Oat3. Data from Ullrich et al. [21] in rat proximal tubules, however, found no inhibition of PAH transport by GSH, although relatively hydrophobic GSH conjugates were moderately inhibitory. This would suggest that Oat1 may not participate in GSH uptake at the BLM. Further study is required to resolve this uncertainty about the role of Oats in GSH uptake.
Three classes of organic anion carriers are present on the renal BLM: The multispecific organic anion transporter family (Oat proteins; Slc22a gene family), the multidrug resistance-associated proteins or ATP-binding cassette proteins (Mrps; Abcc gene family), and the sodium-dicarboxylate 2 carrier (Slc13a gene family). These carriers have been cloned and their cDNA sequences deposited in GenBank™. Substrate specificity and tissue distribution have been described for many of these carriers and several excellent reviews have been published recently on the properties and molecular biology of these proteins [22–29].
Of the Oat proteins (Slc22a gene family), Oat1 is thought to be the primary 2-oxoglutarate (2-OG)/organic anion exchanger on the BLM. Oat1 is considered the “classical” renal organic anion carrier that transports PAH. Additionally, Oat1 transports a diverse array of other anions, including acetylsalicylate, methotrexate, urate, and cephaloridine. Although transport of GSH or GSH S-conjugates has not been demonstrated with Oat1, the finding that cysteine S-conjugates and mercapturates are substrates [30] suggests the possibility that GSH and GSH S-conjugates may also be substrates. The potential role of Oat3 in GSH transport has not been previously studied. Hence, the goal of the present work was to test the hypothesis that rat Oat3 can mediate uptake of GSH across the renal BLM. Results with cDNA-expressed and reconstituted rat Oat3 suggest that this carrier can indeed transport GSH in exchange for other organic anions. Finally, the appropriateness of NRK-52E cells, an immortalized cell line derived from rat renal PT cells, as a model to study GSH transport was characterized by comparing mRNA expression of several transport proteins with that in rat kidney homogenates and by assessing organic anion transport.
2. Materials and methods
2.1. Experimental design
As summarized above, previous studies of both ours and others in isolated PT cells from rat kidney suggested that some of the known organic anion or dicarboxylate carriers on the BLM may function in uptake of intact GSH from the renal periplasmic space into the PT cell. Although many of the initial observations that support this conclusion were made more than twenty years ago, to date a specific carrier that can mediate uptake of GSH across the renal BLM has not been demonstrated. As a first step to provide direct evidence for the function of a known BLM carrier in GSH transport, we focused on Oat3. The rationale for beginning with Oat3 rather than Oat1, is that the known substrate specificity and inhibition pattern observed for GSH transport in renal PT cells are more consistent with those of Oat3. A simple way to determine the ability of a carrier protein to function in transport is to express the protein and then reconstitute it in an artificial membrane. This approach has been widely used and allows one to assess transport function without confounding variables such as metabolism or the function of other, competing transporters. Ultimately, however, it is desirable to study the function of a membrane carrier in an intact cell; this allows one to more readily relate the transport process to various cellular functions and responses. In analogy with our previous studies on the function of renal mitochondrial GSH carriers [31,32], we chose to use NRK-52E cells as the experimental model in which to study the function of BLM carriers in GSH transport. Before using the cell line for transfection of carrier cDNAs, we characterized its baseline function in GSH and organic anion transport and assessed mRNA expression of Oats and other plasma membrane carriers and compared these properties with those found in freshly isolated renal tissue.
2.2. Materials
L-[3H-glycyl]-GSH (44.8 Ci/mmol), p-[glycyl-1-14C]-aminohippuric acid (PAH; 53.1 mCi/mmol), and [U-14C]-2-OG (281 mCi/mmol) were purchased from DuPont NEN (Boston, MA). Restriction enzymes for PCR, other enzymes (e.g., DNA polymerases, T7 RNA polymerase), and plasmids were purchased from New England Biolabs (Boston, MA), Promega (Madison, WI), and Gibco-BRL/Life Technologies (Gaithersburg, MD). PCR primers were custom synthesized by Integrated DNA Technology, Inc. (Coralville, IA). Cloning vectors (pGEM®-T Easy, pRSET, pcDNA3.1/V5-His-TOPO®) were purchased from Promega and Invitrogen (Carlsbad, CA). Dowex-1, Triton X-100, and phospholipids were purchased from Sigma (St. Louis, MO). Reagents for gel electrophoresis (SDS, acrylamide, agarose, buffers) were purchased from Bio-Rad (Richmond, CA) or Sigma. ProBond™ nickel-chelating resin was purchased from Invitrogen. Millicell polycarbonate filters (0.2-μm pore size, 25-mm diameter) for proteoliposome transport studies and polycarbonate Millicell filter inserts (0.4-μm pore size, 30-mm diameter) for polarized growth of cells were purchased from Millipore (Billerica, MA). NRK-52E cells (catalogue number CRL-1571) and cell culture medium (catalogue number 30-2002; Dulbecco’s modified Eagle’s medium with 4 mM L-glutamine adjusted to contain 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 1.0 mM sodium pyruvate), and 10% (w/v) bovine calf serum were purchased from the American Type Culture Collection (Manassas, VA). A polyclonal rabbit anti-rat Oat3 antibody (catalogue number OAT31-S) was purchased from Alpha Diagnostics International, Inc. (San Antonio, TX). Double-distilled, deionized water was used for all experiments. All other chemicals and reagents were purchased from commercial vendors and were of the highest purity available.
2.3. Amplification of rat kidney Oat3 cDNA by RT-PCR and bacterial expression
Total RNA was isolated from renal cortical homogenates of male Fischer 344 rats (200–300 g body weight; Charles River Laboratories, Wilmington, MA) by acid phenol extraction using the TRIZOL® extraction kit (Gibco-BRL/Life Technologies) and was stored at −80°C until needed. Total rat kidney RNA was reverse transcribed with Superscript II reverse transcriptase and amplified with forward and reverse primers based on the complete cDNA sequence (2,157 bp) for kidney Oat3 protein from the Norway rat (Rattus norvegicus; GenBank™ accession number NM_031332). PCR primers were designed with the aid of Oligo 6.76 software (Molecular Biology Insights, Inc., Cascade, CO), based on the knowledge that the coding region is from positions 124 to 1,734, and were as follows: Upper primer = 5′–CAT CTT GCC TGG TGC CAT GAC–3′; positions 108 to 128; lower primer = 5′–TAC TGC TTG GGA TCA GTC TCT TGT G–3′; positions 1,796 to 1,772. RT-PCR was conducted by running 35 cycles of 94°C × 3 min for denaturation and 55°C × 2 min for annealing/extension. Sample was then loaded onto a 1% (w/v) agarose gel, the gel stained with ethidium bromide, and the bands visualized under UV light. The 1,689-bp product was ligated into a T-A cloning vector (pGEM®-T Easy) for transformation. Confirmation of the PCR product was by automated DNA sequencing.
The full-length cDNA for rat kidney Oat3 was subcloned into the pRSET T7 expression vector for high-level expression in E. coli as an N-terminal polyhistidine (His6) fusion product. Bacterial cells containing the overexpressed protein were harvested by centrifugation (5,000g × 5 min at 4°C), supernatants were discarded, and pellets were resuspended in 20 ml of TE-1 buffer (50 mM Tris-HCl, 2 mM EDTA, pH 8.0). Cells were then lysed by incubation for 15 min at 30°C and occasional mixing with 100 μg of lysozyme/ml (freshly prepared in TE-1 buffer) and 0.1% (v/v) Triton X-100. Bacterial DNA in the cell lysate was sheared by sonication, the suspension centrifuged at 12,000g × 15 min, and the pellet resuspended in 2 ml of TE-2 buffer (10 mM Tris-HCl, 0.1 mM EDTA, 1 mM dithioerythritol, pH 7.0) and recentrifuged. The final pellet was resuspended in 2 ml of TE-2 buffer and was solubilized with 1.2% (w/v) sarkosyl dissolved in TE-2 buffer. The inclusion body fraction was isolated by centrifugation of the resuspended pellet at 131,000g × 4.5 h through a step sucrose gradient [12.4 ml of 40% (w/v) sucrose and 18.6 ml of 53% (w/v) sucrose (sucrose solutions prepared in TE-2 buffer)]. The inclusion body pellet was resuspended in 30 ml of TE-2 buffer and centrifuged at 12,000g × 15 min. The Oat3 protein was then extracted by resuspension of the inclusion body pellet in 2 ml of 1.2% (w/v) sarkosyl dissolved in TE-2 buffer and centrifugation at 314,000g × 30 min.
Purity of the Oat3-His6 fusion protein obtained from the inclusion body fraction was enhanced by fractionation of the inclusion body pellet (2 ml, approx. 25 mg protein) on a 1.0 × 6.8 cm column with 4 ml of nickel-chelating resin and equilibrated with ProBond™ buffer (20 mM potassium phosphate, pH 7.8, containing 500 mM NaCl). The column was then washed with ProBond™ buffer adjusted to pH 6.0 (25 ml) and finally with 25-ml each of ProBond™ buffer, pH 6.0, containing 100 mM and then 300 mM imidazole.
2.4. Preparation of proteoliposomes for reconstitution of purified rat Oat3
Liposomes and proteoliposomes were prepared essentially as described previously [33]. Briefly, liposomes were prepared by transferring 700 mg of phosphatidylcholine into a 30-ml polypropylene tube and drying under a stream of nitrogen. After redissolving the dried lipid in diethyl ether and removal of solvent under nitrogen, liposome buffer (7 ml; 120 mM HEPES, 50 mM KCl, 1 mM EDTA, pH 7.0) was added to the dried lipid, the tube was flushed with nitrogen, sealed, and vigorously mixed on a vortex mixer. Lipid was then dispersed in a bath sonicator for approximately 60 min and was stored at −80°C for up to 3 months. Proteoliposomes were prepared by mixing purified carrier protein (200 μl, 50 μg protein) or liposome buffer (for a blank control) with 0.5 ml of liposomes and 50 μl of preloading solution (buffer with or without indicated substrates) in a final volume of 1 ml. After mixing on a vortex mixer, the proteoliposomes were rapidly frozen in liquid nitrogen and stored at −80°C for up to 1 month until use. Immediately prior to assay, the proteoliposome suspension was thawed, sonicated with a bath sonicator at room temperature, placed on ice for 1 min, and then passed through an anion exchange column (Dowex-1, 100-200 mesh, equilibrated with liposome buffer and prepared in a 9-inch Pasteur pipette) to remove external substrate by elution with liposome buffer. The opalescent fraction (approximately 1 ml) containing the proteoliposomes was collected. The proteoliposomes were equilibrated at room temperature for approximately 5 min before being used in transport assays. This freeze-thaw cycle and sonication method gives rise to a heterogeneous population of large, primarily unilamellar proteoliposomes that are suitable for transport studies [34].
2.5. Transport assays in reconstituted proteoliposomes
Transport of substrates (i.e., GSH, PAH) into proteoliposomes was measured by first preincubating 200 μl of proteoliposomes (preloaded with either buffer or 1 mM 2-OG) for 1 min with 12 μl of ‘Reconstitution Buffer’ (120 mM HEPES, 50 mM KCl, 1 mM EDTA, pH 7.4). Competitive inhibitors were added simultaneously with substrates. Transport was initiated by addition of 12 μl of 20X radiolabeled substrate. At various times (30 to 240 sec), 30 μl aliquots were loaded onto Sephacryl S-100 mini-columns, which were prepared in 5.75-inch Pasteur pipettes. These columns were placed in 16 × 125 mm glass test tubes and were centrifuged at 2,300 rpm × 2 min in a tabletop clinical centrifuge. Supernatant from each tube was transferred to a 5-ml scintillation vial, each tube was washed with 200 μl of scintillation fluid, which was then added to scintillation vials.
Uptake rates were calculated by linear curve-fitting as described previously [33,35], plotting ln[Ptotal/(Ptotal − Pt)] vs. time according to Halestrap [36]. Ptotal represents the total uptake of substrate at equilibrium (estimated by exponential decay curve-fitting of time course data) and Pt represents substrate uptake at time t (0 to 5 min). The initial rates of uptake were then determined from the first-order rate equation v = k (Ptotal). As a control, liposomes were reconstituted with either buffer and no protein or protein that was inactivated by boiling for 5 min. These control liposomes exhibited less than 5% of the measured uptake of either GSH or 2-OG (data not shown), similar to previous reconstitution studies [33], indicating that nearly all of the measured substrate transport was due to the function of the reconstituted carrier protein.
2.6. Culture of NRK-52E cells
NRK-52E cells were cultured on either collagen-coated, polystyrene T-75 culture flasks (RT-PCR experiments) or on polycarbonate Millicell filter inserts (0.4-μm pore size, 30-mm diameter) in 35-mm culture dishes with Dulbecco’s modified Eagle’s medium containing 4% (w/v) glutamine, 1.5 g/l sodium bicarbonate, 4.5 g glucose/l, 1 mM sodium pyruvate, and 10% (v/v) bovine calf serum in an atmosphere of 5% CO2, 95% air at 37 C. On reaching confluence (5 to 9 days), subcultures were prepared by a 15-min treatment with 0.02% (w/v) EDTA, 0.05% (w/v) trypsin solution and replating the cells at a density of 4 × 104 cells/cm2.
2.7. Transport measurements in NRK-52E cells
NRK-52E cells were grown to confluence on 0.4-μm pore size Millicell filter inserts in 35-mm polystyrene culture dishes. Because confluence for cells grown on filter inserts cannot be determined visually due to the semi-opaqueness of the filters, we assessed confluence by cultures reaching a maximum protein content per dish. In previous studies [37], we confirmed that this is an accurate criterion for indicating confluence by showing that only very small amounts of 14C-sucrose were recovered in the lower fluid compartment when added to the upper fluid compartment. Filter inserts enable maintenance of epithelial polarity as cells grow with the BBM facing up and the BLM facing down. Addition of substrates to media in the lower compartment enables measurement of transport across the BLM whereas addition of substrates to media in the upper compartment enables measurement of transport across the BBM. This maintenance of polarity is typical of epithelial cells grown in culture and was previously validated by showing that NRK-52E cells grown on semi-permeable supports exhibit alkaline phosphatase and GGT activity only towards substrates present in the upper fluid compartment [37]. Cells were preincubated on the BLM side for 30 min with medium containing either no addition (i.e., buffer control), or 5 mM PAH, 2-OG, or GSH. Media were replaced with fresh media containing the appropriate concentration of substrate and approximately 0.025 μCi of radiolabeled substrate and dishes were incubated at 37°C for the indicated times (5, 10, 15, 30, 60 min). After incubations, membrane filters were removed from inserts, washed in PBS, placed in a liquid scintillation vial with scintillation fluor, and radioactively was measured in a Beckman 3600I liquid scintillation counter. Initial rates of uptake were calculated as described above for the transport studies in proteoliposomes.
2.8. RT-PCR analysis of carrier mRNA expression
mRNA expression of several organic anion carriers in either homogenates of rat kidney cortex or NRK-52E cells was determined essentially as described above except that primers encoded only a portion of the coding region for each carrier. The carriers measured and the primers used are summarized in Table 1. mRNA expression levels for the mitochondrial 2-oxoglutarate carrier (OGC) was determined in NRK-52E cells as a positive control, as done previously [31].
Table 1.
Carriers for which mRNA expression were determined in rat kidney cortex homogenates and NRK-52E cells.
| Gene 4Product/Mr (kDa) | Gene Symbol | Accession Number(s) | PCR Product (bp) | Primers |
|---|---|---|---|---|
| Oat1/60 | Slc22a6 | NM_017224 | 895 | Forward: 5′–GCT GTC CAG ACC CCC GAA GT–3′
Reverse: 5′–GGC ATC CAT TCC ACA TTT AGT GTC A–3′ |
| Oat3/62 | Slc22a8 | NM_031332 | 702 | Forward: 5′–GGA CCC TAG GAC AGA GCA CG–3′
Reverse: 5′–TAC CCA TTC CAC ATT CAA GAT AAC G–3′ |
| NaC2 (SDCT2)/66 | Slc13a3 | NM_022866 | 714 | Forward: 5′–GCG CTG GCC AAG AAG GTG TGG A-3′
Reverse: 5′–CCC GAT GCT GGC TCA GTA GGG AAT G-3′ |
| Oatp1a1 (Oatp1)/74 | Slco1a1 | NM_017111 | 554 | Forward: 5′–AGA CAG CAA AGC AAA GAC TT–3′
Reverse: 5′–ATA TTT CCT ACC ATT ACA CAT ATC C–3′ |
| Oatp1a3_v1
(Oat-k1)/74 |
Slco1a3 | NM_030837 | 754 | Forward: 5′–CGT CCG GTC GCT GAG TTG TC–3′
Reverse: 5′–GGG CCA ATC ATC TTT CCT GTT TCT A–3′ |
| Oatp1a3_v2
(Oat-k2)/55 |
Slco1a3 | AB012662 | 605 | Forward: 5′–TTG TCC TGA AGC TCG GCA GT–3′
Reverse: 5′–GGA GAG TTT TCA GAT TTG GCA AAG T–3′ |
| Mrp2/175 | Abcc2 |
NM_013806
NM_012833 |
533 | Forward: 5′–GAG GAA AAA GTA AAG GAG AAA CAG T–3′
Reverse: 5′–TCA GGA GTC CTC GTA TCA GAG–3′ |
| Mrp5/160 | Abcc5 | AB020209 | 652 | Forward: 5′–CTT GTT TTG CTC CGG GTC CGA TGT T–3′
Reverse: 5′– GCG GCA AAA GAT CCA CAC AAC CCT–3′ |
Primers were designed with the use of Oligo 6.76 and encode of portion of the coding region for each carrier. Abbreviations: Mrp, multidrug resistance-associated protein; NaC2, sodium-dicarboxylate carrier 2; Oat1/3, organic anion transporter 1/3; Oatp, organic anion transporting polypeptide.
2.9. SDS PAGE and Western blotting
For gel electrophoresis, 20 μg of protein was loaded per well onto a 7.5% (w/v) polyacrylamide gel (Bio-Rad, Hercules, CA), and separation was achieved according to the method of Laemmli [38]. Total protein was visualized by staining with Coomassie Brilliant Blue G. For Western blot analysis of Oat3 expression, protein was transferred by electroblotting to a nitrocellulose membrane (MSI, Westborough, MA), blocked with 3% (w/v) bovine serum albumin (Promega, Madison, WI), washed with Tris-buffered saline containing Tween 20 (TTBS), probed with a polyclonal rabbit anti-rat Oat3 antibody (Alpha Diagnostics International, San Antonio, TX), washed with TTBS, and then probed with an anti-rabbit IgG antibody conjugated to alkaline phosphatase (Jackson Immunoresearch, West Grove, PA). Immunoreactive bands were visualized following incubation with a solution containing 5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium (Promega).
2.10. Data analysis
Protein determinations were done using the bicinchoninic acid (BCA) protein determination kit from Sigma, using bovine serum albumin as a standard. Results are expressed as means ± SE. For inhibition studies, significant differences for means were first assessed by a one-way analysis of variance. When significant F values were obtained with the analysis of variance, the Fisher’s protected least-significance t test was performed to determine which means were significantly different from one another, with two-tail probabilities < 0.05 considered significant.
3. Results
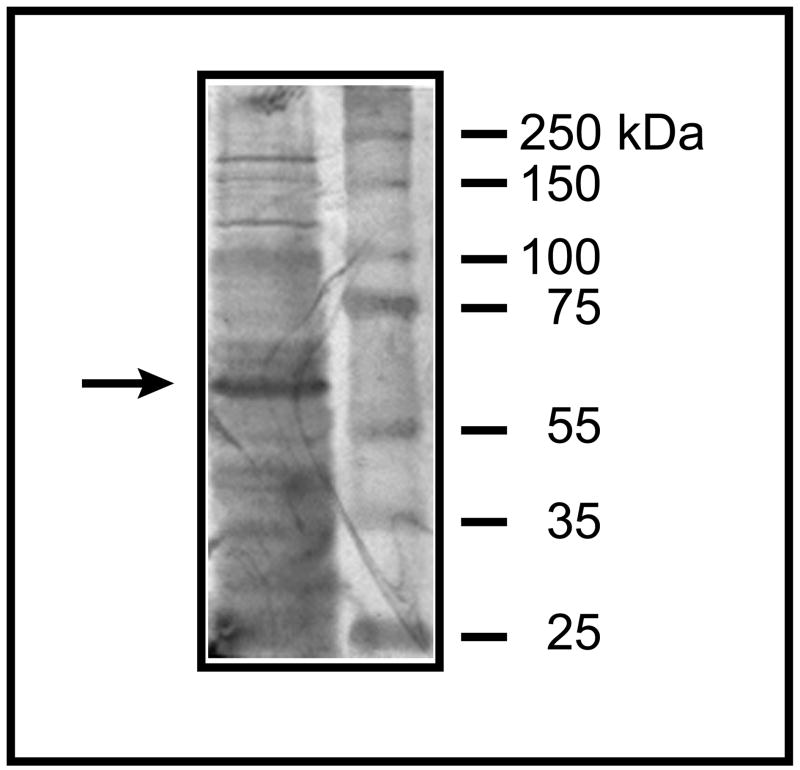
The cDNA sequence from rat kidney Oat3 was amplified by RT-PCR using rat kidney total RNA as a template. Identity of the PCR product was confirmed by automated DNA sequencing. E. coli were transformed with the rat Oat3 cDNA and induced to express the protein as an N-terminal His6-fusion protein (Figure 1). By Western blot analysis, using a polyclonal rabbit anti-rat Oat3 antibody, the predominant protein expressed corresponded to rat Oat3, which has a MW of 62 kDa. Lower molecular weight bands likely correspond to partial synthesis products, which is not uncommon in bacterial systems [39]. The higher molecular weight bands at ~120 kDa and ~180 kDa may be a dimer and tetramer, respectively. Nonetheless, the predominant protein band was observed at 62 kDa.
Figure 1. Bacterial expression of rat Oat3-His6 as assessed by Western blot analysis.
Rat Oat 3 (MW = 62 kDa) was expressed in E. coli as the His6-fusion protein, was isolated from inclusion bodies, and was subjected to SDS-PAGE on a 7.5% (w/v) acrylamide gel. The protein purified from the affinity column was subjected to Western blot analysis using a polyclonal rabbit anti-rat Oat3 antibody (Alpha Diagnostics International). The arrow points to the Oat3 band.
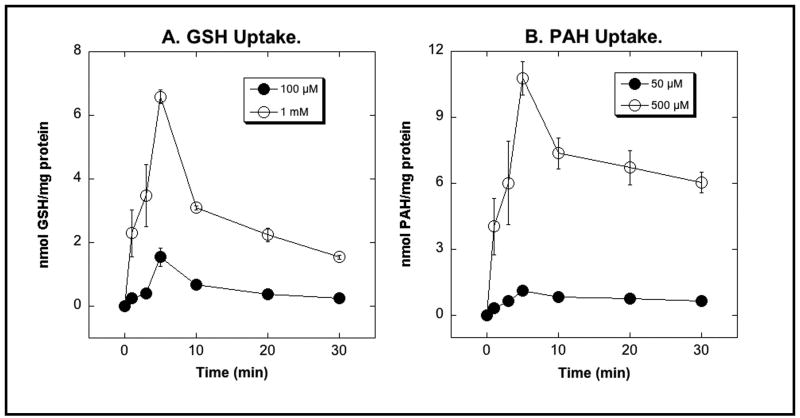
The bacterial expressed protein was isolated from inclusion bodies, further purified by Ni2+-affinity chromatography, and reconstituted into proteoliposomes for determination of transport activity. Proteoliposomes were preloaded with 1 mM 2-OG, because Oat3 functions as a 2-OG – organic anion exchanger [40], before assessing uptake of either GSH or PAH (Figure 2). Time- and concentration-dependent uptake of both GSH and PAH were observed, with a modest overshoot reaching a maximal intravesicular content at 5 min. Initial uptake rates for PAH were significantly higher than those for GSH.
Figure 2. Transport of GSH (A) and PAH (B) by bacterial expressed and reconstituted rat Oat3.
Uptake of the indicated concentrations of [3H]-GSH or [14C]-PAH was measured in proteoliposomes that were preloaded with 1 mM 2-OG. Preloading buffer was replaced with substrate-free buffer before initiation of transport measurements. At the indicated times, aliquots of incubation mixtures were centrifuged through Sephacryl S-100 mini-columns and eluates were placed in scintillation vials for determination of radioactivity. Results are means ± SE of measurements from 3 experiments. Where not visible, error bars are smaller than the size of the data points.
At a 10-fold molar excess, both probenecid (non-selective organic anion transport inhibitor) and furosemide (selective Oat3 inhibitor) significantly inhibited PAH uptake by bacterial expressed and reconstituted rat Oat3 (Table 2). Furosemide was a modestly more effective inhibitor than probenecid, producing 62% and 61% inhibition of 50 μM and 500 μM PAH uptake, respectively, as compared to 43% and 44% inhibition of 50 μM and 500 μM PAH uptake, respectively, by probenecid.
Table 2.
Inhibition of PAH uptake in bacterial expressed and reconstituted Oat3 by probenecid and furosemide.
| 50 μM PAH | 500 μM PAH | |||
|---|---|---|---|---|
| nmol/mg protein | % Control | nmol/mg protein | % Control | |
| Control | 0.55 ± 0.07 | 100 | 4.30 ± 0.21 | 100 |
| + Probenecid | 0.32 ± 0.03* | 57.3 | 2.40 ± 0.21* | 55.8 |
| + Furosemide | 0.21 ± 0.04* | 38.0 | 1.67 ± 0.31* | 38.8 |
Uptake of [3H]-PAH, at a final concentration of either 50 μM or 500 μM, was measured in proteoliposomes incubated for 5 min with either buffer (= Control) or a 10-fold molar excess of either probenecid or furosemide. Results are the means ± SE of measurements from 3 proteoliposome preparations.
Significantly different (P < 0.05) from the corresponding control sample.
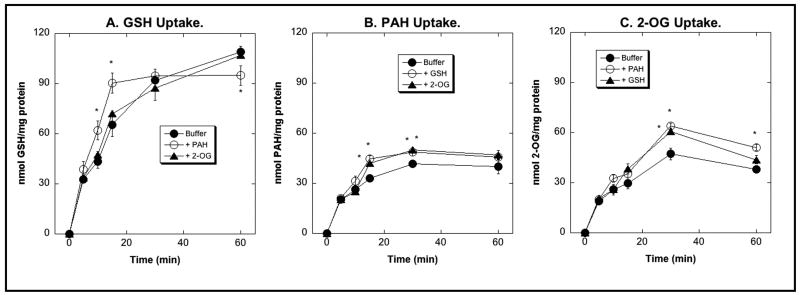
We have previously used NRK-52E cells, an immortalized cell line derived from rat PT cells, as a cellular model to study renal mitochondrial GSH transport [31,32,37]. The rationale for using these cells was that they have relatively low basal levels of mitochondrial GSH transport and that total rat kidney RNA was used as a template to express carrier cDNAs. To assess the appropriateness of these cells as a model in which to study plasma membrane transport processes, uptake of GSH, PAH, and 2-OG across the BLM was studied in cells that were grown on Millicell filter inserts to maintain epithelial polarity (Figure 3). Over the 60-min time course, preloading of NRK-52E cells with 2-OG had no effect on GSH uptake. In contrast, preloading with PAH significantly increased GSH uptake as compared to cells preincubated with only buffer at 10 and 15 min of incubation (Figure 3A). The stimulatory effect, however, was only about 40% at both time points. Preloading of NRK-52E cells with either GSH or 2-OG modestly stimulated PAH uptake at 15 and 30 min as compared to buffer-preincubated cells (Figure 3B), but the stimulatory effect was, again, rather modest, being only 17% and 36%, respectively, for GSH and 27% and 19%, respectively, for 2-OG. Similarly, preloading of NRK-52E cells with either PAH or GSH only modestly stimulated uptake of 2-OG (Figure 3C); PAH increased 2-OG uptake by 36% and 34% at 30 and 60 min, respectively, whereas GSH stimulated 2-OG uptake only at 30 min by 30%. These results suggest that NRK-52E cells possess minimal levels of Oat proteins.
Figure 3. BLM uptake of GSH, PAH, and 2-OG in NRK-52E cells.
NRK-52E cells were grown to confluence on Millicell filter inserts and were preincubated for 30 min by exposure at the BLM side (lower compartment) to either buffer or 5 mM of PAH, 2-OG, or GSH. After replacement of preincubation solution with fresh, substrate-free buffer, uptake of 1 mM [3H]-GSH (A), 1 mM [14C]-PAH (B), or 1 mM [14C]-2-OG (C) was measured at indicated times by scintillation counting. Results are means ± SE of measurements from 3 separate experiments. *Statistically significant (P < 0.05) difference from the corresponding sample preincubated with buffer. Where not visible, error bars are smaller than the size of the data points.
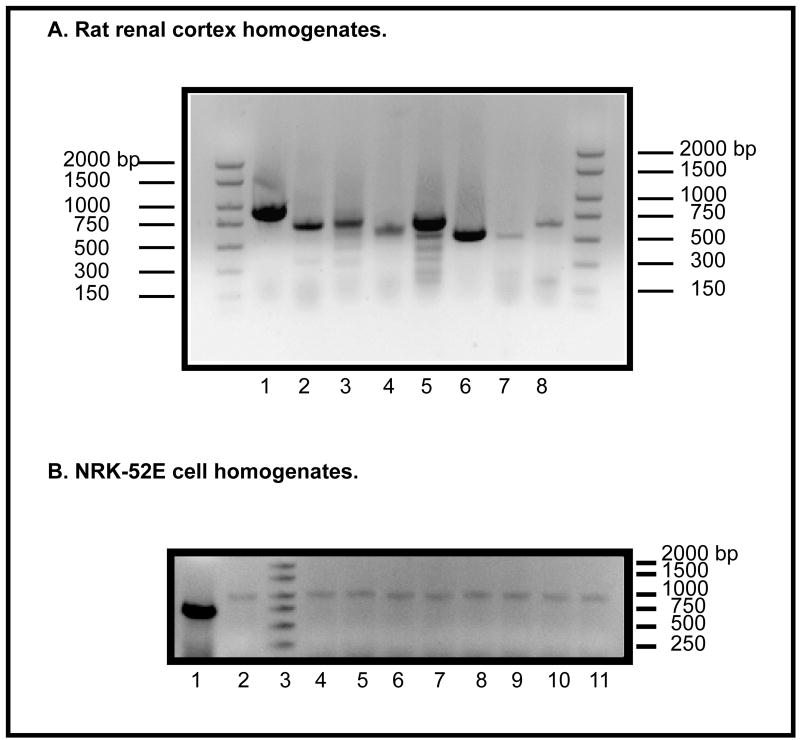
To characterize further the status of NRK-52E cells with respect to organic anion transport, we next determined mRNA expression of eight different plasma membrane carriers, first in rat kidney homogenates (Figure 4A) and then in total extracts of NRK-52E cells (Figure 4B). In agreement with the literature [23–29], Oat1 was clearly the most abundantly expressed carrier in rat kidney. The other two carriers that appeared to be abundantly expressed were NaC2 and Oatp1a1. mRNA for the other carriers (Oat3, Oatp1a3_v1, Oatp1a3_v2, Mrp2, and Mrp5) was also detected, although at modestly lower levels than that of Oat1. In contrast with these results from rat kidney homogenate, mRNA for none of the eight carriers was detected in total extracts of NRK-52E cells. mRNA for the mitochondrial carrier OGC was measured alongside with those of the plasma membrane carriers as a positive control to make sure that the mRNA for a known carrier that is expressed in these cells was detected. Thus, results from RT-PCR agreed with those of transport activity, suggesting that NRK-52E cells have little endogenous organic anion transport function.
Figure 4. RT-PCR analysis of mRNA expression of organic anion transporters from rat kidney cortex (A) and NRK-52E cells (B).
Levels of mRNA were determined by semi-quantitative RT-PCR using total rat kidney cortex RNA as a template and primers for the cDNA of several plasma membrane transporters to express a portion of the coding region of each gene (cf. Table 1). A. DNA markers were run on the right and left lanes. Transporters (size of expected PCR product, bp): Lane 1 = Oat1 (895); lane 2 = Oat3 (702); lane 3 = Oatp1a3_v1/Oat-k1 (754); lane 4 = Oatp1a3_v2/Oat-k2 (605); lane 5 = NaC2 (714); lane 6 = Oatp1a1 (554); lane 7 = Mrp2 (533); lane 8 = Mrp5 (652). B. As a positive control, primers were used to detect the mRNA for the 2-oxoglutarate carrier (OGC) (full-length = 1 kb; partial = 700 bp). Lane 1 = OGC (700 bp); lane 2 = OGC (1 kb); lane 3 = DNA ladder (sizes to right of figure); lane 4 = OGC (1 kb) + Oat1; lane 5 = OGC (1 kb) + Oat3; lane 6 = OGC (1 kb) + Oatp1a3_v1/Oat-k1; lane 7 = OGC (1 kb) + Oatp1a3_v2/Oat-k2; lane 8 = OGC (1 kb) + Oatp1a1; lane 9 = OGC (1 kb) + Mrp2; lane 10 = OGC (1 kb) + Mrp5; lane 11 = OGC (1 kb) + NaC2.
4. Discussion
The kidneys are unique in possessing enzymes to synthesize and degrade GSH and have plasma membrane carriers that mediate both uptake of extracellular GSH and efflux of intracellular GSH. The renal PT cell is the most active nephron cell type for most pathways of drug metabolism and metabolite transport [41]. As such, maintenance of redox status, in which GSH plays a major role, is critical to renal cellular function. GSH concentrations in the renal PT cell are maintained by a balance between processes that consume GSH and those that increase GSH; consumption is largely mediated by oxidation, degradation, and efflux whereas increases occur by de novo synthesis from precursor amino acids or uptake of extracellular GSH. Renal GGT, which is localized on the PT cell BBM and has its active site facing out into the tubular lumen, is responsible for turnover of most of the GSH in the body [1,6,9]. Although glomerular filtration is a significant source of tubular GSH, the majority of the luminal GSH derives from the PT cell, which in turn derives from GSH that is synthesized within the PT cell or is transported from the periplasmic space into the PT cell. Thus, uptake of GSH by transport across the BLM provides, along with intracellular GSH synthesis, the major source of luminal GSH. In times of oxidative or electrophilic stress, uptake of GSH at the BLM can enhance intracellular GSH levels and protect renal PT cells from injury [2,7,20].
The present study sought to better define the mechanism of GSH uptake at the renal BLM by assessing the potential function of Oat3 in the uptake process. We chose Oat3 to study first because previous inhibitor studies in renal PT cells were most consistent with this carrier functioning in BLM GSH uptake. While other carriers also clearly play some role, the present studies represent the first efforts to define the function of a specific carrier protein. Rat Oat3 was expressed in E. coli as an N-terminal His6-tagged protein and was purified from inclusion bodies and application of Ni2+-affinity chromatography. This approach for the purification of bacterially expressed, membrane proteins provides higher purity than simply isolating the expressed protein from inclusion bodies [31,32]. Expression of a His6-tagged protein and application of Ni2+-affinity chromatography with an appropriate elution pH assures that only protein containing the His6-tag is isolated [31,32].
The reconstituted Oat3 was shown to transport both GSH and PAH in exchange for 2-OG in a time- and concentration-dependent manner. Moreover, PAH transport was inhibited by both probenecid and furosemide, consistent with known properties of Oat3-mediated transport of organic anions. Although furosemide is selective for Oat3, estrone sulfate can also be used as a competitive inhibitor to provide additional evidence for the function of Oat3. Rates of Oat3-dependent GSH transport in the proteoliposomes were lower than those for PAH, but were still at high enough levels to be consistent with Oat3 playing a significant role in GSH transport across the BLM.
Studies in models possessing an intact BLM, including isolated membrane vesicles [17,18] and isolated renal PT cells [7,19,20], are consistent with the function of multiple carriers in the BLM transport of GSH. Such redundancy is not unexpected and occurs commonly in biological systems, particularly for organic anion transport [23–29]. Whether or not Oat1 also functions in GSH uptake will need to be directly tested. Additionally, our previously reported Na+-dependence of BLM GSH transport [2,17,18,20] and the sensitivity to dimethylsuccinate [20] suggested the possible involvement of NaC2. This will require further study as well.
Transport of GSH across the BLM in wild-type NRK-52E cells occurred at rates that were comparable to those for other organic anions (cf. Figure 3 and [37]). GSH transport across the BLM in NRK-52E cells occurred at only about 60% of the rate at which it occurs in freshly isolated rat PT cells [20]. This comparison and the present findings of little endogenous Oat1, Oat3, or NaC2 expression in the NRK-52E cells suggest that a significant fraction of GSH transport across the BLM occurs by other carrier(s). Nonetheless, the demonstration here that expressed and reconstituted Oat3 transports GSH, that compounds such as PAH, furosemide, and dimethylsuccinate inhibit GSH transport across the BLM [20], support the conclusion that Oat3 plays a quantitatively significant role in vivo. The relatively modest inhibition of PAH transport by probenecid in Oat3-reconstituted proteoliposomes as compared to what is typically observed may be caused by leakiness of the vesicles or non-carrier-mediated transport that is insensitive to Oat inhibitors.
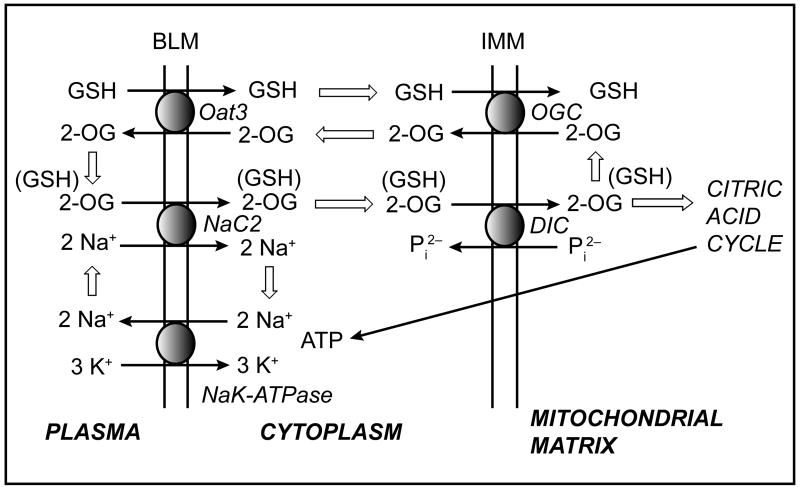
The involvement of Oat3 and possibly NaC2 in the BLM transport of GSH also indicates that GSH transport is closely linked to that of small dicarboxylates, in particular 2-OG. Interactions between GSH transport and that of dicarboxylates also exist at the level of the mitochondrial inner membrane; previous work of ours identified both the 2-oxoglutarate carrier (OGC; Slc25a11) and the dicarboxylate carrier (DIC; Slc25a10) as being responsible for most, if not all, the transport of cytoplasmic GSH into the mitochondrial matrix in rat proximal tubule [31–33,35]. These relationships are illustrated in Figure 5. Flux of 2-OG into and out of the cell at the BLM is controlled by Oat3 and NaC2, which mediate efflux and uptake, respectively. In each case, 2-OG transport is coupled to either uptake of an organic anion or co-transport with sodium ions, respectively. At the inner mitochondrial membrane, 2-OG can exchange with GSH or various dicarboxylates via action of the OGC, or can exchange with inorganic phosphate via action of the DIC. 2-OG and other oxidizable, intermediary metabolites in turn generate ATP via the citric acid cycle, thereby supplying high-energy phosphate for cellular work. One example of such ATP-dependent, cellular work is the (Na++K+)-stimulated ATPase on the BLM, which generates a sodium-ion gradient to drive secondary active transport processes such as the NaC2.
Figure 5. Interplay between GSH transport at the basolateral plasma and inner mitochondrial membranes in renal proximal tubule.
This summary scheme illustrates the interplay between various carriers that may function either directly or indirectly in the transport of GSH across the basolateral plasma membrane (BLM) and the inner mitochondrial membrane (IMM). Thick arrows indicate flux of substrate between pathways. See text for discussion. Abbreviations: DIC, dicarboxylate carrier; NaC2, sodium-dicarboxylate carrier 2; Oat3, organic anion transporter 3; 2-OG, 2-oxoglutarate; OGC, 2-oxoglutarate carrier; Pi2−, inorganic phosphate
One potential implication of the interconnections between transport of GSH and 2-OG is that cellular and mitochondrial GSH concentrations, and thus cellular and mitochondrial redox status, can be modulated by substrate supply. Accordingly, metabolic diseases or other pathological processes that affect intermediary metabolism or mitochondrial oxidative phosphorylation would be expected to alter cellular and mitochondrial GSH status by affecting the function of several transporters.
The present work has provided the first level of validation of the hypothesis that rat Oat3, which is expressed on the BLM of the renal PT cell, can function in GSH uptake. Additional studies are needed to more completely characterize transport kinetics and the relative importance of this carrier among all the potential carriers for GSH uptake at the BLM. One approach that is commonly used to assess carrier function is to express the carrier in an immortalized cell line that possesses low, endogenous activity of the carrier. In such an intact cellular model, function of the carrier can thereby be studied in relation to other cellular processes, such as cellular energetics and stress response. Our previous work used NRK-52E cells to characterize the function of carriers on the inner mitochondrial membrane in the transport of cytoplasmic GSH into the matrix [31,32]. Advantages of NRK-52E cells for study of mitochondrial GSH transport are that these cells have low, endogenous activity of mitochondrial GSH transport, very low activity of GGT, and are derived from rat PT cells [32]. For the present study, these cells have similar advantages, including having very low activity of Oat3 (cf. Figure 3) and no detectable expression of mRNA for either Oat3 or for other putative BLM carriers for GSH (cf. Figure 4B).
In summary, the present work has validated the hypothesis that rat Oat3 can transport GSH and showed that NRK-52E cells have very low endogenous Oat3 activity, suggesting that these cells are suitable models to overexpress putative GSH carriers and study their function in an intact renal PT-derived cell line.
Acknowledgments
This work was supported by a grant to L.H.L. from the National Institute of Diabetes and Digestive Diseases (R01-DK40725). Core facilities funded by the National Institute of Environmental Health Sciences Center for Molecular Toxicology with Human Application (Grant P30-ES06639) at Wayne State University were used for some of these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lash LH, Jones DP, Anders MW. Glutathione homeostasis and glutathione S-conjugate toxicity in the kidney. Rev Biochem Toxicol. 1988;9:29–67. [Google Scholar]
- 2.Lash LH. Role of glutathione transport processes in kidney function. Toxicol Appl Pharmacol. 2005;204:329–342. doi: 10.1016/j.taap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Ballatori N, Dutczak WJ. Identification and characterization of high and low affinity transport systems for reduced glutathione in liver cell canalicular membranes. J Biol Chem. 1994;269:19731–19737. [PubMed] [Google Scholar]
- 4.Kaplowitz N, Aw TY, Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol. 1985;25:715–744. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- 5.Lash LH. Glutathione and other antioxidant defense mechanisms. In: Goldstein RS, editor. Comprehensive Series in Toxicology. Vol. 7. Kidney Toxicology; Elsevier, Oxford: 1997. pp. 403–428. [Google Scholar]
- 6.Hinchman CA, Ballatori N. Glutathione-degrading capacities of liver and kidney in different species. Biochem Pharmacol. 1990;40:1131–1135. doi: 10.1016/0006-2952(90)90503-d. [DOI] [PubMed] [Google Scholar]
- 7.Hagen TM, Aw TY, Jones DP. Glutathione uptake and protection against oxidative injury in isolated kidney cells. Kidney Int. 1988;34:74–81. doi: 10.1038/ki.1988.147. [DOI] [PubMed] [Google Scholar]
- 8.Fonteles MC, Pillio DJ, Jeske AH, Leibach FH. Extraction of glutathione by the isolated perfused rabbit kidney. J Surg Res. 1976;21:169–174. doi: 10.1016/0022-4804(76)90154-2. [DOI] [PubMed] [Google Scholar]
- 9.Griffith OW, Meister A. Glutathione: Interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci USA. 1979;76:5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Häberle D, Wahlländer A, Sies H. Assessment of the kidney function in maintenance of plasma glutathione concentration and redox state in anesthetized rats. FEBS Lett. 1979;108:335–340. doi: 10.1016/0014-5793(79)80558-x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson ME, Bridges RJ, Meister A. Direct evidence for inter-organ transport of glutathione and that the non-filtration mechanism for glutathione utilization involves γ-glutamyl transpeptidase. Biochem Biophys Res Commun. 1980;96:848–853. doi: 10.1016/0006-291x(80)91433-3. [DOI] [PubMed] [Google Scholar]
- 12.Ormstad K, Låstbom T, Orrenius S. Evidence for different localization of glutathione oxidase and γ-glutamyltransferase activities during extracellular glutathione metabolism in isolated perfused kidney. Biochim Biophys Acta. 1982;700:148–153. doi: 10.1016/0167-4838(82)90091-7. [DOI] [PubMed] [Google Scholar]
- 13.Rankin BB, Curthoys NP. Evidence for renal paratubular transport of glutathione. FEBS Lett. 1982;147:193–196. doi: 10.1016/0014-5793(82)81040-5. [DOI] [PubMed] [Google Scholar]
- 14.Rankin BB, Wells W, Curthoys NP. Rat renal peritubular transport and metabolism of plasma [35S]glutathione. Am J Physiol. 1985;249:F198–F204. doi: 10.1152/ajprenal.1985.249.2.F198. [DOI] [PubMed] [Google Scholar]
- 15.Abbott WA, Bridges RJ, Meister A. Extracellular metabolism of glutathione accounts for its disappearance from the basolateral circulation of the kidneys. J Biol Chem. 1984;259:15393–15400. [PubMed] [Google Scholar]
- 16.Inoue M, Shinozuka S, Morino Y. Mechanism of peritubular extraction of plasma glutathione: The catalytic activity of contralumenal γ-glutamyltransferase is prerequisite to the apparent peritubular extraction of plasma glutathione. Eur J Biochem. 1986;157:605–609. doi: 10.1111/j.1432-1033.1986.tb09708.x. [DOI] [PubMed] [Google Scholar]
- 17.Lash LH, Jones DP. Transport of glutathione by renal basal-lateral membrane vesicles. Biochem Biophys Res Commun. 1983;112:55–60. doi: 10.1016/0006-291x(83)91796-5. [DOI] [PubMed] [Google Scholar]
- 18.Lash LH, Jones DP. Renal glutathione transport: Characteristics of the sodium-dependent system in the basal-lateral membrane. J Biol Chem. 1984;259:14508–14514. [PubMed] [Google Scholar]
- 19.Lash LH, Jones DP. Uptake of the glutathione conjugate S-(1,2-dichlorovinyl)glutathione by renal basal-lateral membrane vesicles and isolated kidney cells. Mol Pharmacol. 1985;28:278–282. [PubMed] [Google Scholar]
- 20.Lash LH, Putt DA. Renal cellular transport of exogenous glutathione: Heterogeneity at physiological and pharmacological concentrations. Biochem Pharmacol. 1999;58:897–907. doi: 10.1016/s0006-2952(99)00155-0. [DOI] [PubMed] [Google Scholar]
- 21.Ullrich KJ, Rumrich G, Wieland T, Dekant W. Contraluminal para-aminohippurate (PAH) transport in the proximal tubule of the rat. VI. Specificity: Amino acids, their N-methyl-, N-acetyl- and N-benzoylderivatives; glutathione- and cysteine conjugates, di- and oligopeptides. Pflügers Arch. 1989;415:342–350. doi: 10.1007/BF00370886. [DOI] [PubMed] [Google Scholar]
- 22.Pajor AM. Sodium-coupled transporters for Krebs cycle intermediates. Annu Rev Physiol. 1999;61:663–682. doi: 10.1146/annurev.physiol.61.1.663. [DOI] [PubMed] [Google Scholar]
- 23.van Aubel RAMH, Masereeuw R, Russel FGM. Molecular pharmacology of renal organic anion transporters. Am J Physiol. 2000;279:F216–F232. doi: 10.1152/ajprenal.2000.279.2.F216. [DOI] [PubMed] [Google Scholar]
- 24.Sekine T, Cha SH, Endou H. The multispecific organic anion transporter (OAT) family. Pflüg Arch – Eur J Physiol. 2000;440:337–350. doi: 10.1007/s004240000297. [DOI] [PubMed] [Google Scholar]
- 25.Burckhardt G, Bahn A, Wolff NA. Molecular physiology of renal p-aminohippurate secretion. News Physiol Sci. 2001;16:114–118. doi: 10.1152/physiologyonline.2001.16.3.114. [DOI] [PubMed] [Google Scholar]
- 26.Berkhin EB, Humphreys MH. Regulation of renal tubular secretion of organic compounds. Kidney Int. 2001;59:17–30. doi: 10.1046/j.1523-1755.2001.00461.x. [DOI] [PubMed] [Google Scholar]
- 27.Russel FGM, Masereeuw R, van Aubel RAMH. Molecular aspects of renal anionic drug transport. Annu Rev Physiol. 2002;64:563–594. doi: 10.1146/annurev.physiol.64.081501.155913. [DOI] [PubMed] [Google Scholar]
- 28.Lee W, Kim RB. Transporters and renal drug elimination. Annu Rev Pharmacol Toxicol. 2004;44:137–166. doi: 10.1146/annurev.pharmtox.44.101802.121856. [DOI] [PubMed] [Google Scholar]
- 29.Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev. 2004;84:987–1049. doi: 10.1152/physrev.00040.2003. [DOI] [PubMed] [Google Scholar]
- 30.Pombrio JM, Giangreco A, Li L, Wempe MF, Anders MW, Sweet DH, Pritchard JB, Ballatori N. Mercapturic acids (N-acetylcysteine S-conjugates) as endogenous substrates for the renal organic anion transporter-1. Mol Pharmacol. 2001;60:1091–1099. doi: 10.1124/mol.60.5.1091. [DOI] [PubMed] [Google Scholar]
- 31.Xu F, Putt DA, Matherly LH, Lash LH. Modulation of expression of rat mitochondrial 2-oxoglutarate carrier in NRK-52E cells alters mitochondrial transport and accumulation of glutathione and susceptibility to chemically induced apoptosis. J Pharmacol Exp Ther. 2006;316:1175–1186. doi: 10.1124/jpet.105.094599. [DOI] [PubMed] [Google Scholar]
- 32.Lash LH, Putt DA, Matherly LH. Protection of NRK-52E cells, a rat renal proximal tubular cell line, from chemical induced apoptosis by overexpression of a mitochondrial glutathione transporter. J Pharmacol Exp Ther. 2002;303:476–486. doi: 10.1124/jpet.102.040220. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Putt DA, Lash LH. Enrichment and functional reconstitution of glutathione transport activity from rabbit kidney mitochondria: Further evidence for the role of the dicarboxylate and 2-oxoglutarate carriers in mitochondrial glutathione transport. Arch Biochem Biophys. 2000;373:193–202. doi: 10.1006/abbi.1999.1527. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan RS, Pedersen PL. Isolation and reconstitution of the n-butylmalonate-sensitive dicarboxylate transporter from rat liver mitochondria. J Biol Chem. 1985;260:10293–10298. [PubMed] [Google Scholar]
- 35.Chen Z, Lash LH. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J Pharmacol Exp Ther. 1998;285:608–618. [PubMed] [Google Scholar]
- 36.Halestrap AP. The mitochondrial pyruvate carrier: Kinetics and specificity for substrates and inhibitors. Biochem J. 1975;148:85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lash LH, Putt DA, Hueni SE, Cao W, Xu F, Kulidjian SJ, Horwitz JP. Cellular energetics and glutathione status in NRK-52E cells: Toxicological implications. Biochem Pharmacol. 2002;64:1533–1546. doi: 10.1016/s0006-2952(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 38.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.Palmieri F, Indiveri C, Bisaccia F, Iacobazzi V. Mitochondrial metabolite carrier proteins: Purification, reconstitution, and transport studies. Methods Enzymol. 1995;260:349–369. doi: 10.1016/0076-6879(95)60150-3. [DOI] [PubMed] [Google Scholar]
- 40.Sweet DH, Chan LMS, Walden R, Yang XP, Miller DS, Pritchard JB. Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am J Physiol. 2003;284:F763–F769. doi: 10.1152/ajprenal.00405.2002. [DOI] [PubMed] [Google Scholar]
- 41.Lash LH. Susceptibility to toxic injury in different nephron cell populations. Toxicol Lett. 1990;53:97–104. doi: 10.1016/0378-4274(90)90101-q. [DOI] [PubMed] [Google Scholar]