Abstract
A genetic system has been developed for studying bacterial photosynthesis in the recently described nonsulfur purple photosynthetic bacterium Rhodospirillum centenum. Nonphotosynthetic mutants of R. centenum were obtained by enrichment for spontaneous mutations, by ethyl methanesulfonate mutagenesis coupled to penicillin selection on solid medium, and by Tn5 transposition mutagenesis with an IncP plasmid vector containing a temperature-sensitive origin of replication. In vivo and in vitro characterization of individual strains demonstrated that 38 strains contained mutations that blocked bacteriochlorophyll a biosynthesis at defined steps of the biosynthetic pathway. Collectively, these mutations were shown to block seven of eight steps of the pathway leading from protoporphyrin IX to bacteriochlorophyll a. Three mutants were isolated in which carotenoid biosynthesis was blocked early in the biosynthetic pathway; the mutants also exhibited pleiotropic effects on stability or assembly of the photosynthetic apparatus. Five mutants failed to assemble a functional reaction center complex, and seven mutants contained defects in electron transport as shown by an alteration in cytochromes. In addition, several regulatory mutants were isolated that acquired enhanced repression of bacteriochlorophyll in response to the presence of molecular oxygen. The phenotypes of these mutants are discussed in relation to those of similar mutants of Rhodobacter and other Rhodospirillum species of purple photosynthetic bacteria.
Full text
PDF


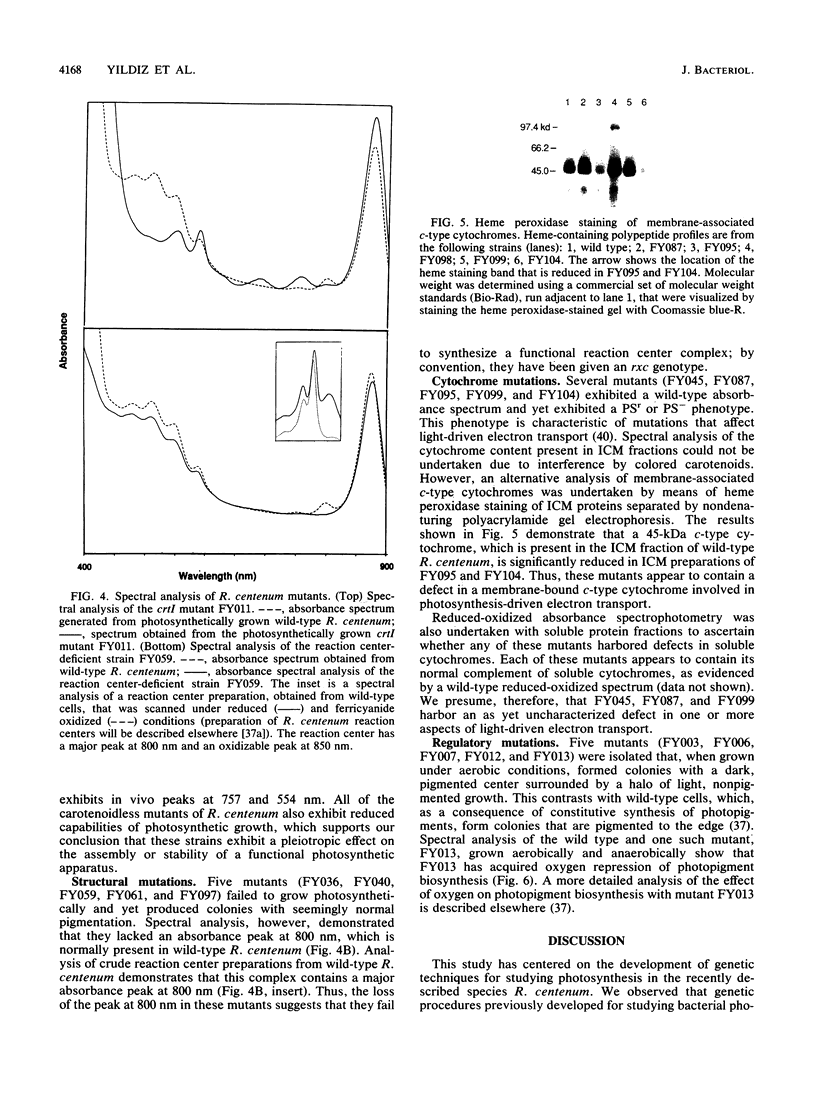
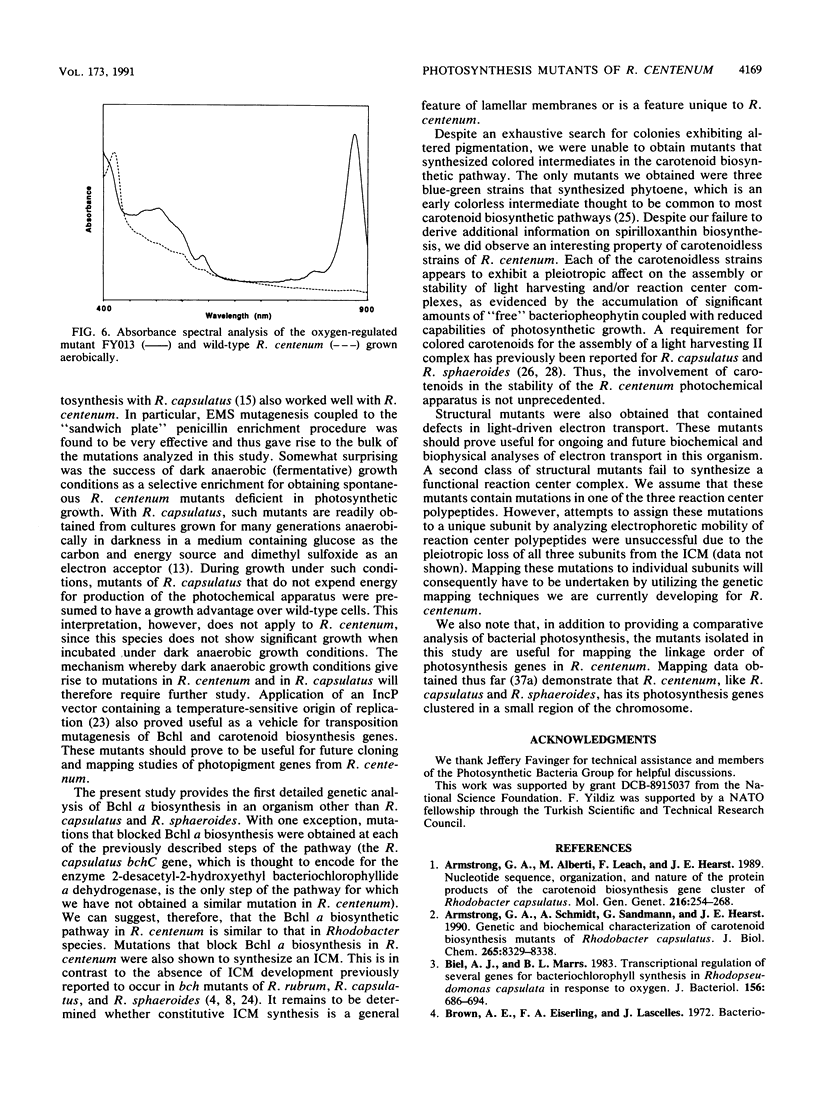

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. A., Alberti M., Leach F., Hearst J. E. Nucleotide sequence, organization, and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus. Mol Gen Genet. 1989 Apr;216(2-3):254–268. doi: 10.1007/BF00334364. [DOI] [PubMed] [Google Scholar]
- Armstrong G. A., Schmidt A., Sandmann G., Hearst J. E. Genetic and biochemical characterization of carotenoid biosynthesis mutants of Rhodobacter capsulatus. J Biol Chem. 1990 May 15;265(14):8329–8338. [PubMed] [Google Scholar]
- Biel A. J., Marrs B. L. Transcriptional regulation of several genes for bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata in response to oxygen. J Bacteriol. 1983 Nov;156(2):686–694. doi: 10.1128/jb.156.2.686-694.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomber S. A., Chaudhri M., Connor A., Britton G., Hunter C. N. Localized transposon Tn5 mutagenesis of the photosynthetic gene cluster of Rhodobacter sphaeroides. Mol Microbiol. 1990 Jun;4(6):977–989. doi: 10.1111/j.1365-2958.1990.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Daldal F., Cheng S., Applebaum J., Davidson E., Prince R. C. Cytochrome c(2) is not essential for photosynthetic growth of Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2012–2016. doi: 10.1073/pnas.83.7.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favinger J., Stadtwald R., Gest H. Rhodospirillum centenum, sp. nov., a thermotolerant cyst-forming anoxygenic photosynthetic bacterium. Antonie Van Leeuwenhoek. 1989 Mar;55(3):291–296. doi: 10.1007/BF00393857. [DOI] [PubMed] [Google Scholar]
- Giuliano G., Pollock D., Scolnik P. A. The gene crtI mediates the conversion of phytoene into colored carotenoids in Rhodopseudomonas capsulata. J Biol Chem. 1986 Oct 5;261(28):12925–12929. [PubMed] [Google Scholar]
- Madigan M., Cox J. C., Gest H. Photopigments in Rhodopseudomonas capsulata cells grown anaerobically in darkness. J Bacteriol. 1982 Jun;150(3):1422–1429. doi: 10.1128/jb.150.3.1422-1429.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B. Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1974 Mar;71(3):971–973. doi: 10.1073/pnas.71.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudek M. R., Richards W. R. A possible alternate pathway of bacteriochlorophyll biosynthesis in a mutant of Rhodopseudomonas sphaeroides. Biochemistry. 1975 Jul 15;14(14):3132–3137. doi: 10.1021/bi00685a015. [DOI] [PubMed] [Google Scholar]
- Richards W. R., Lascelles J. The biosynthesis of bacteriochlorophyll. The characterization of latter stage intermediates from mutants of Rhodopseudomonas spheroides. Biochemistry. 1969 Aug;8(8):3473–3482. doi: 10.1021/bi00836a051. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Yoshikawa M. A series of Tn5 variants with various drug-resistance markers and suicide vector for transposon mutagenesis. Gene. 1987;56(2-3):283–288. doi: 10.1016/0378-1119(87)90145-4. [DOI] [PubMed] [Google Scholar]
- Saunders V. A. Genetics of Rhodospirillaceae. Microbiol Rev. 1978 Jun;42(2):357–384. doi: 10.1128/mr.42.2.357-384.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick J., Drews G. The morphogenesis of the bacterial photosynthetic apparatus. 3. The features of a pheophytin-protein-carbohydrate complex excreted by the mutant M 46 of Rodospirillum rubrum. Biochim Biophys Acta. 1969 Jun 3;183(1):215–229. doi: 10.1016/0005-2736(69)90145-x. [DOI] [PubMed] [Google Scholar]
- Scolnik P. A., Walker M. A., Marrs B. L. Biosynthesis of carotenoids derived from neurosporene in Rhodopseudomonas capsulata. J Biol Chem. 1980 Mar 25;255(6):2427–2432. [PubMed] [Google Scholar]
- Scolnik P. A., Zannoni D., Marrs B. L. Spectral and functional comparisons between the carotenoids of the two antenna complexes of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1980 Dec 3;593(2):230–240. doi: 10.1016/0005-2728(80)90061-4. [DOI] [PubMed] [Google Scholar]
- Sojka G. A., Freeze H. H., Gest H. Quantitative estimation of bacteriochlorophyll in situ. Arch Biochem Biophys. 1970 Feb;136(2):578–580. doi: 10.1016/0003-9861(70)90231-6. [DOI] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N., Clark W. G., Marrs B. L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983 May;154(2):580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Yang Z. M., Bauer C. E. Rhodobacter capsulatus genes involved in early steps of the bacteriochlorophyll biosynthetic pathway. J Bacteriol. 1990 Sep;172(9):5001–5010. doi: 10.1128/jb.172.9.5001-5010.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H. C., Hu N. T., Marrs B. L. Characterization of the gene transfer agent made by an overproducer mutant of Rhodopseudomonas capsulata. J Mol Biol. 1979 Jun 25;131(2):157–168. doi: 10.1016/0022-2836(79)90071-8. [DOI] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata. J Bacteriol. 1976 May;126(2):619–629. doi: 10.1128/jb.126.2.619-629.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. A., Bauer C. E., Williams J. C., Marrs B. L. Genetic evidence for superoperonal organization of genes for photosynthetic pigments and pigment-binding proteins in Rhodobacter capsulatus. Mol Gen Genet. 1989 Jul;218(1):1–12. doi: 10.1007/BF00330558. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Hearst J. E. Genetic-physical mapping of a photosynthetic gene cluster from R. capsulata. Cell. 1984 Jul;37(3):937–947. doi: 10.1016/0092-8674(84)90428-8. [DOI] [PubMed] [Google Scholar]