Abstract
Objective
Standard outcome measures used for amyotrophic lateral sclerosis (ALS) clinical trials, including the ALS Functional Rating Scale-revised (ALSFRS-R), maximal voluntary isometric contraction testing (MVICT), and manual muscle testing (MMT) are limited in their ability to detect subtle disease progression. Electrical impedance myography (EIM) is a new non-invasive technique that provides quantitative data on muscle health by measuring localized tissue impedance. This study investigates whether EIM could provide a new outcome measure for use in ALS clinical trials work.
Methods
Fifteen ALS patients underwent repeated EIM measurements of one or more muscles over a period of up to 18 months and the primary outcome variable, θz-max, measured. The θz-max megascore was then calculated using the same approach as has been applied in the past for MVICT. This and the MMT data were then used to assess each measure’s statistical power to detect a given effect on disease progression in a hypothetical planned clinical therapeutic trial.
Results
θz-max showed a mean decline of about 21% for the test period, averaged across all patients and all tested muscles. The θz-max megascore had a power of 73% to detect a 10% treatment effect in our planned hypothetical trial, as compared to a 28% power for MMT. These results also compared favorably to historical data for ALSFRS-R and MVICT arm megascore from the trial of celecoxib in ALS, where both measures had only a 23% power to detect the same 10% treatment effect.
Conclusions
The θz-max megascore may provide a powerful new outcome measure for ALS clinical trials.
Significance
The application of EIM to future ALS trials may allow for smaller, faster studies with an improved ability to detect subtle treatment effects.
Keywords: Amyotrophic lateral sclerosis, Clinical trials Methodology, Electrical impedance myography
Amyotrophic lateral sclerosis (ALS) remains an ultimately fatal neurodegenerative disease without effective therapy. While recent trials have failed to demonstrate benefit of a variety of therapeutic interventions, including celecoxib, topiramate, creatine (Cudkowicz et al., 2003; Shefner et al., 2004; Cudkowicz et al., 2006), advances in basic science are leading to a proliferation of new agents that will require clinical evaluation. A significant problem in clinical trial design is that large numbers of patients must be studied for long time periods for modest therapeutic benefit to be appreciated, making the design of phase II proof-of-concept trials challenging. For example, the study of topiramate in ALS required 288 patients to be studied over a one-year period in order to obtain a 80% power to detect a slowing of disease progression of 35% (Cudkowicz et al., 2003).
The need for large sample size and long study duration is, in part, related to the nature of ALS itself. First, the progression of the disease is variable: some people deteriorate rapidly whereas others have a protracted course (Brooks et al., 1990). Moreover, the disease is inconsistent in its presentation. It often begins in a restricted fashion, affecting an arm, a leg, or the cranial nerve muscles, before gradually generalizing. Furthermore, some patients have a predominantly upper motor neuron presentation while in others lower motor neuron degeneration predominates.
In addition to these biological variables, the outcome measures themselves are limited in their inherent ability to detect change. Many measures have been shown to decline over time in ALS, with some having quite linear characteristics (Bryan et al., 2003). However, slow rates of decline combined with variability of measurement makes small changes in disease status very difficult to detect. A measure that is highly sensitive to change and reproducible would therefore be of intense interest and substantial benefit.
Electrical impedance myography (EIM) is a non-invasive, painless, quantitative technique for the assessment of muscle that has the potential to provide such an outcome measure for ALS clinical trials (Aaron and Shiffman, 2000; Shiffman et al., 1999; Rutkove et al., 2002). Similar to other bioimpedance-based techniques, EIM relies upon the application and surface measurement of high-frequency, low-intensity electrical current. However, EIM is distinctly different since it focuses on measurement of the impedance in relatively restricted regions of muscle (Rutkove et al., 2002), rather than on large areas of the body. In general, bioimpedance-based methods rely on the concept that tissues can be modeled as networks of resistors and capacitors (Schwan, 1957). In the case of EIM, the lipid bilayers of the muscle cell membranes act as the capacitors, the source of the reactance (X) of the network, and the intra- and extracellular fluids act as the resistors, the source of its resistance (R). The major EIM outcome variable is the phase angle, θ, calculated using the relationship θ = arctan (X/R). Measurements are often taken using multiple electrodes spanning the region of interest, in which case the spatially averaged value, θavg, is used as the principal outcome variable.
Work thus far has shown that θavg is effective in assessing both localized disorders (e.g., radiculopathy) and generalized diseases (e.g., inflammatory myopathy), lower values almost invariably reflecting more serious disease (Rutkove et al., 2005a; Tarulli et al., 2005). In addition, long-term prospective evaluation of a single ALS patient has demonstrated a major, steady decline in θavg with time, consistent with known disease behavior (Rutkove et al., 2002). Although we are refining the technique and its method of application (Esper et al., 2006), we have continued to enroll ALS patients for testing using the original method, given the prominent phase reductions observed in that initial case. In this paper we report data on 15 ALS patients followed longitudinally for up to 18 months, to determine the potential for EIM as a technique for measuring outcomes in ALS clinical trials. We compare these results to manual muscle testing (MMT) for the same cohort of patients as well as to historical data for maximal voluntary isometric contraction testing (MVICT) and ALS functional rating scale-revised (ALSFRS-R) (Cedarbaum et al., 1999). We also assess whether a simplified approach to data acquisition may be sufficiently robust for clinical trials application.
METHODS
Patients
Only subjects who met the El Escorial criteria for probable or definite ALS were included in this study, since only those categories of patients are typically eligible for participation in ALS clinical trials. Those with a clinical diagnosis of primary lateral sclerosis or those with atypical clinical histories or persistently restricted disease (e.g. monomelic amyotrophy) were excluded. Patients with edema (1+ or greater) overlying the muscle(s) to be evaluated were also excluded from the study. Subjects were recruited from the neurology and ALS clinics of Beth Israel Deaconess Medical Center and Massachusetts General Hospital. All subjects participated voluntarily and signed an institutional review board approved informed consent form.
EIM measurements
In this study, data were obtained using either a modified RJL Model 101-A impedance instrument (RJL Electronics, Clinton Township, Michigan) operating at 50 kHz (Shiffman et al., 1999), or a wide range lock-in amplifier (Signal Recovery Model 7280, Advanced Measurement Technology Inc., Oak Ridge, TN, USA) with current supplied from the 1 volt reference channel output (Esper et al., 2006). With the latter device, 50 kHz values were extracted from multiple frequency data. The two systems were calibrated against the same resistor-capacitor network designed to mimic the behavior of typical muscle tissue.
Although EIM can be applied to any superficial muscle group, in this study measurements were restricted to the 5 muscles: biceps, forearm flexors, quadriceps, tibialis anterior, and medial gastrocnemius. Also, we have continued to use electrode configurations developed in our earlier work. For each muscle, a series of strip electrodes (part number 019-766400, Viasys Healthcare/Nicolet Biomedical, Madison, WI, cut to half-length) was placed along the long axis of the limb and two current-injecting electrodes (Disposable Ground Plate Electrodes, part number 019-400500, Viasys Healthcare/Nicolet Biomedical, Madison, WI) were placed at a distance from this array. For upper extremity muscle measurement, the current injecting electrodes were placed on the palms of both hands, and for each lower extremity muscle measurement they were placed on the dorsum of both feet. Although the distance between the voltage and current electrodes thus varied depending on arm or leg length, our previous work suggests that such effects will be negligible (Rutkove et al., 2005b). Figure 1 shows the electrode configuration for tibialis anterior as an example. Specific details for the voltage electrode arrangements have been described previously (Esper et al., 2006).
Figure 1.
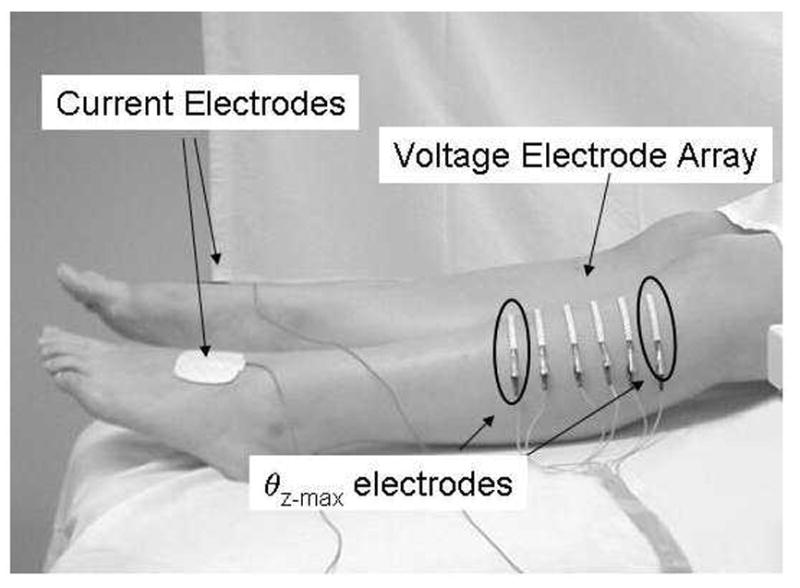
Standard EIM set-up for recording over tibialis anterior. The current injecting electrodes are placed on both feet and the voltage electrode array is positioned over the muscle. The entire array is used to calculate θavg, whereas only the first and last voltage electrodes (circled) are used to calculate θz-max.
Voltages were measured between the most distal voltage electrode and progressively more proximal members of the array, selected by computer controlled relays. The current through the limb was fixed (in the case of the 50 kHz instrument) or measured separately (when the multi-frequency system was used), from which the resistances and reactances were calculated for increasing values of z, the distance along the muscle. We calculated the phase, θ(z) = arctan{X(z)/R(z)} and, from the area under the θ(z) curve, the spatially averaged phase, θavg. Since we were interested in determining whether a simplified version of the technique would suffice for clinical trials, we also examined what we refer to as the “z-max” phase, θz-max, found from the measured voltages between the first and last members of the array, ignoring data from any of the others (Figure 1).
Manual muscle strength testing
MMT was performed using a standard 1–5 modified Medical Research Council scale, with pluses converted to 0.33 above the integer value and minuses to 0.33 below (i.e., a 4-converts into 3.66). Five arm and 5 leg muscle groups were measured: shoulder flexion, elbow flexion and extension, wrist extension and flexion, hip flexion, knee extension and flexion, ankle flexion and extension. Measurements were made by the same examiner on each patient on repeated visits whenever possible. The average value for all 10 muscle groups (global MMT) was calculated and used in analysis.
Data analysis
Most clinical ALS trials are now structured to last no more than about 1–2 years; accordingly, we restricted analysis to data for the first 18 months. (Data for more than 18 months had been only obtained for 3 of the 15 patients.)
Commonly used outcome measures such as MMT, MVICT, and ALSFRS-R yield a summary score for the entire individual, although subscores can also be employed. Thus, although we assessed individual muscle data, we also adopted summary scores appropriate to EIM, creating z-transformed values and a megascore along the lines previously developed for quantitative muscle testing (Andres et al., 1988). In this case, however, the required mean values and standard deviations were taken from data from 106 healthy individuals (aged 18–90 years, mean age 51.5 years) who were studied as part of our ongoing developmental work in EIM. Specifically, the difference between each muscle’s phase and the normal mean for that muscle was expressed as a multiple of the standard deviation for the normal distribution, and these “z-scores” were then averaged across all the muscles to give the megascore for that individual. This approach has the advantage of assuring that the values for all muscles are equally weighted (ie those with a high normal θz-max do not overwhelm those with a low θz-max).
Rather than only comparing changes over time for EIM versus MMT, we chose to incorporate the data obtained here into a power analysis as if we were planning a new placebo-controlled trial, using EIM θz-max megascore along with the other standard measures of disease progression, including MVICT and ALSFRS-R. The basis of the analysis was the assumption that for muscles undergoing active denervation, EIM phase, like most other outcome measures, falls nearly linearly with time (Bryan et al., 2003), and thus the slope of each parameter over time can be calculated and compared. The structure of this planned hypothetical trial was as follows: 100 patients receiving drug, 100 patients receiving placebo, followed for one year with bi-monthly evaluations, with a one-sided 0.05 significance level, and a 2% per month dropout rate. The potential power was calculated, assuming the treatment effect was 30%, 20% and 10% based on the slopes of the EIM megascore and global MMT. The data obtained here were also compared to the ALSFRS-R and MVICT arm strength megascore data obtained from a previous trial of celecoxib in ALS (Cudkowicz et al., 2006).
We assumed the trial would be analyzed using a random effects analysis of variance using PROC MIXED in SAS (SAS Institute Inc., Cary, North Carolina). Again, the basic idea is that patient trajectories are linear, with slopes and intercepts randomly distributed about average values that may depend on treatment. Thus, for each patient, the data over time were fitted to a line and the slopes of those lines used in the analysis. The dependent variables are the outcome measures’ slopes and the independent variable is time. We assumed the covariance was zero. The important factors that affect sample size in such a calculation are threefold. First, there is the mean slope of patients’ trajectories. Secondly, there is the standard deviation of the trajectories about the mean. For clarification, we report the ratio of the standard deviation over the mean slope as the coefficient of variation. Thirdly, there is the standard deviation of patients’ measurement about their trajectories, which we report as the within-patient standard deviation. The extent to which the last parameter affects power depends on the design of the study. As the number of observations or the duration of the observation increase, the effect of the within-patient variation should decrease.
RESULTS
Fifteen patients (10 men and 5 women, mean age 58.7 years, range 37.6 – 74.5 years) met our inclusion criteria and participated in this study. They were followed for an average of 187 days (range 32 to 491), with 10 having 2 visits and 5 having 3 or more. The muscles which were tested depended on the time available and body region most affected by the disease: fourteen patients had biceps studied, 9 forearm flexors, 5 medial gastrocnemius, 13 quadriceps, and 10 tibialis anterior. The behaviors of θz-max and θavg were essentially the same; both showed a mean decline of 21% across all the muscles, although the standard deviation was somewhat greater for θz-max (±16%) than for θavg (±12%). By comparison, global MMT score decreased on average by only 9.5 ± 7.3% for the group.
Having established that much more easily obtained θz-max provided similar data to θavg, we calculated the θz-max megascore for each patient using the previously described approach (Andres et al., 1988) and found that it declined at 0.10 megascore points per month (Figure 2), with a coefficient of variation of 0.21 and a within-patient standard deviation of 0.22. In comparison, MMT declined at a slower mean rate of 0.073 per month (coefficient of variation: 0.64; within-patient standard deviation: 0.092).
Figure 2.
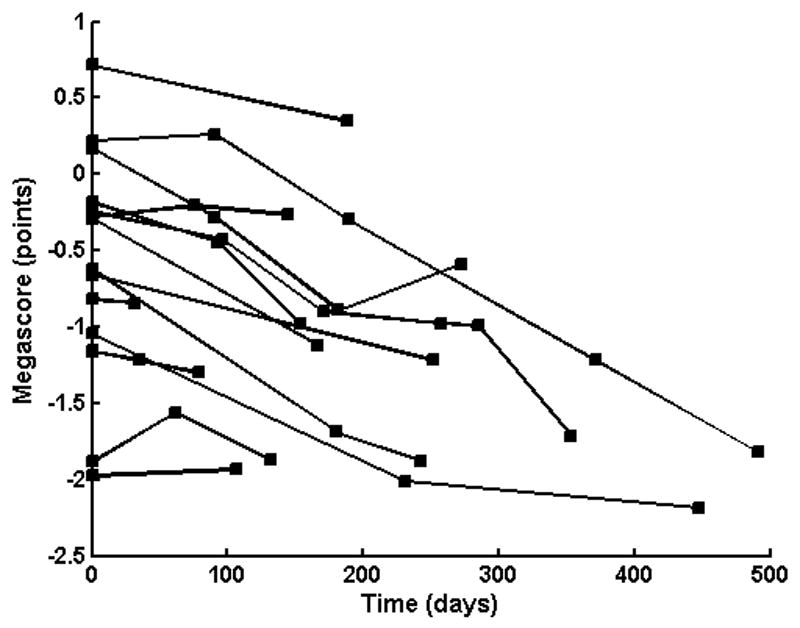
Plot of θz-max megascore over time in each of the 15 ALS patients. The data from two patients are overlying one another (both starting at approximately −1.2 points).
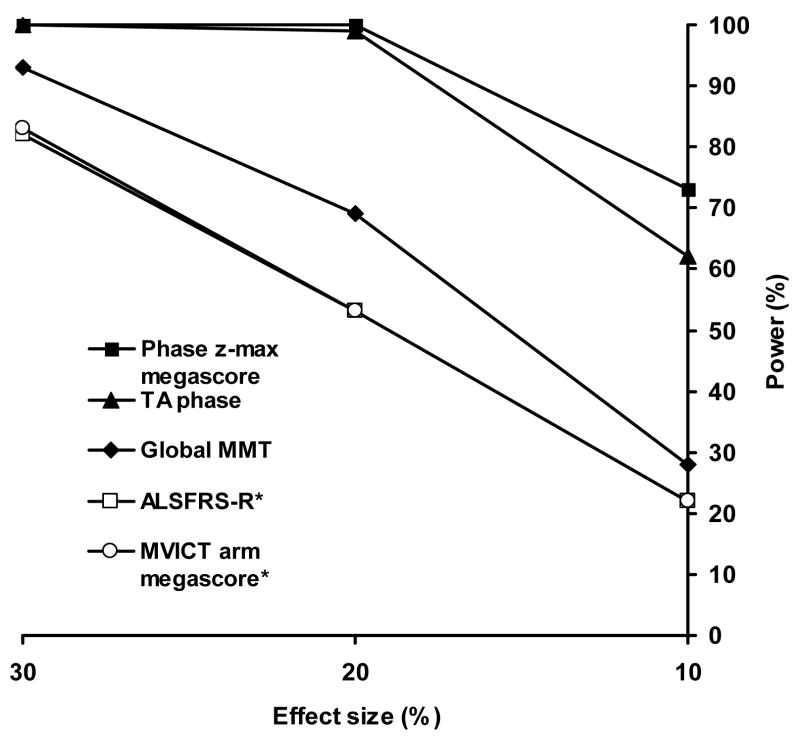
Next, we performed a power analysis as if planning for our hypothetical trial (see Figure 3) comparing EIM’s θz-max meagascore to MMT. This analysis confirmed that the megascore based on θz-max had a much higher power to detect a given treatment effect than MMT (73% versus 28% for the same 10% treatment effect). Although a comparison with historical data needs to be interpreted cautiously, ALSFRS-R (mean decline: 1.07 per month; coefficient of variation: 0.78 and within-patient standard deviation: 2.08) and MVICT megascores (mean decline: 0.095 per month; coefficient of variation: 0.77; within-patient standard deviation: 0.21) obtained in the celecoxib in ALS trial also would have had a considerably weaker power to detect a given treatment effect (Figure 3). Evaluated another way, to achieve EIM’s power of 73%, over 700 patients would be needed in each arm of an ALS trial using either MVICT or ALSFRS-R, for a total of more than 1400 patients vs. 200 patients using the θz-max megascore.
Figure 3.
Plot of potential power for three different effect sizes for each of the measures investigated based on the 1-year study design described in the text. Note that the ALSFRS-R and MVICT megascore values virtually overlie one another. *Data derived from celecoxib in ALS study (Cudkowicz et al, 2006).
Finally, since EIM has been found to be highly reproducible (Rutkove et al., 2006), we were also interested in seeing whether single-muscle EIM data could be used as an outcome measure. We compared the data across subjects for each of the muscles (except for medial gastrocnemius since it was only studied in 5 patients) and did a similar power analysis. Although the data for most muscles fared no better than the standard measures, of interest was the data for tibialis anterior which showed a relatively low coefficient of variation of 0.20 (mean decline: 0.25 per month; within-patient standard deviation: 0.52) and a very similar power to that of the θz-max megascore (Figure 3).
DISCUSSION
These results support the view that EIM has the potential of serving as an effective outcome measure for assessing disease progression in ALS, using the more simply obtained measure θz-max. This was true both in comparison to MMT data from the same group of patients and for historical data for ALSFRS-R and MVICT.
The primary EIM variable that we evaluate here is the phase, in the forms of θavg and θz-max. Although the reactance and resistance can also be examined separately, both are much more dependent on muscle shape and size, being approximately inversely proportional to the cross-sectional area of the measured region. However, these dependences tend to cancel in the calculation of the phase, and thus changes over time for a given subject predominantly reflect variation in the inherent characteristics of the tissue itself rather than simple size or shape effects. We note also that the reduction in phase with increasing disease severity is not specific to neurogenic disorders, since myopathic disorders show a similar change (Tarulli et al., 2005) as does muscle disuse [unpublished results]. In fact, it is possible that the reductions in phase observed here are in part related to axon loss and in part related to the superimposed disuse that affects the muscle as it becomes increasingly functionally impaired, due to both central and peripheral disease effects.
EIM is a new technique that is still under development and is likely unfamiliar to most neurologists; thus, several salient points are worth reviewing. First, as applied in this study, the technique measures the group of muscles underlying the voltage electrodes and not a single muscle. Other EIM approaches may be useful for the study of individual muscles, but those methods are still in relatively early stages of development. Second, the measurements are made from specified landmarks and the electrodes placed accordingly; no specific optimization procedure is required for this form of testing. Third, EIM is quick to perform. Using just 2 voltage electrodes to perform a measurement takes no more than about 3 minutes and the entire group of 5 muscles studied here can be completed within 15–20 minutes. Fourth, EIM can be applied successfully to the cranial nerve muscles (e.g. masseter) or thoracic muscles (e.g. thoracic paraspinal muscles), and thus be of use in patients with weakness affecting those regions, a definite advantage over conventional outcome measures.
An established characteristic of ALS is the linear behavior of commonly used outcome measures as functions of time, as a muscle or group of muscles deteriorates. Correspondingly, the effect of a hypothetical drug treatment can be taken as the percentage change in the slope of the chosen outcome measure, e.g. the phase megascore in the case of EIM, as was done here. In power calculations, the important factors for determining sample sizes for a specified effect would then be patient-to-patient variations in slope and the variations of slope with time for individual patients. The first of these simply represents the different rates of disease progression in ALS, as measured by the given technique, while the second reflects a combination of measurement errors and genuine departures from strict linear behavior. In addition, since some muscles will be in the process of active deterioration while others will not, there is a risk of underestimating the slope of decline in any given patient by mixing the effects of those actively deteriorating with those muscles that are yet to be affected or those in which no functioning motor units remain. This issue, of course, applies to the hypothetical EIM drug test power calculations presented here as well as to the global MMT results.
However, there is in principle no reason why the reliance on muscle-averaged slopes must inevitably be invoked. For this reason, we also evaluated a single-muscle approach to disease assessment, which would be considerably simpler to implement than a multiple-muscle approach. Although this implies representing the disease course for the entire body by the behavior of a single muscle, such an approach is taken routinely in motor unit number estimation where a single hand muscle is assessed repeatedly over time. Of course, such a single-muscle approach would demand very high reproducibility, but EIM has been shown to have extremely high reproducibility at the single-muscle level in biceps, quadriceps, and tibialis anterior (Rutkove et al., 2006), and with careful technique it is likely to demonstrate high reproducibility elsewhere as well. In contrast, single-muscle reproducibility for MMT and MVICT is relatively low, necessitating data collection from multiple muscles and averaging of the results to help reduce scatter. We should note that in the present single-muscle comparisons, it is not clear why tibialis anterior fared better than the other muscles, but that may relate to the possibility of disease progression occurring most substantially in that region during the study period in these patients. In any case, further EIM research may consider alternatives to the megascore approach, such as following multiple separate muscles phases normalized to their first visit values, which would allow the active stage of deterioration in individual muscles to be compared between patients.
Like any measure, EIM has its limitations, and a variety of factors can affect the reliability of the results. For example, edema and artificial joints may produce spuriously low values for the phase, and thus limbs impacted by these factors will need to be excluded from study. We also note that EIM is position-dependent and that major changes in the angle of a joint at the time of measurement may cause considerable variability. Similarly, placing the voltage electrodes in the same positions at each measurement session can be very important. For example, phase may change up to 11% for a 1 cm distal-proximal shift of the electrode array (Rutkove et al., 2005b). The use of small tattoos to assist in repeated electrode placements can certainly help overcome this problem. These factors, in truth, may account for much of the observed variability in the EIM measurements seen in Figure 2, since none of these patients improved clinically. We also note that the phase changes reported here are far beyond those found for normal subjects followed over comparable time periods (Aaron et al., 2006, Rutkove et al., 2006), so are highly unlikely to be due to an effect of aging alone.
A more general area of interest concerns the nature of the relationship between muscle deterioration and changes in the EIM phase. First, it is likely that the changes in θz-max observed in ALS patients are predominantly due to muscle fiber denervation and atrophy and not that due to reinnervation, but this is yet to be proven and is currently the subject of investigation in rats (see Nie et al., 2006). Still, one cannot exclude the possibility that accompanying changes in the connective tissue, the state of hydration, or even the blood supply to the muscle may contribute to the reductions in θz-max that occur with disease progression. One point, however, is clear. Despite the measurements being taken via surface electrodes, the skin and subcutaneous fat contribute minimally to the EIM data. The four-electrode technique, coupled with distant placement of the current electrodes and high-impedance input circuitry, reduces the contribution of the skin and the subcutaneous fat to a negligible level (Shiffman et al, 1999). Moreover, of all tissues in a limb, muscle contributes by far the greatest extent to the EIM data since is has a very low resistivity (Faes et al., 1999) and relativity large volume, thus serving as the preferential path for electrical current flow.
Regardless of the actual mechanism underlying EIM phase reduction, this study shows that θz-max has substantial potential to serve as an outcome measure in ALS clinical trials. Given the robustness of the data, our next step will be to perform a dedicated investigation prospectively comparing EIM against existing outcome measures of disease progression, including survival, ALSFRS-R, MVICT, and MUNE, using a larger group of patients at multiple centers and with several testing sessions per patient. The results should help determine whether EIM can be effectively and meaningfully used as a new outcome measure for ALS clinical trials work.
Acknowledgments
This study was supported by the National Institutes of Health, Grant RO1-NS42037-01A2 and Grant RR01032 to the Beth Israel Deaconess Medical Center General Clinical Research Center.
Footnotes
Disclosure: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaron R, Shiffman CA. Using localized impedance measurements to study muscle changes in injury and disease. Ann N Y Acad Sci. 2000;904:171–180. doi: 10.1111/j.1749-6632.2000.tb06443.x. [DOI] [PubMed] [Google Scholar]
- Aaron R, Esper GJ, Shiffman CA, Bradonjic K, Lee KS, Rutkove SB. Effects of age on muscle as measured by electrical impedance myography. Physiol Meas. 2006;27:953–9. doi: 10.1088/0967-3334/27/10/002. [DOI] [PubMed] [Google Scholar]
- Andres PL, Finison LJ, Conlon T, Thibodeau LM, Munsat TL. Use of composite scores (megascores) to measure deficit in amyotrophic lateral sclerosis. Neurology. 1988;38:405–8. doi: 10.1212/wnl.38.3.405. [DOI] [PubMed] [Google Scholar]
- Brooks B, Depaul R, Tan Y, Sanjak M, Sufit R, Robbins J. Controlled clinical trials in neurological disease. In: Porter R, Schoenberg B, editors. Motor neuron disease. Norwell, MA: Kluwer Academic Publishers; 1990. pp. 249–281. [Google Scholar]
- Bryan WW, Hoagland RJ, Murphy J, Armon C, Barohn RJ, Goodpasture JC, Miller RG, Parry GJ, Petajan JH, Ross MA, Stromatt SC. Can we eliminate placebo in ALS clinical trials? Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:11–5. doi: 10.1080/14660820310006661. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Cudkowicz ME, Shefner JM, Schoenfeld DA, Brown RH, Jr, Johnson H, Qureshi M, Jacobs M, Rothstein JD, Appel SH, Pascuzzi RM, Heiman-Patterson TD, Donofrio PD, David WS, Russell JA, Tandan R, Pioro EP, Felice KJ, Rosenfeld J, Mandler RN, Sachs GM, Bradley WG, Raynor EM, Baquis GD, Belsh JM, Novella S, Goldstein J, Hulihan J. A randomized, placebo-controlled trial of topiramate in amyotrophic lateral sclerosis. Neurology. 2003;61:456–64. doi: 10.1212/wnl.61.4.456. [DOI] [PubMed] [Google Scholar]
- Cudkowicz ME, Shefner JM, Schoenfeld DA, Zhang H, Andreasson KI, Rothstein JD, Drachman DB. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol. 2006;60:22–31. doi: 10.1002/ana.20903. [DOI] [PubMed] [Google Scholar]
- Esper GJ, Shiffman CA, Aaron R, Lee KS, Rutkove SB. Assessing neuromuscular disease with multifrequency electrical impedance myography. Muscle Nerve. 2006;34:595–602. doi: 10.1002/mus.20626. [DOI] [PubMed] [Google Scholar]
- Faes TJ, van der Meij HA, de Munck JC, Heethaar RM. The electric resistivity of human tissues (100 Hz-10 MHz): a meta-analysis of review studies. Physiol Meas. 1999;20:R1–10. doi: 10.1088/0967-3334/20/4/201. [DOI] [PubMed] [Google Scholar]
- Nie R, Chin AB, Lee KS, Sunmonu NA, Rutkove SB. Electrical impedance myography: transitioning from human to animal studies. Clin Neurophys. 2006;117:1844–9. doi: 10.1016/j.clinph.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Rutkove SB, Aaron R, Shiffman CA. Localized bioimpedance analysis in the evaluation of neuromuscular disease. Muscle Nerve. 2002;25:390–7. doi: 10.1002/mus.10048. [DOI] [PubMed] [Google Scholar]
- Rutkove SB, Esper GJ, Lee KS, Aaron R, Shiffman CA. Electrical impedance myography in the detection of radiculopathy. Muscle Nerve. 2005;32:335–341. doi: 10.1002/mus.20377. [DOI] [PubMed] [Google Scholar]
- Rutkove SB, Lee KS, Shiffman CA, Aaron R. Test-Retest Reproducibility of 50 kHz Linear-Electrical Impedance Myography. Clin Neurophys. 2006;117:1244–8. doi: 10.1016/j.clinph.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Rutkove SB, Partida RA, Esper GJ, Aaron R, Shiffman CA. Electrode position and size in electrical impedance myography. Clin Neurophys. 2005;116:290–299. doi: 10.1016/j.clinph.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Schwan HP. Electrical properties of tissue and cell suspensions. Adv Biol Med Phys. 1957;5:147–209. doi: 10.1016/b978-1-4832-3111-2.50008-0. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Cudkowicz ME, Schoenfeld DA, Conrad T, Taft J, Chilton M, Urbinelli L, Qureshi M, Zhang H, Pestronk A, Caress JB, Donofrio P, Sorenson E, Bradley W, Lomen-Hoerth C, Pioro EB, Rezania K, Ross M, Pascuzzi R, Heiman-Patterson T, Tandan R, Mitsumoto H, Rothstein J, Smith-Palmer T, MacDonald D, Burke D. A clinical trial of creatine in ALS. Neurology. 2004;63:1656–61. doi: 10.1212/01.wnl.0000142992.81995.f0. [DOI] [PubMed] [Google Scholar]
- Shiffman CA, Aaron R, Amoss V, Therrien J, Coomler K. Resistivity and phase in localized BIA. Phys Med Biol. 1999;44:2409–2429. doi: 10.1088/0031-9155/44/10/304. [DOI] [PubMed] [Google Scholar]
- Tarulli A, Esper GJ, Lee KS, Aaron R, Shiffman CA, Rutkove SB. Electrical impedance myography in the bedside assessment of inflammatory myopathy. Neurology. 2005;65:451–452. doi: 10.1212/01.wnl.0000172338.95064.cb. [DOI] [PubMed] [Google Scholar]