Abstract
CD147 is a type I transmembrane protein previously identified as a signal transducing receptor for extracellular cyclophilins. CD147-expressing cells exhibit a characteristic activation of extracellular-signal regulated kinase 1 and 2 (ERK1/2) in response to stimulation with cyclophilin A (CypA). CD147 was also shown to enhance HIV-1 infection in a CypA-dependent fashion, but the role of signaling in this activity of CD147 has not been investigated. In this report, we demonstrate that neither mutations incapacitating signaling response of CD147 to CypA stimulation, nor inhibitor of ERK activation, reduced susceptibility of cells to HIV-1 infection. Surprisingly, truncation of the cytoplasmic tail of CD147 did not abolish signaling response to CypA, but reduced infection by HIV-1 to the level observed in control cells. These results indicate that CD147 enhances HIV-1 replication in a signaling-independent fashion through specific events mediated by the cytoplasmic domain of the protein.
Keywords: HIV-1, CD147, cyclophilin A, ERK, signaling, cytoplasmic domain, co-factor, replication
Introduction
Cyclophilin A (CypA) is a ubiquitously distributed intracellular protein possessing peptidyl-prolyl cis-trans isomerase activity [1]. This activity enables CypA to assist protein folding and function as a chaperone during various cellular processes [2]. CypA also binds with high affinity to immunosuppressive drug cyclosporin A (CsA), and this binding is required for the immunosuppressive effect of CsA [3].
In addition to its intracellular functions, CypA can be secreted into the extracellular environment and has been shown to induce chemotaxis of monocytes, neutrophils, and T lymphocytes [4-8]. The chemotactic activity of CypA is likely related to its ability to initiate signaling response in target cells, characterized by activation of the extracellular signal regulated kinase 1 and 2 (ERK1/2)-dependent pathway [9; 10]. These features suggest that CypA, and also CypB that shares many of CypA activities [4; 6; 11], can be considered as mediators of intercellular communication [12].
The extracellular activities of cyclophilins imply existence of a cyclophilin receptor. Our studies identified CD147 as an essential component of the cell-surface signaling receptor to CypA and CypB [10; 11; 13]. This notion has been supported in a number of subsequent publications [14-16]. CypA is incorporated into HIV-1 particles during virus morphogenesis through a specific interaction with the CA domain of the Gag precursor polyprotein [17-20] and plays an essential role in the early steps of the HIV-1 life cycle [21; 22]. Biochemical studies indicate that CypA is exposed on the viral surface [23; 24] and thus may signal through CD147. CD147 has been shown also to stimulate, in a CypA-dependent manner, an early step of HIV-1 replication [13], however, the role of signaling events in this activity of CD147 has not been investigated. In this report, we provide evidence that signaling from CD147 is not required for its activity in HIV-1 infection. Unexpectedly, the cytoplasmic domain of CD147 is essential for stimulation of HIV-1 infection, although it is unnecessary for CD147 signaling activity.
Materials and Methods
CD147ΔC cloning
Construct encoding CD147 lacking the cytoplasmic tail (CD147ΔC) was prepared by PCR using direct primer 5'-gctaagcttgccaccatggcggctgcgctgttc-3' and reverse primer 5'-gaaggatcctcactaccggcgcttctcgtagatgaagatgat-3'. This cDNA encodes two stop-codons (TAG and TGA) after the fourth residue (Arg232) of the cytoplasmic domain of CD147. It was cloned between the HindIII and BamHI sites of pcDNA3.1+/Zeo (Invitrogen) and introduced into CHO-K1 cells.
CD147-expressing CHO cells
CHO-K1 cells were transfected using Fugene 6 (Roche) according to manufacturer's protocol. Transfected cells were cloned by limiting dilution and cultured in the presence of 50 μg/ml zeomycin. Individual clones were analyzed for CD147 expression by FACS using FITC-conjugated anti-CD147 antibody (Ancell).
HIV-1 infection
CHO-K1 cells were infected with luciferase-expressing HIV-1 recombinant (5 ng of p24 per 106 cells) [25; 26] pseudotyped with Env of amphotropic MuLV [27]. After 4 days, cells were washed and lysed in reporter lysis buffer (Promega), and the luciferase activity was measured in relative light units using a Dynex MLX microplate luminometer.
Analysis of signaling
Serum-starved cells were treated with 1 μg/ml of recombinant CypA prepared as previously described [10].Cell lysates were separated on 10% SDS-PAGE and analyzed by Western blotting using antibodies specific for the nonphosphorylated and phosphorylated forms of ERK1/2 MAP kinase (New England Biolabs) following the protocol provided by the manufacturer.
Results and Discussion
Signaling is not required for CD147-mediated enhancement of HIV infection
To address the role of signaling in the activity of CD147 as a co-factor in HIV-1 infection, we took advantage of our finding that mutation of Pro180Gly181 to alanines (PG180,181AA) in the extracellular domain of CD147 disrupted the ability of CD147 to initiate signaling responses to CypA stimulation [10]. We investigated activity of this mutant using a previously described approach which relies on infection of CD147-transfected CHO cells with HIV-1 construct pseudotyped with an amphotropic MuLV envelope [13]. Such pseudotyped virus is going through a one-cycle replication in CHO cells. Importantly, this approach has been previously validated as an unbiased test for CypA-CD147 interactions [13].
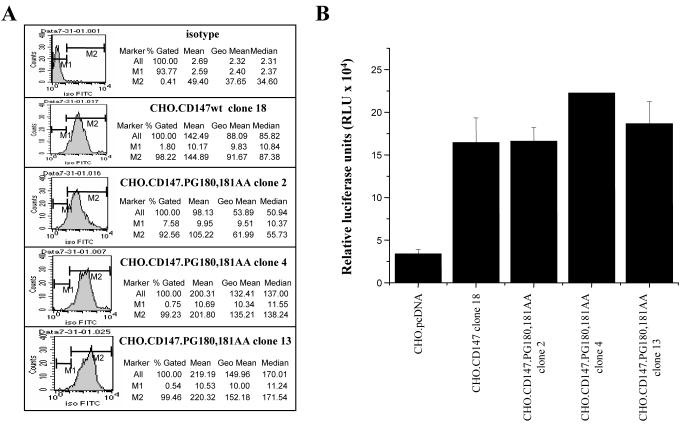
CHO cells were transfected with vectors expressing either wild-type CD147 or PG180,181AA mutant, and clones stably expressing these proteins were selected. One clone expressing the wild-type CD147 (CHO.CD147wt) and three clones expressing the mutant protein (CHO.CD147 PG180,181AA) were selected based on the highest level of CD147 expression (Fig. 1A). Cells were infected with luciferase-expressing HIV-1 construct pseudotyped with MuLV envelope, and HIV-1 replication was monitored by luciferase expression. As shown in Figure 1B, luciferase activity in clones expressing CD147.PG180,189AA was similar to activity in cells transfected with the wild-type CD147 and was substantially higher than in cells transfected with an empty vector. This result indicates that signaling is not necessary for CD147 activity during HIV-1 infection.
Figure 1. Enhancement of HIV-1 infection by a non-signaling CD147 mutant.
A. Analysis of CD147 expression on stable clones of CD147wt and CD147.PG180,181AA-transfected CHO cells by flow cytometry. Cells were stained with FITC-conjugated anti-CD147 mAb, and analyzed on BD FACScan. B. Analysis of HIV-1 replication by luciferase activity. Stable clones of CHO cells transfected with wild-type (CHO.CD147wt) or non-signaling CD147 (CHO.CD147PG180,181AA) or with an empty vector pcDNA3.1 (CHO.pcDNA) were inoculated with luciferase-expressing HIV-1 pseudotyped with an envelope of amphotropic murine leukemia virus (MuLV). Luciferase expression was measured on day 4 post-infection. Results and presented as mean ± SE of three independent wells.
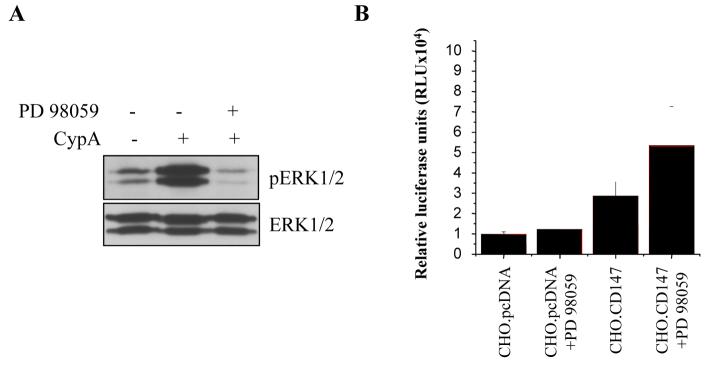
To further validate this conclusion, we used PD98059, a selective inhibitor of MEK activity [28], which is a key event in a signaling cascade initiated by CypA from CD147 [10]. Results presented in Figure 2A demonstrate that PD98059 eliminated CypA-dependent ERK phosphorylation in CD147-transfected CHO cells. However, the inhibitor did not reduce the stimulating effect of CD147 on HIV-1 infection (Fig. 2B). This stimulating effect was weaker in this experiment than in the experiment shown in Figure 1 because uncloned CD147-transfected CHO cells were used here.
Figure 2. Inhibition of ERK1/2 activation by PD 98059 does not reduce the enhancing effect of CD147 on HIV-1 replication.
A. Serum-starved CD147-expressing CHO cells (clone 18) were stimulated or not with CypA in the presence or absence of MEK inhibitor PD 98059 (10 μM), lysed and analyzed by Western blotting using anti-ERK (ERK1/2) (bottom panel) or anti-phosphoERK (pERK1/2) monoclonal antibodies (upper panel). Results are for one representative experiment out of two performed. B. CHO.pcDNA or CHO.CD147 (clone 18) cells were pre-treated with 10 μM PD98059 dissolved in DMSO or with DMSO alone for 1 hr, inoculated with luciferase-expressing HIV-1 pseudotyped with an envelope of amphotropic murine leukemia virus (MuLV), and analyzed for luciferase expression after 4 days of incubation in the medium without drug. Results are presented as mean ± SE of three independent wells for one representative experiment of two performed.
Taken together, these results indicate that ERK-mediated signaling is not involved in CD147-dependent enhancement of HIV-1 infection.
Cytoplasmic domain of CD147 is necessary for enhancement of HIV-1 infection, but not for signaling
Most transmembrane receptors transmit signals via their cytoplasmic domains. Since signaling response is not required for CD147 activity in HIV-1 infection, we expected that truncation of the cytoplasmic tail of the protein will not affect HIV-1 infection but will eliminate ERK1/2 activation after CypA stimulation. To test this forecast, we constructed cDNA encoding a truncated version of CD147 (CD147ΔC) lacking most of the cytoplasmic domain (Fig. 3A) and introduced it into CHO cells. For further analysis we picked two CD147ΔC clones (#3 and #9) expressing similar levels of CD147 on the cell surface as the cells transfected with the wild-type CD147 (Fig. 3B). In contrast to our expectations, CHO.CD147ΔC cell clones demonstrated the same level of ERK1/2 activation as the wild-type CD147 clone after being stimulated with recombinant CypA (Fig. 3C). This result suggests that the cytoplasmic domain of CD147 is not necessary for CypA-induced ERK activation. The mechanism of signaling from CD147 might involve two different signal transduction pathways: one mediated by the cytoplasmic tail of the protein and the other - by CD147-associated transmembrane proteins, such as integrins or syndecans, as suggested previously [14; 29]. Indeed, downregulation of syndecan-1 has been shown to prevent CypB-induced ERK activation [14]. It remains to be determined whether signaling events can really be transduced through the cytoplasmic tail of CD147.
Figure 3. Cytoplasmic domain of CD147 is not required for signaling but is necessary for the enhancing effect of CD147 on HIV-1 replication.
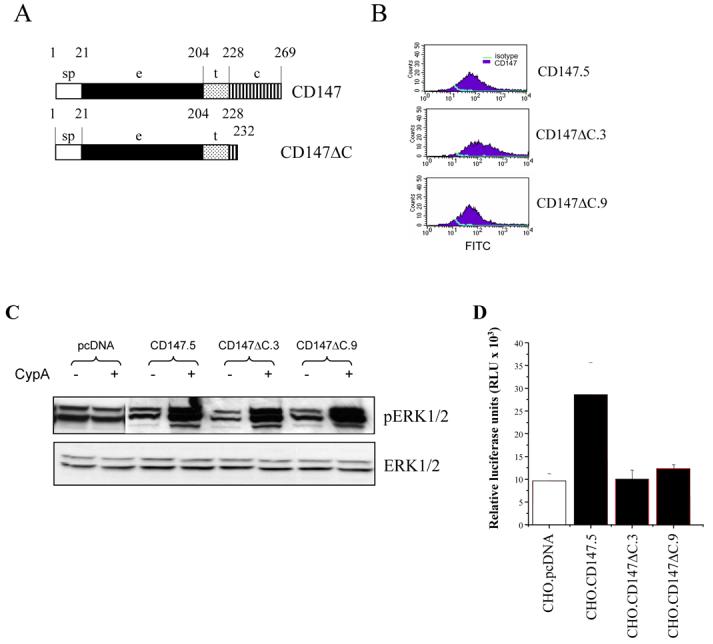
A. Schematic representation of the wild-type and truncated constructs of CD147. B. Analysis of CD147 expression on stable clones of CD147 (clone 5) and CD147ΔC-transfected (clones 3 and 9) CHO cells by flow cytometry. Cells were grown overnight (80% confluency), stained with FITC-conjugated anti-CD147 mAb, and analyzed on BD FACScan. C. Analysis of ERK activation. Serum-starved clones expressing wild-type (CHO.CD147.5) or mutant versions of CD147 with truncated cytoplasmic tail (CHO.CD147ΔC3 and CHO.CD147ΔC9) were stimulated or not with CypA, lysed and analyzed by Western blotting as in Fig. 2A. D. Stable clones of CHO cells transfected with wild-type CD147 (CHO.CD147.5), mutant CD147 carrying truncated cytoplasmic tail (CHO.CD147ΔC.3 and CHO.CD147ΔC.9), or empty vector pcDNA3.1zeo (CHO.pcDNA) were inoculated with luciferase-expressing HIV-1 and analyzed as in Fig. 1B.
Analysis of HIV-1 infection showed that CHO.CD147ΔC-expressing cells were much less susceptible to HIV-1 infection than cells transfected with the wild-type CD147 and exhibited only a marginal increase in HIV-1 replication over the level observed in cells transfected with an empty pcDNA3.1 vector (Fig. 3D). This result indicates that the cytoplasmic domain of CD147 is necessary for the activity of CD147 in HIV-1 infection. This domain might be used as a docking site for molecules important for the early steps of HIV replication. For instance, it might interact with actin filaments, thus facilitating association of the HIV-1 reverse transcription complex with the cytoskeleton [30], which is critical for intracellular trafficking of the viral nucleoprotein complex [31].
Taken together, results of this study suggest that the cytoplasmic tail of CD147 is necessary for its enhancing effect on HIV-1 infection, but is dispensable for the cyclophilin-induced signaling activity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur.J.Biochem. 1993;216:689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- 2.Gothel SF, Marahiel MA. Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell Mol.Life Sci. 1999;55:423–436. doi: 10.1007/s000180050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Albers MW, Wandless TJ, Luan S, Alberg DG, Belshaw PJ, Cohen P, MacKintosh C, Klee CB, Schreiber SL. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992;31:3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- 4.Allain F, Vanpouille C, Carpentier M, Slomianny MC, Durieux S, Spik G. Interaction with glycosaminoglycans is required for cyclophilin B to trigger integrin-mediated adhesion of peripheral blood T lymphocytes to extracellular matrix. Proc.Natl.Acad.Sci U.S.A. 2002;99:2714–2719. doi: 10.1073/pnas.052284899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherry B, Yarlett N, Strupp A, Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc.Natl.Acad.Sci.U.S.A. 1992;89:3511–3515. doi: 10.1073/pnas.89.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Q, Leiva MC, Fischkoff SA, Handschumacher RE, Lyttle CR. Leukocyte chemotactic activity of cyclophilin. J.Biol.Chem. 1992;267:11968–11971. [PubMed] [Google Scholar]
- 7.Seko Y, Fujimura T, Taka H, Mineki R, Murayama K, Nagai R. Hypoxia followed by reoxygenation induces secretion of cyclophilin A from cultured rat cardiac myocytes. Biochem.Biophys.Res.Commun. 2004;317:162–168. doi: 10.1016/j.bbrc.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Damsker J, Bukrinsky MI, Constant SL. Preferential chemotaxis of activated human CD4+ T cells by extracellular cyclophilin A. J.Leuk.Biol. 2007 doi: 10.1189/jlb.0506317. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ.Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 10.Yurchenko V, Zybarth G, O'Connor M, Dai WW, Franchin G, Hao T, Guo H, Hung HC, Toole B, Gallay P, Sherry B, Bukrinsky M. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J.Biol.Chem. 2002;277:22959–22965. doi: 10.1074/jbc.M201593200. [DOI] [PubMed] [Google Scholar]
- 11.Yurchenko V, O'Connor M, Dai WW, Guo H, Toole B, Sherry B, Bukrinsky M. CD147 is a signaling receptor for cyclophilin B. Biochem.Biophys.Res.Commun. 2001;288:786–788. doi: 10.1006/bbrc.2001.5847. [DOI] [PubMed] [Google Scholar]
- 12.Bukrinsky MI. Cyclophilins: unexpected messengers in intercellular communications. Trends Immunol. 2002;23:323–325. doi: 10.1016/s1471-4906(02)02237-8. [DOI] [PubMed] [Google Scholar]
- 13.Pushkarsky T, Zybarth G, Dubrovsky L, Yurchenko V, Tang H, Guo H, Toole B, Sherry B, Bukrinsky M. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc.Natl.Acad.Sci U.S.A. 2001;98:6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pakula R, Melchior A, Denys A, Vanpouille C, Mazurier J, Allain F. Syndecan-1/CD147 association is essential for cyclophilin B-induced activation of p44/42 mitogen-activated protein kinases and promotion of cell adhesion and chemotaxis. Glycobiology. 2007;17:492–503. doi: 10.1093/glycob/cwm009. [DOI] [PubMed] [Google Scholar]
- 15.Boulos S, Meloni BP, Arthur PG, Bojarski C, Knuckey NW. Peroxiredoxin 2 overexpression protects cortical neuronal cultures from ischemic and oxidative injury but not glutamate excitotoxicity, whereas Cu/Zn superoxide dismutase 1 overexpression protects only against oxidative injury. J.Neurosci.Res. 2007 doi: 10.1002/jnr.21429. in press. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Zhai Q, Bharadwaj U, Wang H, Li F, Fisher WE, Chen C, Yao Q. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer. 2006;106:2284–2294. doi: 10.1002/cncr.21862. [DOI] [PubMed] [Google Scholar]
- 17.Luban J, Bossolt KL, Franke EK, Kalpana GV, Goff SP. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 18.Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh CT, Sodroski J, Gottlinger HG. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994;372:363–365. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- 19.Colgan J, Yuan HE, Franke EK, Luban J. Binding of the human immunodeficiency virus type 1 Gag polyprotein to cyclophilin A is mediated by the central region of capsid and requires Gag dimerization. J.Virol. 1996;70:4299–4310. doi: 10.1128/jvi.70.7.4299-4310.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franke EK, Yuan HE, Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994;372:359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- 21.Braaten D, Franke EK, Luban J. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J.Virol. 1996;70:3551–3560. doi: 10.1128/jvi.70.6.3551-3560.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherry B, Zybarth G, Alfano M, Dubrovsky L, Mitchell R, Rich D, Ulrich P, Bucala R, Cerami A, Bukrinsky M. Role of cyclophilin A in the uptake of HIV-1 by macrophages and T lymphocytes. Proc.Natl.Acad.Sci.U.S.A. 1998;95:1758–1763. doi: 10.1073/pnas.95.4.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saphire AC, Bobardt MD, Gallay PA. Host cyclophilin A mediates HIV-1 attachment to target cells via heparans. EMBO J. 1999;18:6771–6785. doi: 10.1093/emboj/18.23.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saphire AC, Bobardt MD, Gallay PA. Human immunodeficiency virus type 1 hijacks host cyclophilin A for its attachment to target cells. Immunol.Res. 2000;21:211–217. doi: 10.1385/IR:21:2-3:211. [DOI] [PubMed] [Google Scholar]
- 25.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1--infected individuals. J.Exp.Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC- CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 27.Alfano M, Pushkarsky T, Poli G, Bukrinsky M. The B-Oligomer of pertussis toxin inhibits Human Immunodeficiency Virus type 1 replication at multiple stages. J.Virol. 2000;74:8767–8770. doi: 10.1128/jvi.74.18.8767-8770.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J.Biol.Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 29.Berditchevski F, Chang S, Bodorova J, Hemler ME. Generation of monoclonal antibodies to integrin-associated proteins. Evidence that alpha3beta1 complexes with EMMPRIN/basigin/OX47/M6. J.Biol.Chem. 1997;272:29174–29180. doi: 10.1074/jbc.272.46.29174. [DOI] [PubMed] [Google Scholar]
- 30.Bukrinskaya A, Brichacek B, Mann A, Stevenson M. Establishment of a functional human immunodeficiency virus type 1 (HIV- 1) reverse transcription complex involves the cytoskeleton. J.Exp.Med. 1998;188:2113–2125. doi: 10.1084/jem.188.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iordanskiy S, Bukrinsky M. Reverse transcription complex: the key player of the early phase of HIV replication. Future Virol. 2007;2:49–64. doi: 10.2217/17460794.2.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]