Abstract
Eating and anxiety disorders are more prevalent in females, increase during adolescence, and are associated with early pubertal development. This study examined whether timing of puberty onset is associated with disordered eating and anxiety in a large sample of post-pubertal male and female undergraduate students. Self-report questionnaires assessed timing of puberty, disordered eating, anxiety, alcohol use, personality, and sensation seeking. Females scored significantly higher on measures of disordered eating (binge eating, dietary restraint, eating concerns, and weight and shape concerns) and anxiety (state and trait anxiety) than did males. In addition, early maturing women and men scored significantly higher on measures of disordered eating and anxiety than on-time or late maturing women and men. Measures of alcohol use, sensation seeking, and personality characteristics differed in males and females but did not vary with pubertal timing. Findings suggest that early puberty is associated with disordered eating and anxiety, and this association may be due to an organizational effect of pubertal hormones. Despite important differences in body fat composition, both males and females experiencing early puberty had an increased incidence of disordered eating. The fact that early puberty was associated with increased eating and anxiety symptoms in both sexes suggests that puberty may influence these symptoms through both biological and psychosocial mechanisms.
Keywords: Puberty Timing, Disordered Eating, Anxiety, Organizational Effects, Sex Differences, Sexual Development
INTRODUCTION
The incidence of disordered eating increases dramatically at puberty and rarely occurs in prepubertal individuals (Hayward et al., 1997; American Psychiatric Association, 2000). In addition, puberty onset marks a developmental shift in the relationship between biological and environmental influences on disordered eating. Twin studies demonstrate that genetic influences on disordered eating increase, while environmental factors decrease, during puberty (Klump et al., 2000, 2003; in press). Finally, both disordered eating and anxiety disorders, which are frequently comorbid with disordered eating, are more common in females than in males (Lucas et al., 1991; American Psychiatric Association, 2000). These prevalence patterns suggest a possible role for pubertal neuroendocrine changes in the etiology or expression of disordered eating and anxiety symptoms.
Pubertal increases in circulating sex steroids could influence psychological symptoms through either activational (i.e. transient or reversible) and/or organizational (i.e. permanent or long lasting) mechanisms. At puberty, testosterone increases in boys, and estradiol and progesterone begin to be secreted cyclically in girls. These increases in circulating steroids directly modulate psychology and behavior, including measures of eating behavior and anxiety. Circulating estrogen levels correlate with overall levels of disordered eating in women (Klump et al., 2006) and inhibit food intake in rodents (reviewed by Geary, 2001; Eckel, 2004). In addition, circulating steroids act organizationally on adolescent brain development, permanently altering psychological traits and behavior. In particular, the absence of steroid hormones during adolescence alters a variety of adult sexually dimorphic behaviors in animal models (reviewed by Sisk et al., 2003; Romeo, 2003; Sisk & Zehr, 2005; Schulz & Sisk, 2006). These findings led us to examine how pubertal hormones might influence human adolescent brain development, which is a protracted process that spans more than a decade (Gogtay et al., 2002). The animal literature suggests that the potential for hormone-dependent organization is greater in the adolescent brain than in the adult brain (Sisk & Zehr, 2005; Schulz & Sisk, 2006), leading to the prediction that in humans, the timing of pubertal increases in steroid hormones relative to the degree of brain maturation will contribute to individual differences in adult psychological characteristics and behavior.
During adolescence, pubertal timing and the onset of pubertal neuroendocrine events are associated with the relative risk for psychopathology in girls and boys (Graber et al., 1997, 2004, Ge et al., 2001, Kaltiala-Heino et al., 2003a, 2003b). For example, degree of pubertal development in early adolescent girls predicts disordered eating one year later (Keel et al., 1997). In addition, 12 year-old girls symptomatic for eating disorders have greater breast and pubic hair development than do nonsymptomatic girls of the same age (Killen et al., 1992, 1994, 1996), and postpubertal adolescent girls report higher levels of disordered eating than their prepubertal same-aged peers (Koff & Rierdan, 1993). Furthermore, both adolescent girls and boys who mature earlier than their peers have a higher incidence of bulimic behavior compared to girls and boys who mature on time or later (Kaltiala-Heino et al., 2001). Similar to disordered eating behavior, adolescent girls who are more physically developed show a higher incidence of panic attacks (Hayward et al., 1992). However, because subjects in these studies vary in degree of pubertal development, these studies can not distinguish between transient activational effects of steroids on disordered eating and anxiety symptoms and long-term organizational effects of pubertal steroids on psychological development.
If steroids influence adult behavior via organizational effects, then one would predict that individual differences based on pubertal timing would still be observable long after the completion of puberty. More specifically, since organizational effects depend on the timing of hormone exposure and cause long-lasting or permanent changes in brain and behavior, changes due to hormone exposure earlier in development will remain in adulthood.
The current study tested whether the timing of puberty influences disordered eating and anxiety in young adults, consistent with an organizational effect of steroids. In this study, young adult participants were asked to report whether pubertal milestones, such as breast development in women or facial hair growth in men, were achieved earlier than, at a time similar to, or later than their peers. Early, on-time, and later maturing post-pubertal women and men were then compared to determine whether pubertal timing was associated with a variety of psychological traits in young adulthood, including disordered eating, anxiety, personality, sensation seeking, and alcohol use. These were chosen to include measures that are linked to pubertal development (disordered eating, anxiety) or adolescent behavior (sensation seeking, alcohol use) and measures that are not linked to puberty (personality). Finding no association between pubertal timing and psychological traits in young adulthood would suggest that gonadal hormones do not have organizational effects, while a significant association between pubertal timing and psychological measures in young adulthood would be consistent with the hypothesis that gonadal hormones have organizational effects on these symptoms during adolescence.
METHODS
Participants
The initial sample included 750 female (age range = 17–36 years, M = 19.35, SD = 1.60) and 750 male (age range = 18 – 32 years, M = 19.77, SD = 1.75) undergraduate students at a large Midwestern university. Due to missing data (see below), the final sample of participants included 717 women and 643 men who responded to measures of pubertal development. Participants were recruited from introductory psychology courses and received course credit for their participation. All research was approved by the institutional human subjects review board. The majority (81.5%) of all participants reported a Caucasian ethnic background. The remaining participants reported African American (6.2%), Asian (6.0%), Hispanic (2.9%), Native American (0.2%), Pacific Islander (0.6%), or “other” (2.7%) ethnic backgrounds. Socioeconomic status (SES), assessed by parental income, was largely in the middle-to-upper level income classes. Specifically, 83.7% of male participants and 82.7% of female participants reported parental income greater than $40,000/year. Participants who were currently or previously married (2.6% of the sample) were not included in the assessment of SES since parental income was likely not an adequate proxy for their SES.
Measures
Participants completed all measures electronically as part of the University’s on-line volunteer research pool. Questionnaires obtained demographic information as well as information on pubertal development, disordered eating, anxiety, personality, sensation seeking, and alcohol use. With the exception of pubertal development measures (see below), missing data was addressed using a prorating system. Participants with missing data that constituted less than 10% of the responses within a scale received a prorated score for that scale. Participants missing more than 10% of responses were dropped from analyses of that scale. In total, less than 2.1% of scores on measures of disordered eating, anxiety, personality, sensation seeking, and alcohol use were coded as missing in both the male and female samples.
Demographic Questionnaire
A general demographic questionnaire collected information on age, ethnicity, marital status, parental income, height, and weight.
Pubertal Development
Participants retrospectively answered questions on pubertal development using a modified version of the Pubertal Development Scale (PDS; Petersen et al., 1988). The original version of the PDS is used with adolescents to assess current pubertal development of a variety of secondary sex characteristics. With permission from the authors (A. Petersen, personal communication, May 19, 2006), we designed a modified version of the PDS to retrospectively assess timing of pubertal development in post-pubertal adults. Participants reported whether their pubertal development occurred much earlier than others (1), somewhat earlier (2), about the same time (3), somewhat later (4), much later (5), or they did not know. For female participants, six aspects of pubertal development were assessed, including onset of menses, breast development, growth spurt, appearance of body hair (axial and pubic), skin changes (e.g., acne), and overall development (i.e., “In general, do you think your development was any earlier or later than most other girls?”). For male participants, seven aspects of pubertal development were assessed: appearance of facial hair, voice changes, growth spurt, appearance of body hair (axial and pubic), skin changes including acne, spontaneous erections, nocturnal emissions and overall development. Responses (1–5) were summed for the 6 aspects of development in women and 7 aspects of development in men to create a total score of pubertal development and a continuous measure of pubertal timing.
For most response items, participants recalled the timing of pubertal development relative to their peers. However, two response items produced a large number of missing responses, timing of body hair appearance for females and occurrence of nocturnal emissions for males. Although individuals vary in the timing and duration of pubertal changes, different aspects of pubertal development progress in a fairly standard manner. Thus, missing scores on these two items were replaced with the average score from the remaining 5 items for females and 6 items for males. Individuals who were missing data, as a result of skipping an item or responding “don’t know”, to any other item on the pubertal development scale were excluded from analyses. This resulted in final sample sizes of 717 females and 643 males with pubertal development scores (95.6% and 85.7% of the initial sample respectively). Within this final sample, 10% of females (73 of 717) received prorated scores on the timing of body hair, and 30% of males (193 of 643) received prorated scores on the timing of nocturnal emissions. Quartiles for the summed total score of pubertal development were used to categorize early (lowest quartile, n= 218 women, n=180 men), on-time (middle two quartiles, n=304 women, n=291 men), and late (highest quartile, n=195 women, n=172 men) pubertal timing.
Although previous studies have demonstrated that perception of pubertal timing relative to peers is highly correlated with actual pubertal timing (Dubas et al., 1991; Graber et al., 1997), participants were also asked to report specific ages of pubertal developmental markers (e.g. facial hair growth or breast development). With the exception of age at menarche, the majority of female and male participants did not recall specific ages for pubertal developmental markers. For those women who did recall age at menarche, categorization based on the continuous pubertal development score corresponded well with categorization of pubertal timing based on normative data in a Western society (Kaltiala-Heino, et al., 2003b).
The original PDS (Petersen et al., 1988) showed good psychometric properties. In previous studies (Petersen et al., 1988), internal consistency of the PDS was adequate (α = 0.76 to 0.83 in adolescent girls; α = 0.68 to 0.78 in adolescent boys). In addition, findings have shown that the PDS correlates highly (r = 0.61 – 0.67) with physician ratings of pubertal development (Brooks-Gunn et al., 1987) as well as with interviewer ratings (median r = 0.70; Petersen et al., 1988). The modified version of the PDS also demonstrated good psychometric properties. In this study, the modified PDS demonstrated good internal consistency (α = 0.80 in women; α = 0.84 in men), suggesting that participants were consistent in their retrospective reports of several pubertal development markers. The modified measure also demonstrated excellent test-retest reliability (r = 0.87 for females; r = 0.83 for males) in a subset of our participants (n = 110 females; n = 70 males) who were re-administered the modified PDS 2–6 weeks (average time = 19 days) after the initial administration.
Disordered Eating
Two questionnaires were used to assess disordered eating: the Eating Disorder Examination Questionnaire (EDE-Q; Fairburn & Beglin, 1994) and the Binge Eating Scale (BES; Gormally et al., 1982). The EDE-Q (Fairburn & Beglin, 1994) contains four subscales that examine Eating Concerns (i.e., preoccupation with food, eating in secret, and guilt about eating), Restraint (i.e., restraint over eating, avoidance of eating), Shape Concerns (i.e., desire for a flat stomach, importance of body shape, and fear of gaining weight), or Weight Concern (i.e., importance placed on weight, dissatisfaction with weight, and desire to lose weight). Each of the EDE-Q items asks about attitudes and behaviors over the last 28 days and is rated on a 7-point scale, where higher scores denote higher levels of the specified disordered eating behavior. The BES (Gormally et al., 1982) is a 16-item self-report questionnaire designed to assess binge eating in terms of behavioral (e.g., eating in secret, eating quickly, overeating until feeling nauseous) and emotional (e.g., feelings of guilt or lack of control) manifestations of a binge. Participants select the response that best describes their eating attitudes and behaviors from a series of differently weighted statements, ranging from 0 to 3. A total score is computed by summing the weights for each of the 16 items. A high score on the BES scale suggests more severe binge eating problems. Previous research has demonstrated good reliability and validity for the EDE-Q (Black & Wilson, 1996; Fairburn & Beglin, 1994; Luce & Crowther, 1999) and the BES (Gormally et al., 1982). In the current study, internal consistencies were good for all E-DEQ subscales (α = 0.82 – 0.92 for female sample; α = 0.79 – 0.89 for male sample) and the BES total score (α = 0.89 for female sample; α = 0.88 for male sample).
Body Mass Index
Body Mass Index (BMI) frequently varies with disordered eating (e.g. Erol et al., 2006). Thus, participants’ BMI (body weight (kg) divided by height squared (m2)) was calculated from self-reports of body weight and height. Previous studies have indicated that self-reported height and weight are valid and reliable measures of actual height and weight (Stunkard & Albaum, 1981; Palta et al., 1982). Since BMI significantly correlated with disordered eating variables in the present study (r’s = 0.13 – 0.27; p’s <0.001) but was not a dependent variable of interest, BMI was included as a covariate in analyses of disordered eating.
Anxiety
The State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983) assessed anxiety in participants. The STAI is a self-report measure containing separate scales for state (i.e. transitory and temporary dispositions, how one feels “right now” or “at this moment”) versus trait anxiety (i.e. stable and enduring dispositions, how one feels “in general”). Previous research has documented the reliability and validity of both STAI scales (Spielberger et al., 1983). In addition, internal consistencies were excellent for both State (α = 0.92 for male sample; α = 0.93 for female sample) and Trait (α = 0.90 for male and female samples) anxiety subscales.
Personality Characteristics
The 50-item International Personality Item Pool (IPIP; Goldberg, 2001; Goldberg et al., 2006) was used to assess personality characteristics: Extroversion, Agreeableness, Conscientiousness, Emotional Stability (considered the opposite of Neuroticism), and Intellect. Each item included a phrase describing people’s behaviors (e.g., “I am the life of the party”) and was rated on a 5-point scale ranging from very inaccurate (1) to very accurate (5). The IPIP scales relate strongly to the dimensions of personality assessed by the NEO Five Factor Inventory (NEO-FFI; Costa & McCrae, 1992) and Eysenck Personality Questionnaire – Revised Short Form (EPQ- R Short Form; Eysenck et al., 1985) which are two leading personality questionnaires (Gow et al., 2005). The IPIP scales demonstrated good internal consistency in previous research (Goldberg, 2001; Gow et al., 2005) as well as in the current study (α = 0.74 – 0.85 for male sample; α = 0.76 – 0.87 for female sample).
The Sensation Seeking Scale Form V (SSS-V; Zuckerman et al., 1978) was used to assess dimensions of sensation-seeking on four subscales. The subscales, each comprised of 10 forced choice items, include Boredom Susceptibility (i.e., assesses one’s dislike towards repetitions of experience and restlessness when things are unchanging), Disinhibition (i.e., assesses levels of social disinhibition in regards to drinking, sex, and partying behaviors), Experience Seeking (i.e., assesses the desire to engage in novel experiences and an unconventional lifestyle), and Thrill and Adventure Seeking (i.e., assesses the desire to engage in physical activities involving elements of speed or danger). A total overall sensation seeking score is computed by summing across the four subscales. The Disinhibition, Thrill and Adventure Seeking, and Total Score have shown good internal consistency and 3-week test-retest reliability (Zuckerman, 1979), whereas the Experience Seeking and Boredom Susceptibility subscales have demonstrated somewhat lower reliability (Zuckerman, 1979). Similar to previous studies, internal consistencies were adequate for the Disinhibition subscale (α = 0.72 for female sample; α = 0.69 for male sample), Thrill and Adventure Seeking subscale (α = 0.77 for female sample; α = 0.73 for male sample), and overall Total Score (α = .80 for female sample; α = 0.76 for male sample) in this study, but low for the Experience Seeking and Boredom Susceptibility subscales (all α’s < 0.56). As a result, Experience Seeking and Boredom Susceptibility subscales were not further analyzed in the present study.
Alcohol Use
The Alcohol Use Disorders Identification Test (AUDIT; Saunders et al. 1993a, 1993b) assessed recent alcohol use (e.g., “how often do you have a drink containing alcohol?”), alcohol dependence symptoms, and alcohol related problems (e.g., “have you or someone else been injured as a result of your drinking?”). The total score on the AUDIT reflects extent of alcohol involvement on a broad continuum of severity. The AUDIT has been shown to accurately detect alcohol dependence in university students (Fleming et al., 1991) and screens for potentially hazardous drinking behaviors (Bohn et al., 1995). AUDIT scores also correlate well with measures of drinking consequences, attitudes towards drinking, vulnerability to alcohol dependence, reasons for drinking, and negative mood states after drinking (Bohn et al., 1995). Internal consistency for the AUDIT total score was good for both the male and female samples in this study (α = 0.83 for female sample; α = 0.84 for male sample).
Statistical Analysis
Statistical analysis investigated the relationship of sex and pubertal timing to responses on questionnaires. Analysis of covariance (ANCOVA) tested measures of disordered eating for main effects of timing (early, on-time, and late puberty) and sex (male, female) with BMI as a covariate. This analysis factors out the correlation of BMI with disordered eating measures and tests for remaining relationships among sex and pubertal timing on variables of interest. The EDE-Q eating concern subscale had a positively skewed distribution and was log transformed before analysis, however figures and tables for this subscale include the untransformed means for ease of interpretation. For measures of anxiety, personality characteristics, and alcohol use, relationships between timing (early, on-time, and late puberty) and sex (male, female) were analyzed using analysis of variance (ANOVA). Tukey HSD post-hoc tests were used to follow-up significant main effects of pubertal timing. A p ≤ 0.05 was considered significant in all analyses.
RESULTS
Measures of disordered eating differed across both pubertal timing and sex (Table 1). In general, individuals who matured early had higher scores than did individuals who matured on-time or late. Specifically, early maturing individuals had significantly higher levels of EDE-Q dietary restraint, more shape concerns, and more weight concerns as well as a trend towards significantly more eating concerns than on-time or late maturing individuals (Figure 1, Table 1). Furthermore, females had significantly higher scores than males across all EDE-Q measures, indicating more eating concern, more dietary restraint, more shape concern, and more weight concern. None of the EDE-Q subscales showed an interaction of pubertal timing and sex (Table 1). Scores on the BES questionnaire showed a remarkably similar pattern of results (Figure 1, Table 1). Early maturing individuals had significantly higher BES scores than on-time individuals (Tukey HSD, p<0.05) as well as a trend towards significantly higher scores than late individuals (Tukey HSD, p= 0.06), and females had significantly higher BES scores than did males.
Table 1.
Test statistics for comparisons of mean levels of disordered eating and anxiety between males and females with early, on-time, and late pubertal development.
| BMI F(df, df) | Sex F (df, df) | Timing F (df, df) | Interaction F (df, df) | |
|---|---|---|---|---|
| EDE-Q – Eating Concern | 24.55 (1,1314)*** | 148.23 (1, 1314)*** | 2.69 (2, 1314)† | 0.49 (2, 1314) |
| EDE-Q – Shape Concern | 50.99 (1,1313)*** | 337.85 (1, 1313)*** | 3.86 (2, 1313)* | 0.09 (2, 1313) |
| EDE-Q – Restraint | 16.50 (1,1315)*** | 82.17 (1, 1315)*** | 3.76 (2, 1315)* | 0.11 (2, 1315) |
| EDE-Q – Weight Concern | 58.43 (1,1314)*** | 331.02 (1, 1314)*** | 4.70 (2, 1314)** | 0.27 (2, 1314) |
| Binge Eating Scale | 40.23 (1,1295)*** | 136.76 (1,1295)*** | 5.60 (2,1295)** | 0.29 (2,1295) |
| STAI – State Anxiety | N/A | 3.98 (1, 1344)* | 3.58 (2, 1344)* | 0.28 (2, 1344) |
| STAI – Trait Anxiety | N/A | 25.72 (1, 1344)*** | 4.22 (2, 1344)* | 0.73 (2, 1344) |
Note. Means and standard deviations are presented in Figure 1.
p<0.10;
p<0.05;
, p<0.01;
p<0.001, N/A=Not Applicable
Figure 1.
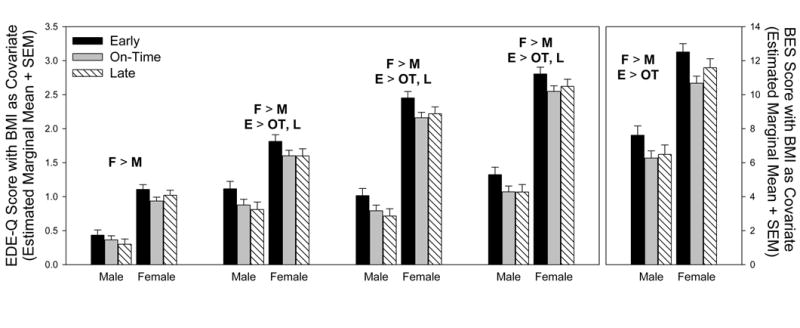
Scores on the Eating Disorder Examination Questionnaire (EDE-Q) and Binge Eating Scale (BES) varied with both sex and timing of pubertal onset. Across EDE-Q subscales and the BES, females had higher scores than did males. Furthermore, early-maturing individuals, whether male or female, scored highest on all measures of disordered eating. No measures had a significant interaction between pubertal timing and sex, and all measures used body mass index (BMI) as a covariate in the analysis. Significant differences between females (F) and males (M) and between early (E), on-time (OT), and late (L) maturers are indicated in the figure. Analyses are based on 171–173 early maturing men, 278–284 on-time maturing men, 165–169 late maturing men, 208–212 early maturing women, 293–299 on-time maturing women, and 187–190 late maturing women.
Measures of anxiety also varied with both pubertal timing and sex (Table 1). Participants who matured early had significantly higher scores on measures of both state anxiety and trait anxiety than did participants who matured on-time (Figure 2, Table 1). In addition, females scored significantly higher than males in measures of both state and trait anxiety (Figure 2, Table 1).
Figure 2.
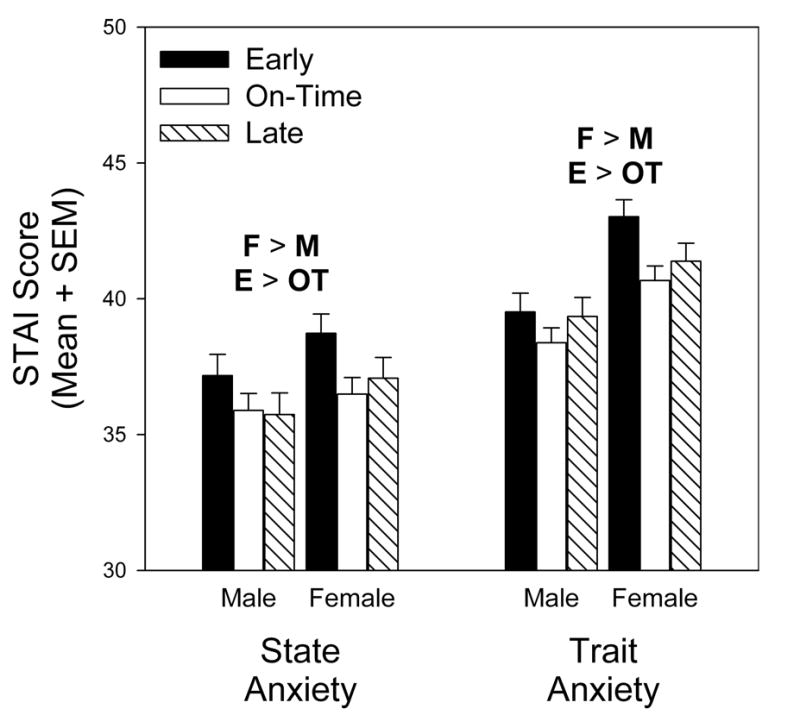
Scores on the State-Trait Anxiety Inventory (STAI) varied with both sex and timing of pubertal onset. On both state and trait subscales, females had higher scores than did males. In addition, early-maturing individuals, whether male or female, scored highest on anxiety measures. Significant differences between females (F) and males (M) and between early (E), on-time (OT), and late (L) maturers are indicated in the figure. Analyses are based on 180/179 (State/Trait) early maturing men, 288 on-time maturing men, 172 late maturing men, 217 early maturing women, 300/301 on-time maturing women, and 193 late maturing women.
Measures of personality, sensation seeking, and alcohol use showed expected sex differences, but did not vary based on pubertal timing (see Table 2). Scores on IPIP extroversion, agreeableness, and conscientiousness items were significantly higher in females than in males (Table 2). Scores on IPIP emotional stability, characterized as the opposite of neuroticism, and intellect items were significantly higher in males than in females. Across all sensation seeking measures, males had significantly higher scores than did females (Table 2). Scores on the AUDIT, a survey designed to assess alcohol use, were also significantly higher in males than in females.
Table 2.
Mean Differences in Personality, Sensation Seeking, and Substance Use for Men and Women who had Early, On Time, and Late Pubertal Development.
| Pubertal Timing | Sex F (df, df) | Timing F (df, df) | Interaction F (df, df) | |||
|---|---|---|---|---|---|---|
| Early n = 267 | On Time n = 420 Mean (Std. Error) | Late n = 263 | ||||
| IPIP – Agreeableness
Males Females |
39.55 (0.42)
41.30 (0.38) |
38.50 (0.33)
41.34 (0.32) |
38.53 (0.43)
41.39 (0.40) |
63.86 (1, 1345)*** | 1.09 (2, 1345) | 1.35 (2, 1345) |
| IPIP – Conscientiousness
Males Females |
30.04 (0.42)
32.05 (0.38) |
30.25 (0.33)
31.80 (0.33) |
29.81 (0.43)
31.53 (0.41) |
31.15 (1, 1344)*** | 0.55 (2, 1344) | 0.20 (2, 1344) |
| IPIP – Emotional Stability
Males Females |
33.45 (0.54)
27.90 (0.50) |
33.56 (0.43)
29.63 (0.42) |
32.37 (0.56)
28.95 (0.53) |
112.01 (1, 1344)*** | 2.68 (2, 1344) | 2.28 (2, 1344) |
| IPIP – Extroversion
Males Females |
34.24 (0.54)
34.17 (0.49) |
33.39 (0.43)
34.75 (0.42) |
32.98 (0.55)
34.77 (0.52) |
6.47 (1, 1344)** | 0.20 (2, 1344) | 1.78 (2, 1344) |
| IPIP – Intellect
Males Females |
37.72 (0.43)
36.38 (0.39) |
37.31 (0.34)
36.38 (0.33) |
37.84 (0.44)
36.46 (0.42) |
14.32 (1, 1343)*** | 0.34 (2, 1343) | 0.23 (2, 1343) |
| SSS – Disihibition
Males Females |
5.59 (0.19)
4.93 (0.17) |
5.73 (0.15)
4.61 (0.15) |
5.31 (0.20)
4.84 (0.18) |
27.73 (1, 1327)*** | 0.51 (2, 1327) | 2.04 (2, 1327) |
| SSS – Thrill and Adventure Seeking
Males Females |
6.94 (0.19)
6.08 (0.17) |
6.87 (0.15)
6.33 (0.15) |
7.14 (0.20)
6.69 (0.18) |
18.75 (1, 1341)*** | 2.63 (2, 1341) | 0.68 (2, 1341) |
| SSS – Sensation Seeking Total Score
Males Females |
21.48 (0.45)
18.82 (0.40) |
21.34 (0.35)
18.54 (0.34) |
21.17 (0.46)
19.50 (0.43) |
51.24 (1, 1331)*** | 0.52 (2, 1331) | 1.09 (2, 1331) |
| AUDIT – Alcohol Use
Males Females |
10.64 (0.49)
7.08 (0.44) |
10.54 (0.38)
7.40 (0.38) |
9.73 (0.50)
7.69 (0.47) |
64.19 (1, 1339)*** | 0.18 (2, 1339) | 1.37 (2, 1339) |
p<.05,
p< .01,
p<.001
DISCUSSION
This study suggests that early puberty is associated with long-term risk for disordered eating and anxiety. Specifically, young adults who characterized themselves as maturing early relative to their peers had significantly higher scores on measures of disordered eating and trait anxiety than did individuals who characterized themselves as maturing on-time or late. Pubertal timing did not relate to measures of alcohol use, personality, or sensation seeking in young adults. Thus, the relationships of pubertal timing were specific to disordered eating and anxiety rather than generalized to sexually dimorphic psychological traits.
During puberty, circulating gonadal steroids increase in boys and girls. These steroid hormones could act through activational and/or organizational mechanisms on disordered eating behavior to produce differences between prepubertal and postpubertal individuals during the transition to reproductive maturity. In particular, estradiol correlates with both food intake (Gong et al., 1989; Lyons et al., 1989; Buffenstein et al., 1995; Dye & Blundell, 1997) and disordered eating symptoms (Lester et al., 2003; Klump et al., 2006; Edler et al., 2007), demonstrating an activational effect of steroids on eating behaviors. Thus, adolescent girls in a more advanced pubertal stage, with concomitantly higher levels of reproductive hormones, have higher levels of disordered eating than adolescent girls in a less advanced pubertal stage (Killen et al., 1992, 1994, 1996; Koff & Rierdan, 1993; Kaltiala-Heino et al., 2001). If the developing adolescent brain is also sensitive to organizational effects of hormones, then differences based on the timing of puberty onset and steroid hormone changes would be predicted to continue well after puberty is complete, into young adulthood. This study demonstrates that young adult men and women who mature early score higher on measures of disordered eating symptoms than on-time and later maturing men and women, a finding that is consistent with an organizational effect of pubertal steroids. Since activational and organizational actions of steroids are not mutually exclusive, both may contribute to disordered eating symptoms. Animal models have also demonstrated that steroid hormones have activational effects on food intake (reviewed by Asarian & Geary, 2006), but animal studies are still needed to demonstrate whether pubertal steroid hormones have organizational effects on feeding behavior.
Early puberty may influence later behaviors via hormone-dependent remodeling of the adolescent brain. During adolescence, synaptic connections and neural circuits are still remarkably plastic. For example, neuron number in the prefrontal cortex differs in adolescent and adult rats (Markam et al., 2007), and neurons of the medial amygdala differ in structure among pre-, mid-, and late adolescent male hamsters (Zehr et al., 2006). Furthermore, adolescent rodents show differential responsiveness to anxiogenic situations, which is directly related to different effects of neurosteroids on cell excitability in adolescents and adults (Shen et al., 2007). In humans, there are dramatic adolescent decreases in the percentage of gray matter in frontal and temporal lobes (reviewed in Lenroot & Giedd, 2006), brain regions which are linked with disordered eating behavior (Uher & Treasure 2005) and anxiety (Garakani et al., 2006). The amygdala, which is a potential site for estrogen modulation of feeding behavior (Geary, 2001), is sensitive to circulating steroids (e.g. Jasnow et al., 2006), shows steroid-dependent neurogenesis (Fowler et al., 2005), and develops novel neuronal connections during adolescence (e.g. Cunningham et al., 2002). The human amygdala also undergoes structural and functional changes over adolescent development (Giedd et al., 1996; Killgore et al., 2001), and in early and late maturing individuals, the amygdala would therefore be intercepted by steroid hormones at different developmental stages. Since the product of the interaction between hormones and the changing amygdala likely depends on when the interaction occurs, this potential mechanism could account for the observed effects of early puberty on disordered eating symptoms.
Interestingly, early puberty had a similar relationship to disordered eating and anxiety in males and females, even though pubertal gonadal hormone secretions differ. While circulating reproductive hormones increase sharply during puberty in both males and females, adolescent testes primarily secrete testosterone while adolescent ovaries secrete estrogen and progesterone. Since testosterone is metabolized in the brain to estrogen, one possibility is that the early adolescent brain is sensitive to estrogen in both males and females. Alternatively, steroid hormones may act on the adolescent brain via different mechanisms in men and women. Gonadectomy increases food intake in female rodents but decreases food intake in male rodents (reviewed in Asarian & Geary, 2006), indicating that circulating steroids have opposite effects on feeding behavior in males and females. Furthermore, in females, estradiol levels negatively correlate with meal size (Asarian & Geary, 2006) and directly regulate total food intake (Wade, 1975; Varma et al., 1999). In contrast, testosterone acts in males not on meal size but on meal frequency (Gentry & Wade, 1976; Chai et al., 1999). Thus the neural circuits involved in eating behavior may be sensitive to the effects of endogenous steroids in both males and females, even though specific mechanisms affecting eating behavior may differ.
Alternative mechanisms, more peripheral or psychosocial in nature, could also account for the relationship between early puberty and disordered eating observed in this study. The timing of puberty onset is associated with a variety of factors, including prenatal variables, early childhood experiences, nutrition, social class, physical environment, and genetic background (Parent et al., 2003; Ong et al., 2006; van den Berg, et al., 2006; van Weissenbruch & Delemarre-van de Waal, 2006), any of which might in turn mediate the link between early puberty and disordered eating. In particular, increased body size and/or childhood obesity could lead to both an earlier onset of puberty (e.g. Frisch & Revelle, 1970) and some forms of disordered eating in adults, as being overweight in childhood is a documented risk factor for bulimia nervosa (Haines & Neumark-Sztainer, 2006). However, recent prospective studies show that adult BMI is explained solely by prepubertal body mass rather than by early menarche (Must et al., 2005). In the current study, we used adult BMI as a covariate in analyses of pubertal timing. Since prepubertal and adult BMI are highly correlated (Must et al., 2005), this covariate not only removes variance due to adult BMI from the analysis but also acts as a proxy for prepubertal BMI.
A second explanation for the pubertal increase in disordered eating is based on social pressures to attain a thin, lean, body type. In developing girls, adiposity increases dramatically with changes in reproductive hormone secretion. The social pressure hypothesis posits that in social and societal contexts valuing thinness, the pubertal increase in adiposity in turn results in increased disordered eating symptoms. In fact, early maturing girls do have greater body dissatisfaction and worse body image, which likely stem from pubertal changes in body composition (Koff & Rierdan, 1993; Siegel et al., 1999; Ohring et al., 2002; McCabe & Ricciardelli, 2004). The results of the present study do not conflict with this explanation in women. However, in contrast to girls, Western societal ideals for the male body type favor lean muscle mass and athletic builds. In developing boys, increases in testosterone increase lean muscle mass. Since this change in body composition matches that of the social and societal ideals, this psychosocial explanation would predict that early maturing boys would be the least likely to display disordered eating. In this study, early maturing males, like early maturing females, showed the highest levels of disordered eating, contradicting this common explanation. Evidence for relationships between early pubertal timing and other psychosocial mediating variables, including self-esteem and self-consciousness (Graber et al., 1997), has also not been found in males. Indeed, in previous research, males who mature late relative to their peers reported more self-consciousness and greater psychosocial problems than males who mature on time (Graber et al., 1997). In the current study, males maturing late did not differ from those maturing on-time in disordered eating. Thus, psychosocial explanations based on societal ideals for body type do not fully account for the relationship between pubertal timing and disordered eating in women and men. It is most likely that disordered eating results from a complex interaction of both psychosocial and biological factors, an idea which is further supported by examining the incidence of disordered eating across cultures and history (Keel & Klump, 2003). Although the results of this study are consistent with an organizational effect of hormones on disordered eating and anxiety, the use of a retrospective, self-report of pubertal timing and cross-sectional study design limits our ability to draw firm conclusions about the exact mechanisms relating pubertal timing and young adult psychological characteristics.
Anxiety traits were also associated in this and previous studies with early puberty onset. In previous studies, both adolescent boys and girls with very early menarche or oigarche (first ejaculation) have higher anxiety than do boys and girls with typical onset of menarche and oigarche (Kaltiala-Heino et al., 2003b). Anxiety and disordered eating are highly comorbid, have a shared familial transmission (Keel et al. 2005), and are thought to share a common etiology. Thus, it is perhaps not surprising that both disordered eating and anxiety were associated with early puberty. However, a shared etiology would also suggest that interactions between pubertal steroids and anxiety should be investigated further.
In contrast to disordered eating and anxiety, other sexually dimorphic traits such as alcohol use, personality measures, and sensation seeking did not vary with pubertal timing in young adults. Substance use and sensation seeking increase at puberty, with postpubertal individuals engaging in higher levels of the behaviors than prepubertal individuals (Martin et al., 2002; Lanza & Collins, 2002; Patton et al., 2004; Chung et al., 2005; Burt et al., 2006; Costello et al., 2007). However, this study found no evidence that early puberty onset has a long-term relationship to the expression of these behaviors in adulthood. In addition, studies have found conflicting results on how these behaviors vary with circulating steroid levels. For example, measures of sensation seeking have been found to be negatively associated with estradiol levels in women (Balada et al., 1993), positively correlated with testosterone in men (Aluja & Torrubia, 2004; Aluja & Garcia, 2005), or found not to vary with testosterone in either sex (Rosenblitt et al., 2001). Thus, influence of steroids, through either organizational or activational mechanisms, on alcohol use, personality measures, or sensation seeking remains to be fully elucidated.
In summary, studies of disordered eating and anxiety related behaviors have traditionally dichotomized social and biological influences on their development. While this study is not consistent with some psychosocial theories of etiology of disordered eating in adolescents (namely that of increased adiposity and body image at puberty), the most likely explanation for the development of disordered eating or anxiety related symptoms is one which encompasses many different dimensions of development. Individuals with different genetic compositions will necessarily differ in pubertal timing, hormone levels, metabolic rate, and personality type; thus genes predispose an individual to experience a particular biological or social environment (Scarr & McCartney, 1983). Likewise, direct physiological influences on psychological traits are expressed within a social and physical environment. This study demonstrates that early puberty onset puts both men and women at risk for disordered eating and anxiety symptoms. Whether hormones act directly, by organizing neural circuits or synaptic connections, or indirectly by altering the social interactions of adolescents, the timing of pubertal maturation alters psychological traits over the long term. Since the associations of pubertal timing with psychological traits were specific to disordered eating and anxiety, these findings suggest that these psychological symptoms are particularly influenced by the interaction of physical development and experience that occur during adolescence.
Acknowledgments
This research was funded in part by F32-MH068975 (JLZ), T32-MH070343 (KMC), and R01-MH068764 (CLS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aluja A, Garcia LF. Sensation seeking, sexual curiosity and testosterone in inmates. Neuropsychobiology. 2005;51:28–33. doi: 10.1159/000082852. [DOI] [PubMed] [Google Scholar]
- Aluja A, Torrubia R. Hostility-aggressiveness, sensation seeking, and sex hormones in men: Re-exploring their relationship. Neuropsychobiology. 2004;50:102–107. doi: 10.1159/000077947. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2004. [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos T Roy Soc B. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balada F, Torrubia R, Arque JM. Gonadal Hormone Correlates of Sensation Seeking and Anxiety in Healthy-Human Females. Neuropsychobiology. 1993;27:91–96. doi: 10.1159/000118960. [DOI] [PubMed] [Google Scholar]
- Black C, Wilson G. Assessment of eating disorders: Interview versus questionnaire. Int J Eat Disord. 1996;20:43–50. doi: 10.1002/(SICI)1098-108X(199607)20:1<43::AID-EAT5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): Validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:23–432. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child Dev. 1987;58:829–841. [PubMed] [Google Scholar]
- Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle - a retrospective analysis, with implications for appetite research. Physiol Behav. 1995;58:1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- Burt SA, McGue M, DeMarte JA, Krueger RF, Iacono WG. Timing of menarche and the origins of conduct disorder. Arch Gen Psychiatry. 2006;63:890–6. doi: 10.1001/archpsyc.63.8.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Chai JK, Blaha V, Meguid MM, Laviano A, Yang ZJ, Varma M. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am J Physiol Reg I. 1999;276:R1366–R1373. doi: 10.1152/ajpregu.1999.276.5.R1366. [DOI] [PubMed] [Google Scholar]
- Chung H, Park Y, Lanza ST. Latent transition analysis with covariates: pubertal timing and substance use behaviours in adolescent females. Stat Med. 2005;24:2895–2910. doi: 10.1002/sim.2148. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Sung M, Worthman C, Angold A. Pubertal maturation and the development of alcohol use and abuse. Drug Alcohol Depend. 2007;88(Suppl 1):S50–9. doi: 10.1016/j.drugalcdep.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116–30. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dubas JS, Graber JA, Petersen AC. A longitudinal investigation of adolescents changing perceptions of pubertal timing. Dev Psychol. 1991;27:580–586. [Google Scholar]
- Dye L, Blundell JE. Menstrual cycle and appetite control: implications for weight regulation. Hum Reprod. 1997;12:1142–51. doi: 10.1093/humrep/12.6.1142. [DOI] [PubMed] [Google Scholar]
- Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav. 2004;82:35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med. 2007;37:131–41. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Erol A, Toprak G, Yazici F. Psychological and physical correlates of disordered eating in male and female Turkish college students. Psychiat Clin Neuros. 2006;60:551–557. doi: 10.1111/j.1440-1819.2006.01557.x. [DOI] [PubMed] [Google Scholar]
- Eyesenck SBG, Eysenck HJ, Barrett P. A revised version of the Psychoticism scale. Pers Indiv Differ. 1985;6:21–30. [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? Int J Eat Disord. 1994;16:363–370. [PubMed] [Google Scholar]
- Fleming MF, Barry KL, MacDonald R. The alcohol use disorders identification test (AUDIT) in a college sample. Int J Addict. 1991;26:1173–1185. doi: 10.3109/10826089109062153. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Johnson F, Wang Z. Estrogen regulation of cell proliferation and distribution of estrogen receptor-alpha in the brains of adult female prairie and meadow voles. J Comp Neurol. 2005;489(2):166–79. doi: 10.1002/cne.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch RE, Revelle R. Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science. 1970;169(943):397–9. doi: 10.1126/science.169.3943.397. [DOI] [PubMed] [Google Scholar]
- Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. Mt Sinai J Med. 2006;73(7):941–9. [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol. 2001;37:404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Geary N. Estradiol, CCK and satiation. Peptides. 2001;22:1251–1263. doi: 10.1016/s0196-9781(01)00449-1. [DOI] [PubMed] [Google Scholar]
- Gentry RT, Wade GN. Sex differences in sensitivity of food intake, body weight, and running wheel activity to ovarian steroids in rats. J Comp Physiol Psych. 1976;90:747–754. doi: 10.1037/h0077246. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366(2):223–30. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd J, Rapoport JL. Brain development in healthy, hyperactive, and psychotic children. Arch Neurol. 2002;59:1244–8. doi: 10.1001/archneur.59.8.1244. [DOI] [PubMed] [Google Scholar]
- Goldberg LR. International Personality Item Pool: a scientific collaboratory for the development of advanced measures of personality traits and other individual differences[Online] 2001 Available http://ipip.ori.org/ipip.
- Goldberg LR, Johnson JA, Eber HW, Hogan R, Ashton MC, Cloninger CR, et al. The International Personality Item Pool and the future of public-domain personality measures. J Res Pers. 2006;40:84–96. [Google Scholar]
- Gong EJ, Garrel D, Calloway DH. Menstrual cycle and voluntary food intake. Am J Clin Nutr. 1989;49:252–258. doi: 10.1093/ajcn/49.2.252. [DOI] [PubMed] [Google Scholar]
- Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Whiteman MC, Pattie A, Deary I. Goldberg’s IPIP five-factor markers: Internal consistency and concurrent validity in Scotland. Pers Indiv Differ. 2005;39:317–329. [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? J Am Acad Child Psy. 1997;36:1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood? J Am Acad Child Psy. 2004;43:718–726. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- Haines J, Neumark-Sztainer D. Prevention of obesity and eating disorders: a consideration of shared risk factors. Health Educ Res. 2006;21(6):770–82. doi: 10.1093/her/cyl094. [DOI] [PubMed] [Google Scholar]
- Hayward C, Killen JD, Wilson DM, Hammer LD, Litt IF, Kraemer HC, Haydel F, Varady A, Taylor CB. Psychiatric risk associated with early puberty in adolescent girls. J Am Acad Child Psy. 1997;36:255–262. [PubMed] [Google Scholar]
- Hayward C, Killen JD, Hammer LD, Litt IF, Wilson DM, Simmonds B, Taylor CB. Pubertal stage and panic attack history in sixth- and seventh-grade girls. Am J Psychiatry. 1992;149:1239–43. doi: 10.1176/ajp.149.9.1239. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2006;49(2):197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Kosunen E, Rimpela M. Pubertal timing, sexual behaviour and self-reported depression in middle adolescence. J Adolescence. 2003a;26:531–545. doi: 10.1016/s0140-1971(03)00053-8. [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Marttunen M, Rantanen P, Rimpela M. Early puberty is associated with mental health problems in middle adolescence. Soc Sci Med. 2003b;57:1055–1064. doi: 10.1016/s0277-9536(02)00480-x. [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Rimpela M, Rissanen A, Rantanen P. Early puberty and early sexual activity are associated with bulimic-type eating pathology in middle adolescence. J Adolescent Health. 2001;28:346–352. doi: 10.1016/s1054-139x(01)00195-1. [DOI] [PubMed] [Google Scholar]
- Keel PK, Fulkerson JA, Leon GR. Disordered eating precursors in pre- and early adolescent girls and boys. J Youth Adolescence. 1997;26:203–216. [Google Scholar]
- Keel PK, Klump KL. Are eating disorders culture-bound syndromes? Implications for conceptualizing their etiology. Psychol Bull. 2003;129:747–69. doi: 10.1037/0033-2909.129.5.747. [DOI] [PubMed] [Google Scholar]
- Keel PK, Klump KL, Miller KB, McGue M, Iacono WG. Shared transmission of eating disorders and anxiety disorders. Int J Eat Disord. 2005;38:99–105. doi: 10.1002/eat.20168. [DOI] [PubMed] [Google Scholar]
- Killen JD, Hayward C, Litt I, Hammer LD, Wilson DM, Miner B, Taylor B, Varady A, Shisslak C. Is puberty a risk factor for eating disorders. AMA Am J Dis Child. 1992;146:323–325. doi: 10.1001/archpedi.1992.02160150063023. [DOI] [PubMed] [Google Scholar]
- Killen JD, Hayward C, Wilson DM, Taylor CB, Hammer LD, Litt I, Simmonds B, Haydel F. Factors associated with eating disorder symptoms in a community sample of 6th and 7th grade girls. Int J Eat Disord. 1994;15:357–367. doi: 10.1002/eat.2260150406. [DOI] [PubMed] [Google Scholar]
- Killen JD, Taylor CB, Hayward C, Haydel KF, Wilson DM, Hammer L, Kraemer H, Blair Greiner A, Strachowski D. Weight concerns influence the development of eating disorders: A 4-year prospective study. J Consult Clin Psych. 1996;64:936–940. doi: 10.1037//0022-006x.64.5.936. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12(2):427–33. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Klump KL, Perkins P, Burt SA, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychol Med. doi: 10.1017/S0033291707000189. in press. [DOI] [PubMed] [Google Scholar]
- Klump KL, Gobrogge KL, Perkins PS, Thorne D, Sisk CL, Breedlove SM. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychol Med. 2006;36:539–546. doi: 10.1017/S0033291705006653. [DOI] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Age differences in genetic and environmental influences on eating attitudes and behaviors in preadolescent and adolescent female twins. J Abnorm Psychol. 2000;109:239–251. [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Differential heritability of eating attitudes and behaviors in prepubertal versus pubertal twins. Int J Eat Disord. 2003;33:287–292. doi: 10.1002/eat.10151. [DOI] [PubMed] [Google Scholar]
- Koff E, Rierdan J. Advanced pubertal development and eating disturbance in early adolescent girls. J Adolescent Health. 1993;14:433–439. doi: 10.1016/1054-139x(93)90113-4. [DOI] [PubMed] [Google Scholar]
- Lanza ST, Collins LM. A mixture model of discontinuous development in heavy drinking from ages 18 to 30: The role of college enrollment. J Stud Alcohol. 2006;67:552–561. doi: 10.15288/jsa.2006.67.552. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: relation to menstrual-cycle phase and cortisol levels. Psychol Med. 2003;33:51–60. doi: 10.1017/s0033291702006815. [DOI] [PubMed] [Google Scholar]
- Lucas AR, Beard CM, Ofallon WM, Kurland LT. 50-Year trends in the incidence of anorexia nervosa in Rochester, Mn - a population-based study. Am J Psychiat. 1991;148:917–922. doi: 10.1176/ajp.148.7.917. [DOI] [PubMed] [Google Scholar]
- Luce KH, Crowther JH. The reliability of the Eating Disorders Examination: Self-report questionnaire version (EDE-Q) Int J Eat Disord. 1999;25:349–351. doi: 10.1002/(sici)1098-108x(199904)25:3<349::aid-eat15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Lyons PM, Truswell AS, Mira M, Vizzard J, Abraham SF. Reduction of food-intake in the ovulatory phase of the menstrual cycle. Am J Clin Nutr. 1989;49:1164–1168. doi: 10.1093/ajcn/49.6.1164. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144(3):961–8. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J Am Acad Child Psy. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- McCabe MP, Ricciardelli LA. A longitudinal study of pubertal timing and extreme body change behaviors among adolescent boys and girls. Adolescence. 2004;39:145–66. [PubMed] [Google Scholar]
- Must A, Naumova EN, Phillips SM, Blum M, Dawson-Hughes B, Rand WM. Childhood overweight and maturational timing in the development of adult overweight and fatness: the Newton Girls Study and its follow-up. Pediatrics. 2005;116(3):620–7. doi: 10.1542/peds.2004-1604. [DOI] [PubMed] [Google Scholar]
- Ohring R, Graber JA, Brooks-Gunn J. Girls’ recurrent and concurrent body dissatisfaction: correlates and consequences over 8 years. Int J Eat Disord. 2002;31:404–15. doi: 10.1002/eat.10049. [DOI] [PubMed] [Google Scholar]
- Ong KK, Ahmed ML, Dunger DB. Lessons from large population studies on timing and tempo of puberty (secular trends and relation to body size): the European trend. Mol Cell Endocrinol. 2006;254–255:8–12. doi: 10.1016/j.mce.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Palta M, Prineas RJ, Berman R, Hannan P. Comparison of self-reported and measured height and weight. Am J Epidemiol. 1982;115:223–230. doi: 10.1093/oxfordjournals.aje.a113294. [DOI] [PubMed] [Google Scholar]
- Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- Patton GC, McMorris BJ, Toumbourou JW, Hemphill SA, Donath S, Catalano RF. Puberty and the onset of substance use and abuse. Pediatrics. 2004;114:E300–E306. doi: 10.1542/peds.2003-0626-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status - reliability, validity, and initial norms. J Youth Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Puberty: A period of both organizational and activational effects of steroid hormones on neurobehavioural development. J Neuroendocrinol. 2003;15:1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- Rosenblitt JC, Soler H, Johnson SE, Quadagno DM. Sensation seeking and hormones in men and women: Exploring the link. Horm Behav. 2001;40:396–402. doi: 10.1006/hbeh.2001.1704. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Amundsen A, Grant M. Alcohol consumption and related problems among primary health care patients: WHO Collaborative Project on early detection of persons with harmful alcohol consumption I. Addiction. 1993a;88:349–362. doi: 10.1111/j.1360-0443.1993.tb00822.x. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption II. Addiction. 1993b;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype → environment effects. Child Dev. 1983;39:440–450. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006;254–255:120–6. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at alpha4beta2delta GABA(A) receptors triggers anxiety at puberty. Nat Neurosci. 2007;10(4):469–77. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Yancey AK, Aneshensel CS, Schuler R. Body image, perceived pubertal timing, and adolescent mental health. J Adolescent Health. 1999;25:155–165. doi: 10.1016/s1054-139x(98)00160-8. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Schulz KM, Zehr JL. Puberty: a finishing school for male social behavior. Ann N Y Acad Sci. 2003;1007:189–98. doi: 10.1196/annals.1286.019. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg RE, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stunkard AJ, Albaum JM. The accuracy of self-reported weights. Am J Clin Nutr. 1981;83:1297–1305. doi: 10.1093/ajcn/34.8.1593. [DOI] [PubMed] [Google Scholar]
- Uher R, Treasure J. Brain lesions and eating disorders. J Neurol Neurosurg Psychiatry. 2005;76(6):852–7. doi: 10.1136/jnnp.2004.048819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma M, Chai JK, Meguid MM, Laviano A, Gleason JR, Yang ZJ, Blaha V. Effect of estradiol and progesterone on daily rhythm in food intake and feeding patterns in Fischer rats. Physiol Behav. 1999;68:99–107. doi: 10.1016/s0031-9384(99)00152-3. [DOI] [PubMed] [Google Scholar]
- van den Berg SM, Setiawan A, Bartels M, Polderman TJ, van der Vaart AW, Boomsma DI. Individual differences in puberty onset in girls: Bayesian estimation of heritabilities and genetic correlations. Behav Genet. 2006;36:261–70. doi: 10.1007/s10519-005-9022-y. [DOI] [PubMed] [Google Scholar]
- van Weissenbruch MM, Delemarre-van de Waal HA. Early influences on the tempo of puberty. Horm Res. 2006;65(Suppl 3):105–11. doi: 10.1159/000091514. [DOI] [PubMed] [Google Scholar]
- Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J Comp Physiol Psych. 1975;88:183–193. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 2006;66(6):578–90. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking: Beyond the optimum level of arousal. Hillsdale: Erlbaum; 1979. [Google Scholar]
- Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America: cross-cultural, age, and sex comparisons. J Consult Clin Psych. 1978;46:139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]


