Abstract
Objective
International research programs have contributed to the creation of operationally defined criteria to identify individuals at risk for schizophrenia. Although there has been substantial progress in the prospective study of the schizophrenia prodrome, the utility of current diagnostic criteria remains questionable because of the relatively low base rates of incident psychoses, the high false-positive rate and ethical concerns regarding the treatment of individuals at risk. The identification of brain based neurocognitive vulnerability markers for schizophrenia may contribute to the development of an at risk algorithm with greater predictive accuracy.
Methods
Forty subjects at risk (AR) for schizophrenia, 15 in their first episode (FE) of schizophrenia, and 36 healthy comparison (HC) subjects were administered a neurocognitive battery that assessed the domains of processing speed, working memory, verbal episodic memory, executive functioning and general intelligence.
Results
At baseline, AR subjects showed neurocognitive deficits across all domains compared to HC subjects that were less severe than those observed in the FE sample. In preliminary analyses, AR subjects who later converted to psychosis (N=5) had greater neurocognitive impairment at baseline evaluation compared to those individuals who remained “at risk” at follow-up.
Conclusions
Neurocognitive deficits may be important in the pathogenesis of early psychosis and could help to define individuals at greatest risk for schizophrenia. Continued research in larger cohorts is needed to test the validity of this neurocognitive profile and its utility as a vulnerability marker.
Keywords: Prodromal, Endophenotype, Neuropsychological
1. Introduction
Over the last decade, there have been considerable efforts to prospectively identify young people at risk for developing schizophrenia. There is evidence suggesting that longer durations of untreated psychosis is integrally related to poorer outcomes. Therefore, if the early prodromal phase of schizophrenia can be reliably predicted and/or recognized there is hope that early intervention efforts may delay or prevent the onset of psychosis or at least reduce the morbidity of the illness.
Improved understanding of the schizophrenia prodrome is slowly emerging as the result of international prodromal research programs, including the Cognitive Assessment and Risk Evaluation (CARE) Program at the University of California, San Diego. These programs have contributed to the development of operationally defined “prodromal criteria” that include attenuated or transient psychotic symptoms and/or familial risk plus a decline in overall global functioning that aim to identify a group of individuals at high risk for developing psychosis. Although there has been substantial progress made in the ability to prospectively identify individuals at risk for psychosis, the clinical utility of assessment measures alone remains suboptimal since the false-positive rate is over 50% in most studies and the incidence of “conversion” to psychosis over even moderately long study durations is relatively low (Haroun et al., 2006).
The high false-positive rate raises ethical concerns regarding pharmacologic treatment of individuals who are identified as “at risk” but may not be in the prodromal phase of the illness (Corcoran et al., 2005; Haroun et al., 2006; McGlashan, 2001; McGorry et al., 2001). Therefore, improved predictive accuracy for distinguishing individuals at imminent risk for developing psychosis is needed. The identification of brain based neurobiological or neurocognitive vulnerability markers for schizophrenia may contribute to the development of an “at risk algorithm” with greater predictive accuracy.
1.1. Neurcognitive Markers
Neurocognitive dysfunction has been well established in schizophrenia and is an uncontested core component of the illness. A broad array of neurocognitive deficits across multiple domains, including motor abilities, learning/memory, executive functions, attention, language, spatial abilities, and general intelligence has been well documented (Heinrichs & Zakanis, 1998). Similar neurocognitive impairments, particularly deficits in verbal memory, executive functioning, working memory and attention, have been detected at the first episode of psychosis (Bilder et al., 2000). Neurocognitive dysfunction has also been demonstrated in nonpsychotic first-degree relatives of patients with schizophrenia (Cornblatt & Obuchowski, 1997) and individuals with schizotypal personality disorder (Cadenhead et al., 1999; Siever & Davis, 2004), both of which have been shown to be at greater risk of developing psychosis. Detection of impairment prior to onset of psychosis suggests that some neurocognitive abnormalities precede and are not solely a consequence of psychosis, and thus may represent a trait marker for schizophrenia.
Evidence of neurocognitive impairment in individuals putatively in the prodromal phase of schizophrenia is rapidly emerging (Brewer et al., 2006a; Francey et al., 2005; Hawkins et al., 2004; Keefe, 2006; Lencz et al., 2006a). Preliminary results indicate that at risk individuals generally perform intermediate to healthy controls and first-episode psychosis patients, particularly on measures of verbal learning/memory, attention, executive abilities and general intellectual functioning (Bartok et al., 2005; Brewer et al., 2005; Brewer et al., 2006a; Cosway et al., 2000; Francey et al., 2005; Gschwandtner et al., 2003; Hambrecht et al., 2002; Hawkins et al., 2004; Keefe et al., 2006; Lencz et al., 2006b; Wood et al., 2003). Measures of verbal memory and spatial working memory in particular, as well as olfactory identification have demonstrated utility in predicting the transition to psychosis (Brewer et al., 2005; Brewer et al., 2003; Lencz et al., 2006b; Wood et al., 2003). Thus, consistent with a neurodevelopmental model of schizophrenia, neurocognitive dysfunction may precede the onset of psychosis and prove to be an effective vulnerability marker, especially when combined with other known neurobiological markers: P50 event related potential suppression (Myles-Worsley et al., 2004), prepulse inhibition of the startle response (Abel et al., 2004; Cadenhead et al., 2000; Tenn et al., 2005), anti-saccade (O’Driscoll et al., 1998; Thaker et al., 2000; Thaker & Ross, 1998), mismatch negativity (Brockhaus-Dumke et al., 2005) and sub-threshold psychotic symptoms, enhancing our ability to identify individuals truly in the prodromal phase of schizophrenia (Haroun et al., 2006).
Although considerable progress as been made, replication of the few published reports is needed in order to accurately characterize the neurocognitive profile of those in the prodromal phase of psychosis. Additional studies are needed in order to establish specific neurocognitive impairments that are sensitive enough to serve as effective predictors of psychosis. Studies utilizing a broad neuropsychological battery among a sample that includes at risk individuals in addition to those who have recently progressed to psychosis (e.g., Keefe et al., 2006) would allow for the examination into the trajectory of psychotic illnesses.
Here we compare the baseline neurocognitive profiles of individuals at risk (AR) for developing psychosis, to those who have experienced their first-episode (FE) of psychosis, and healthy comparison (HC) subjects in the CARE (Cognitive Assessment and Risk Evaluation) program at the University of California, San Diego. Our a priori hypothesis was that the AR sample would have neurocognitive deficits when compared to the HC group across multiple domains that are intermediate to those observed in FE patients. Additionally, we aimed to identify neurocognitive vulnerability markers for psychosis that would help predict psychotic outcome in the AR sample. Preliminary data are presented that compare baseline neurocognitive performance of AR subjects who later converted to psychosis (true prodromals) to that of subjects who remained at risk at one year follow up.
2. Method
2.1. The CARE Program
The CARE Program provides assessment, evaluation, and referral for individual treatment as needed, for individuals between the ages of 12 and 30, who are putatively at risk for, or experiencing early symptoms of schizophrenia (Seeber & Cadenhead, 2005). After extensive community outreach and education, individuals are referred from various professionals in the community (i.e., physicians, mental health practitioners, school personnel). After telephone screening, individuals deemed appropriate for the clinical research program provide informed consent (IRB# 050650) and undergo an initial clinical evaluation. Family history of psychiatric illness is collected via interviews with family members. Qualifying subjects complete a battery of neurocognitive and psychophysiological tests. Participants are followed for two years, with monthly clinical follow-up visits; the formal testing is re-administered at six month intervals.
2.2. Program Inclusion Criteria
Inclusion criteria for the At Risk (AR) group were based on The Structured Interview for Prodromal Syndromes (SIPS: (Miller et al., 2003)) and our established criteria (see Table 1) (Seeber & Cadenhead, 2005). The AR sample includes individuals who are clinically at high risk for psychosis, because they have had a recent onset (<1 year) of subsyndromal psychotic symptoms, and/or they are genetically at high risk because they have a family history of schizophrenia in a first degree relative or a diagnosis of schizotypal personality disorder and a recent deterioration in functioning. The CARE criteria differ slightly from the Scale of Prodromal Symptoms (SOPS, (Miller et al., 2004)) in that we also utilize disorganized behavior as a criteria for the prodromal syndrome and psychosis. Additionally, the “deterioration in functioning” definition for the genetic risk group also includes new onset of symptoms such as depression or anxiety. Finally, a negative symptom group is also included.
Table 1.
Criteria for At Risk Groups and Psychotic Conversion Criteria in the Cognitive Assessment and Risk Evaluation (CARE) Program.
| At Risk Groups |
|---|
| Brief Psychosis Group |
|
| Subsyndromal Group |
|
| Genetic Risk and Deterioration Group |
|
| Negative Symptoms Group |
|
| Psychotic Conversion Criteria |
|
P1-P5 – Psychosis Items 1 through 5 from the Structured Interview for Prodromal Syndrome (SIPS)
D1-D5 – Disorganized Items 1 through 5 from the SIPS
N1-N6 – Negative Symptom Items 1 through 6 on the SIPS
Individuals in the First Episode group (FE) had experienced their first psychotic episode within the past year. Each of these individuals met DSM-IV diagnostic criteria for Schizophrenia per the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) upon entry to the program.
Those who met criteria for the FE or AR group were accepted into the program irrespective of previous or current treatment. Additionally, pharmacological treatment was initiated on an individual basis if warranted; however in most cases, baseline testing was completed prior to commencement of medications.
Individuals in the AR and FE groups were ineligible for participation if they had a history of head injury (loss of consciousness > 15 minutes or neurological sequelae), current drug abuse/dependence (as defined by DSM-IV and a urine toxicology screen), neurological disorder, current use of stimulant medication, or an IQ below 80 (per prior assessment or general IQ assessment below). Of note, there were no individuals excluded from this study due to low IQ.
The healthy comparison group (HC) consisted of individuals who responded to community advertisements, and who were comparable to the AR and FE subjects with respect to age, gender, ethnicity and parental education. In addition to the exclusion criteria used for the program participants, HC individuals were also excluded if they were currently taking psychiatric medications, met criteria for an AXIS I disorder, or had a personal history of psychiatric illness, learning disability or a family history of psychotic illnesses. Finally, HC subjects were excluded if they met criteria for a Cluster A personality disorder or showed evidence of prodromal symptoms. These exclusionary criteria are similar to other prodromal studies.
2.3. Clinical Measures
The SIPS was administered to all AR and HC individuals to assess level of risk for psychosis. The SIPS rating scale was developed specifically for use in at risk populations and assesses the presence, type, severity, frequency, and duration of prodromal symptoms as well as the degree of distress from the symptoms. Axis I diagnoses (for all groups) were assessed using the Structured Clinical Interview for DSM-IV Axis I disorders (SCID I). Axis II disorders were assessed using the Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II) for HC subjects and the Structured Interview for DSM-IV Personality Disorders (Pfohl et al., 1995) for AR subjects. The Scale for the Assessment of Negative Symptoms (SANS), the Scale for the Assessment of Positive Symptoms (SAPS) and the Brief Psychiatric Rating Scale (BPRS) were used to further evaluate clinical symptoms in AR and FE groups. Current level of functioning was assessed with the Modified Global Assessment of Functioning (GAF-M: (Hall & Parks, 1995)). The highest GAF score from the past year was determined retrospectively. Family history of psychiatric illness was assessed, after receiving consent to contact a relative, using the Family History Research Diagnostic Criteria (Andreasen et al., 1977).
2.4. Neurocognitive Measures
The neurocognitive battery was part of a larger battery of tests that included psychophysiological measures of brain inhibitory functioning, measures of attention and visual information processing. The neurocognitive battery was designed to minimize practice effects over repeated administration and assess five different neurocognitive domains: processing speed, working memory, verbal episodic memory, executive functioning and general intelligence. These neurocognitive domains have been identified based on demonstrated impairments in schizophrenia spectrum populations (Cadenhead et al., 1999; Goldberg et al., 1995; Voglmaier et al., 1997). Each neurocognitive domain was comprised of the following subtests:
Processing Speed
The total raw score from Color Naming subtest from the Stroop Color and Word Test (Golden, 1978), and the total correct on the Numerical Attention Test (Franklin et al., 1988).
Working Memory
The total raw score on the Letter Number Sequencing (LNS) subtest from the Wechsler Memory Scale – Third Edition (WMS-III) (Wechsler, 1997b), and the total raw score from Spatial Span backward condition from the WMS-III (Wechsler, 1997b).
Verbal Episodic Memory
Total immediate and long-delay recall scores from the Hopkins Verbal Learning Test – Revised (HVLT-R) (Benedict & Zgaljardic, 1998) comprised this domain. The HVLT-R was used because there are 6 equivalent forms and our goal was to minimize practice effects over repeated testing.
Executive Functioning
The total number of perseverative responses on the 64-card version of the Wisconsin Card Sorting Test (WCST) (Heaton et al., 1993) and the Interference score from the Stroop Color and Word Test (Golden, 1978).
General Intelligence
The total raw scores from the Vocabulary subtest and Block Design subtests from the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) (Wechsler, 1997a) provided measures of verbal and non-verbal IQ.
2.5. Formation of Composite Scores
Z-scores for all variables were computed based on the mean and standard deviation of the healthy comparison group: (X– mean of HC group)/SD of HC group. As needed, z-scores were transformed so that higher scores were indicative of better performance. Composite scores for each neurocognitive domain were then created by averaging the z-scores of contributing variables using equal weights. A global neurocognitive performance index was created by averaging the five composite scores.
Contributing variables for each domain were chosen on a theoretical basis. Each composite score was comprised of two contributing variables (see Table 3). The small sample size in this study did not allow for confirmation of the factor structure underlying each of the composite scores; however published literature has confirmed the appropriateness of similar neurocognitive domain composite scores (Bilder et al., 2000; Voglmaier et al., 1997).
Table 3.
Individual neurocognitive variable means and significance testing of Healthy Comparison (HC), At Risk (AR) and First Episode (FE) subjects at baseline assessment
| Domain | Measure | Group | N | Mean | SD | F[df] | p |
|---|---|---|---|---|---|---|---|
| Processing
Speed |
Stroop Color Naming | HC | 35 | 78.46 | 12.34 | 14.35[2,87] | .000a,b,c |
| AR | 39 | 69.85 | 10.80 | ||||
| FE | 14 | 60.50 | 7.45 | ||||
| Numeric Attention Total
Seconds |
HC | 36 | 170.97 | 46.39 | 1.66[2,87] | .197 | |
| AR | 40 | 186.75 | 63.66 | ||||
| FE | 12 | 204.58 | 72.60 | ||||
| Working
Memory |
Letter Number Sequencing
Total |
HC | 36 | 13.50 | 3.52 | 8.12[2,89] | .001a,b |
| AR | 40 | 11.03 | 2.54 | ||||
| FE | 14 | 10.86 | 1.99 | ||||
| WMS-III Spatial Span
Backwards |
HC | 36 | 10.00 | 1.87 | 7.06[2,89] | .001a,b | |
| AR | 40 | 8.45 | 2.29 | ||||
| FE | 14 | 8.07 | 1.86 | ||||
| Verbal Episodic
Memory |
Hopkins Verbal Learning | HC | 36 | 28.81 | 4.17 | 8.24[2,88] | .001a,b |
| Test - Revised | AR | 39 | 26.13 | 3.76 | |||
| Total Recall | FE | 14 | 23.57 | 5.98 | |||
| Hopkins Verbal Learning | HC | 36 | 10.42 | 1.40 | 6.15[2,88] | .003a,b | |
| Test - Revised | AR | 39 | 9.28 | 2.54 | |||
| Delayed Recall | FE | 14 | 8.21 | 2.29 | |||
| Executive
Functioning |
Wisconsin Card Sort Test | HC | 36 | 7.89 | 4.65 | 4.61[2,90] | .012b |
| Perseverative Responses | AR | 40 | 11.28 | 8.47 | |||
| FE | 15 | 14.93 | 11.43 | ||||
| Stroop Interference | HC | 35 | 47.80 | 9.94 | 4.90[2,87] | .010a,b | |
| AR | 39 | 43.28 | 10.27 | ||||
| FE | 14 | 38.21 | 9.58 | ||||
| General
Intelligence |
WAIS III Vocabulary | HC | 35 | 49.77 | 7.00 | 13.01[2,87] | .000a,b,c |
| AR | 40 | 41.15 | 12.55 | ||||
| FE | 13 | 33.15 | 12.77 | ||||
| WAIS III Block Design | HC | 36 | 49.00 | 10.48 | 3.16[2,90] | .047b | |
| AR | 40 | 46.45 | 12.10 | ||||
| FE | 15 | 39.93 | 13.53 |
Post hoc F tests Healthy Comparison vs At Risk, p<0.05
Post hoc F tests Healthy Comparison vs First Episode, p<0.05
Post hoc F tests At Risk vs First Episode, p<0.05
2.6. Statistical Analyses
All data were assessed for normality and homogeneity of variance. Univariate analyses (as well as nonparametic tests if indicated) were used to compare the global neurocognitive performance index and neurocognitive domains across the three groups. Multivariate tests were not used because the cell size of the FE group was too small to provide adequate power. Conservative P values of <0.01 were used to correct for multiple comparisons. Post-hoc Dunnett t-tests (or Mann Whitney U tests) were performed to determine whether the AR and FE groups differed from the HC sample. Follow-up analyses were also performed to assess the effect of age and parental education on the neurocognitive measures as well as antipsychotic medication within the AR sample. Additional preliminary analyses, including the Jonckheere Test for Ordered Alternatives (Jonckheere, 1954), were performed to compare the pattern of performance of the AR subgroup who had later converted to psychosis to those AR subjects who remained at risk at one year follow-up, the FE, and HC groups.
3. Results
This paper reports the baseline neurocognitive results of a sample of 91 adult participants, age 16 to 30 (AR = 40, FE = 15, HC = 36). The younger adolescent participants (< age 16) were not included in the current analyses due to the small sample size and the administration of different tests (e.g., WISC-III subtests).
3.1. Program Participant Characteristics
Demographic and clinical characteristics of this sample are summarized in Table 2. The three groups (AR, FE, HC) were not significantly different in age, parental education, gender, handedness, or ethnicity. The AR and FE groups had similar family histories. The AR and FE groups presented with similar overall levels of global functioning (GAF) and clinical symptoms as measured by the BPRS and SANS. The FE sample had more positive psychotic symptoms, as assessed by the global SAPS, compared to the AR group (t[53] = 3.32, p <0.002). These sample characteristics support the developmental model that negative symptoms precede positive symptoms in the pathogenesis of psychotic disorders (Cornblatt et al., 2002).
Table 2.
Demographic and clinical characteristics of the sample.
| Healthy Comparison
N=36 |
At Risk
N=40 |
First Episode
N=15 |
|
|---|---|---|---|
| Age – Mean (SD) | 21.8 (3.4) | 20.8 (3.5) | 21.5 (5.2) |
| Parental Education – Mean (SD) | 15.1 (2.8) | 15.5 (2.3) | 14.4 (2.5) |
| Gender - % Male | 53% | 51% | 55% |
| Ethnicity - % Caucasian | 55% | 53% | 47% |
| Handedness - % Right | 94% | 95% | 93% |
| Family History of Psychosis 1st Degree - % | - | 20% | 27% |
| Family History of Psychosis 2nd Degree or greater - % | - | 35% | 20% |
| Psychotropic Treatment - % | - | 53% | 73% |
| Antipsychotic Treatment - % | - | 30% | 73%a |
| Average weeks on Antipsychotics (range) | - | 17.6 (19.1)
(1-52) |
18.2 (15.1)
(1 -44) |
| GAF – Mean (SD) | - | 53.5 (10.2) | 47.6 (7.4) |
| BPRS Total – Mean (SD) | - | 16.2 (6.3) | 18.6 (8.0) |
| Global SAPS – Mean (SD) | - | 5.0 (2.8) | 8.7 (5.5)a |
| Global SANS – Mean (SD) | - | 5.9 (3.8) | 8.7 (4.8) |
| Structure Interview for Prodromal States Total – Mean (SD) | - | 34.6 (16.0) | - |
At Risk versus First Episode, p < 0.05
The AR and FE samples did not significantly differ in the number taking a psychotropic medication (21/40 AR versus 11/15 FE). Of the 21 AR participants receiving a psychotropic medication at baseline, 11 were receiving more than one. Significantly more FE participants (11/15) were receiving antipsychotic treatment at the time of their baseline evaluation compared to the AR participants (12/40); although average length of antipsychotic treatment was similar for both groups (FE: 18.1 weeks, AR: 17.6 weeks). All of those in the FE group were receiving atypical antipsychotic medications (risperidonel=7; olanzapine=3; ziprasidone=3). Several of the FE participants were receiving other medications (3 were also receiving an antidepressant, 2 were on an anticholinergic agent and 1 was receiving a benzodiazepine). Similarly, all twelve of the AR participants were receiving atypical antipsychotic medications (risperidone=7; olanzapine=3; quetiapine=1; aripiprizole=1). Additionally, there were nine AR participants receiving other psychotropic medications (antidepressants=8; benzodiapine=1). Post-hoc analyses revealed that although AR participants receiving an antipsychotic had more symptoms as indicated by Global SAPS, Global SANS, and Total SIPS (all NS) compared to the AR subjects not on an antipsychotic, a lower GAF score was the only measure that statistically differentiated the groups (F[1,39]=4.95, p<.05).
3.2 Neurocognitive Results
In this sample of 91 participants, there were nine who were missing one of the two measures from which one of the neurocognitive domain composite scores was derived. In each of these cases, their domain composite score was comprised of the one measure that was available. There were three participants who were missing both measures that comprised one domain composite score; for these cases, the score was imputed by averaging the remaining four domain composite scores, this average was then used for the missing domain composite score in the analysis as described in Twisk and de Vente (Twisk & de Vente, 2002). Consequently, the following baseline results represent data from the whole sample of 91 participants (AR = 40, FE = 15, HC = 36).
There were significant group effects on the global neurocognitive performance index (F[2,91]=16.930, p<0.001) (see Figure 1). Follow-up Dunnett t-tests indicated that the AR and FE groups differed significantly from the HC sample (p<0.001). A Kruskal-Wallis test also showed a significant group effect (X2[2]=31.93, p<0.001) and follow-up Mann-Whitney tests between AR and FE subjects versus HC were also significant (p<0.001).
Figure 1.
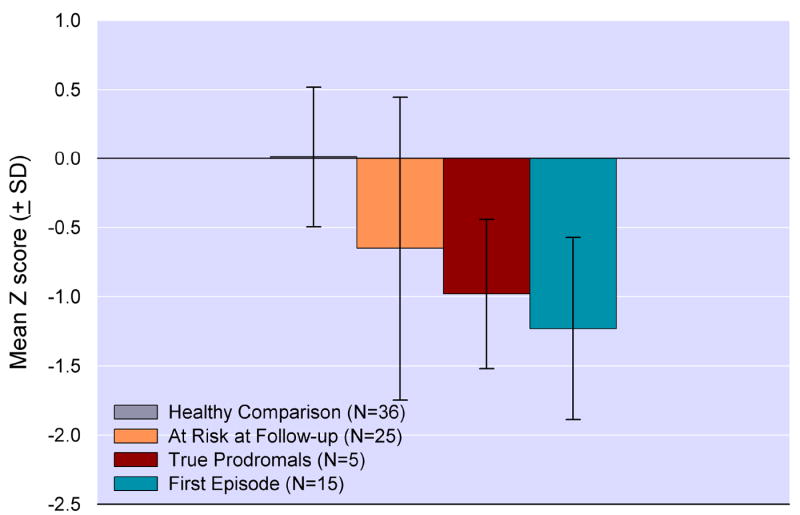
Mean Global Neurocognitive Performance Index (SD) at baseline assessment across groups (F[2,91]=16.930, p<0.001). Post-hoc tests indicated that the at risk and first episode groups differed significantly from the healthy comparison sample (p<0.001).
Significant group differences were present for each of the neurocognitive domains: 1) Processing Speed: F(2,91)=6.86, p<0.002, (Dunnett t-test AR vs HC: p<0.02; FE vs HC: p<0.002); 2) Working Memory: F(2,91)=12.24, p<0.001, (Dunnett t-test AR vs HC: p<0.001; FE vs HC: p<0.001); 3) Verbal Episodic Memory: F(2,91)=12.24, p<0.001, (Dunnett t-test AR vs HC: p<0.015; FE vs HC: p<0.001); 4) Executive Functioning: F(2,91)=6.69, p:<0.002, (Dunnett t-test AR vs HC: p<0.05; FE vs HC: p<0.002); and 5) Intellectual Functioning: F(2,91)=12.42, p<0.001, (Dunnett t-test AR vs HC: p<0.01; FE vs HC: p<0.01). Kruskal-Wallis and follow-up Mann-Whitney tests were also significant for the Executive Functioning (X2[2]=13.52, p<0.001, AR vs HC: p<0.015, FE vs HC: p<0.001) and Intellectual Functioning (X2[2]=18.40, p<0.001, AR vs HC: p<0.01, FE vs HC: p<0.001) domains. Figure 2 displays the group means across neurocognitive domains. Table 3 displays the means of the individual test variables and univariate tests.
Figure 2.
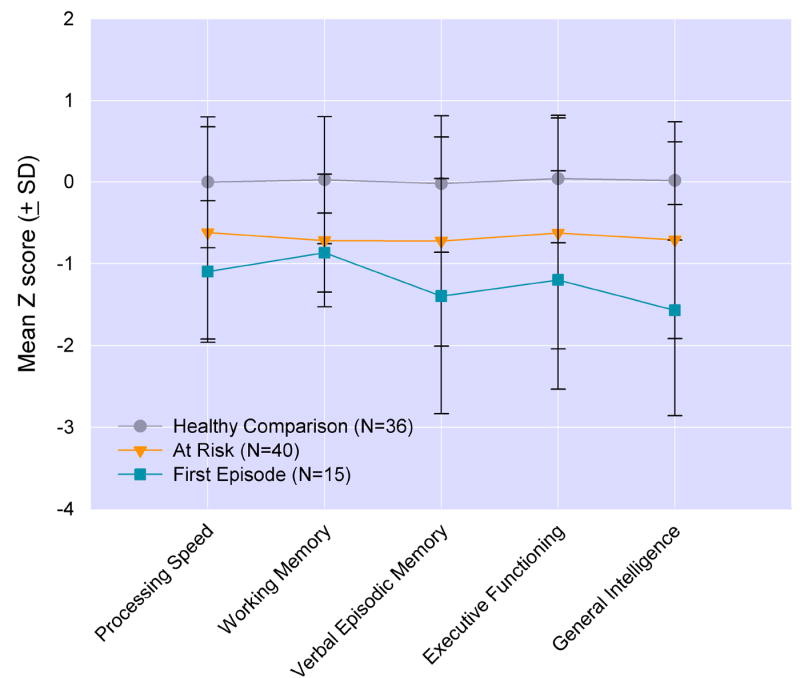
Means (SD) of neurocognitive domains from multivaritate analysis at baseline assessment. Significant group differences were present for each of the neurocognitive domains (p’s<0.002) and post hoc analyses showed significant group differences between at risk and first episode versus healthy comparison subjects (p’s<0.05).
Analyses were unchanged when gender or parental education were added as a covariates into the analyses. Among the AR group, those who were taking antipsychotic medications (n=12) performed significantly worse, compared to the sample not taking antipsychotic medication, on the overall neurocognitive performance index (F [1,40]=6.14, p<.05), as well as the following individual cognitive domains: Attention (F[1,40]=4.58, p<.05); Working Memory (F[1,40]=5.69, p<.05); Executive Function (F[1,40]=5.60, p<.05); and Processing Speed (F[1,40]=7.09, p<.05).
3.3 Preliminary Analyses of Psychotic Conversion
Preliminary analyses that compare the baseline neurocognitive performance of participants who later converted to psychosis (true prodromals) to that of individuals who remained “at risk” at one year follow-up revealed that of the 30 AR participants who received one year follow-up, 5 (17%) had converted to psychosis (3 schizophrenia, 1 bipolar manic with psychosis, 1 psychosis NOS), while 25 (83%) remained at risk. Of the remaining 25 AR participants who received follow-up at one year, four no longer met criteria for an Axis I diagnosis, while the rest remained comorbid for a variety of diagnoses (7 mood disorder, 7 anxiety disorder, 4 mood + anxiety, 3 bipolar). We have previously reported on the demographic (older age, no family history of psychosis, more likely to be taking an antipsychotic), clinical symptom ratings (greater severity of ideas of reference, suspiciousness, disorganized thought) and other risk factors (history of substance abuse) that distinguished those who converted to psychosis at one year and those who had not (Haroun et al., 2006). While it is recognized that the number of individuals who actually converted is small, and conclusions based on this small sample are premature, it is mentioned here because it replicates findings from previous studies (Brewer et al., 2005; Lencz et al., 2006a) and supports this general line of inquiry as well as the comparability of this sample to other studies of high-risk individuals.
As seen in Figure 3, the true prodromal sample’s baseline performance on the neurocognitive performance index fell between the FE sample and the non-converted AR sample. The small number of true prodromals precludes meaningful parametric analyses of individual group differences, but the trend of group differences was evaluated using the nonparametric Jonckheere Test for ordered alternatives (Jonckheere, 1954), a between group trend test assessing the hypotheses that the median levels of neuropsychological performance decrease in an orderly fashion from HC to non-converted to true prodromals to FE. The Jonckheere Test was highly significant, indicating the presence of a monotonic trend among the four groups (see Figure 3, J* statistic = 5.63, p<0.00001).
Figure 3.
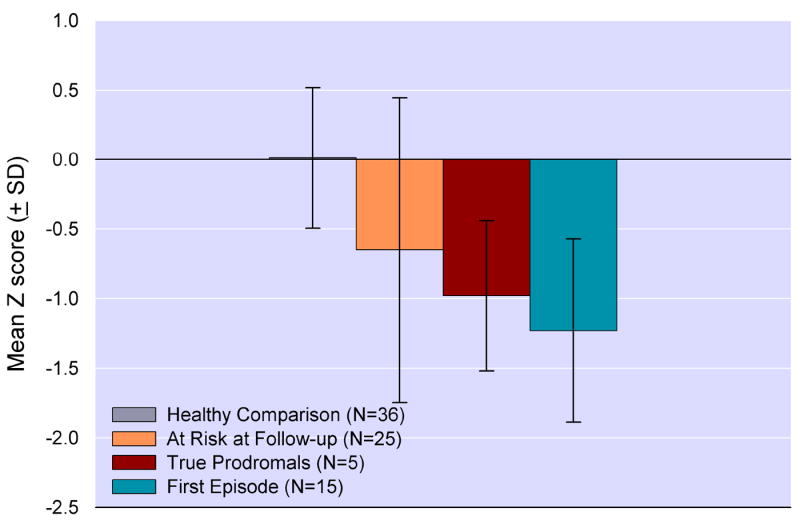
Mean Global Neurocognitive Performance Index (SD) at baseline assessment. At risk subjects are divided into those who were “true prodromals”, who had converted to psychosis, and those who remained At Risk, because they had not experienced a psychotic conversion, at 1 year follow-up. The at risk groups were predicted to perform intermediate to the healthy comparison and first episode schizophrenia patients using the Jonckeere Statistic for Non-Linear Trends (J* statistic = 5.63, p<0.00001).
Although the true prodromals showed summary neuropsychological impairment that was intermediate in severity between impairment of the FE and AR groups, the pattern of the group performances on the individual ability areas is also of interest (see Figure 4). Relative to the HCs, the true prodromals evidence impairment that was of the same magnitude as that of the FE group on verbal episodic memory (Cohen d, effect size = 1.34 and 1.01 vs HCs respectively) (Cohen, 1988) and general intelligence (effect size = 1.28 and 1.34 vs HCs respectively). The general intelligence impairment in the true prodromals and FE subjects appears to be accounted for primarily by the vocabulary subtest (effect size = 1.4 and 1.4 vs HCs respectively) rather than the block design subtest (effect size = .52 and .71 vs HCs respectively). On the other hand, the true prodromals showed comparable results to those of the AR group (and better than the FE group) on Processing Speed and Executive Functioning, and all of the three clinical groups showed approximately equal impairment relative to HCs on Working Memory (effect size range = 0.79 to 1.03).
Figure 4.
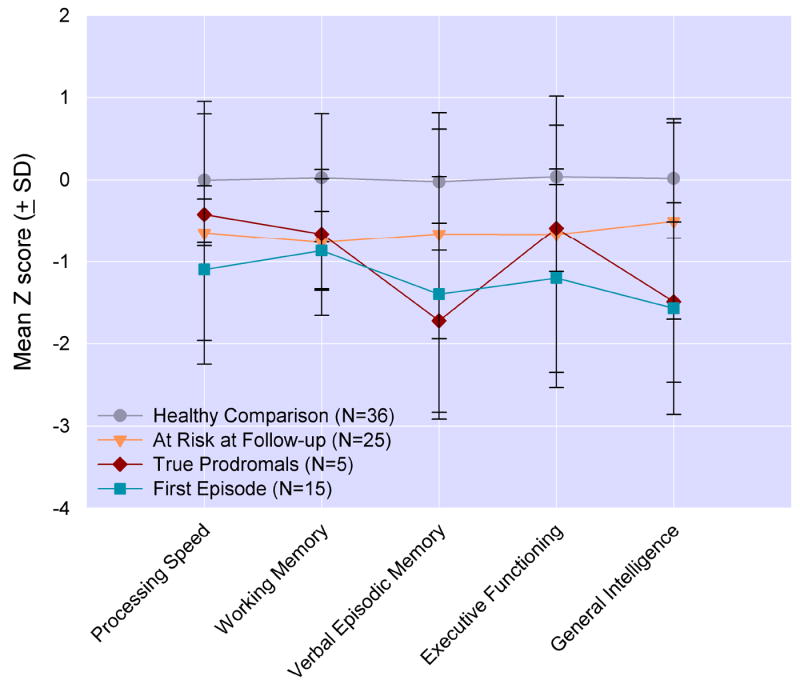
Means (SD) of neurocognitive domains from the baseline assessment. At Risk subjects are divided into those who were “true prodromals”, who had converted to psychosis, and those who remained At Risk, because they had not experienced a psychotic conversion, at 1 year follow-up. Relative to the healthy comparison subjects, the true prodromals evidence impairment that was of the same magnitude as that of the FE group on verbal episodic memory (Cohen d, effect size = 1.34 and 1.01 vs healthy comparison subjects respectively) and general intelligence (effect size = 1.28 and 1.34 vs healthy comparison subjects respectively). All three clinical groups showed approximately equal impairment relative to healthy comparison subjects on Working Memory (effect size range = 0.79 to 1.03).
4. Discussion
Subjects at risk for developing psychosis have neurocognitive deficits across multiple domains consistent with the pattern seen in patients experiencing their first episode of schizophrenia. These baseline findings support previous reports in putatively prodromal samples (Brewer et al., 2006b; Hawkins et al., 2004; Keefe et al., 2006; Lencz et al., 2006a) and generally implicate dysfunction in the frontotemporal regions of the brain, consistent with imaging studies in first episode samples (Morey et al., 2005; Pantelis et al., 2003). Additionally, these findings reinforce the notion that neurocognitive impairment may be important in the pathogenesis of psychosis and the general pattern of progressively poorer performance on neurocognitive measures seen among at risk individuals, first episode psychosis patients and patients with chronic schizophrenia reflects the observed disease progression in the early stages of schizophrenia (Thompson et al., 2001). Furthermore, these results suggest that a neurocognitive profile reflecting impairment across multiple domains may help to characterize those putatively in a prodromal stage of psychosis.
Preliminary inspection of the neurocognitive data of five at risk participants who converted to psychosis one year later demonstrated that as a group they fell between the first episode sample and the non-converted at-risk sample. The true prodromals had the largest effect sizes versus the healthy comparison subjects in the verbal episodic memory and general intellectual functioning domains, of the same magnitude observed in the first episode sample, suggesting that these domains may potentially serve as more specific risk factors for the development of psychosis. The finding of greater deficits in verbal versus non-verbal intelligence measures further suggests that there may be lifelong poor verbal episodic memory (learning) that may account for the impairment in the general intelligence domain. Albeit premature, this notion is consistent with reports indicating that decline in areas of verbal memory and general intellectual functioning correlate with the worsening, or onset of psychotic symptoms (Brewer et al., 2005; Byrne et al., 1999; Cosway et al., 2000; Lencz et al., 2006a). The small sample of converted participants precludes any meaningful conclusions but we believe it is noteworthy that the pattern of performance observed in this sample replicates recent reports by Brewer (Brewer et al., 2005) and Lencz (Lencz et al., 2006a) who reported that at risk subjects who later converted to psychosis demonstrated verbal learning deficits at baseline assessment as well as other reports indicating that impairments in attention do not predict psychosis (Francey et al., 2005).
One limitation of the current study, inherent in all prodromal research, is that it is unknown how many of the at risk individuals in this sample will go on to develop psychosis (true prodomals) and how many may be false positives and never destined to become psychotic. Because the CARE Program offers intervention and treatment as necessary, it is not possible to know if such intervention efforts successfully prevented the development of more significant psychopathology, particularly the onset of psychosis in some true prodromal participants.
Twelve of the 40 at risk participants were receiving an antipsychotic medication at the time of baseline assessment. These individuals performed worse than those at risk participants not receiving an antipsychotic on the global neurocognitive performance index, as well as the individual domains of Attention, Processing Speed, Executive Functions and Working Memory. There is little evidence that the newer atypical antipsychotic medications have a deleterious effect on neurocognition (Kern et al., 2006; Meltzer & McGurk, 1999), consequently, it is believed that more impaired performance among those receiving an antipsychotic is likely reflective of the lower level of functioning and increased symptoms seen in this group. Interesting, all AR participants performed similarly on Verbal Episodic Memory and Intellectual Functioning domains, the same domains that demonstrated utility in predicting transition to psychosis. This further supports the notion that impaired performance in these domains may serve as a specific risk marker for the development of psychosis.
The inclusion of first episode patients along with at risk participants is an important strength of this study. Only two other reports have included a first episode sample for comparison of neurocognitive data (Francey et al., 2005; Keefe et al., 2006). However, unlike these samples, the groups in this study are similar on all relevant demographic characteristics, thereby reducing the possibility of such factors confounding the results. Assessment of at risk individuals, first episode patients and healthy controls on the same broad cognitive battery allows for a more meaningful investigation into the progression of psychotic illnesses.
Prospective research takes considerable time and studying the precursors of an illness with a low base rate, such as schizophrenia, is particularly challenging. This area of research is still in its infancy and available information thus far is generally based on small and potentially variable samples. As our knowledge of the psychotic prodrome continues to evolve, all empirical findings in this area are valuable contributions. The current study is an important addition to the literature as it replicates previous findings, further corroborating the presence of neurocognitive dysfunction across a number of domains in individuals putatively at risk for developing psychosis and identifies the domains of verbal episodic memory and general intellectual functioning as potential risk markers that may contribute to the development of an algorithm of risk that can help to identify individuals who are at greatest risk for psychosis and in need of preventative interventions (Addington et al., In Press). Continued research is needed to assess the predictive validity of neurocognitive functioning not only for the later development of psychosis but also social and global functioning.
Acknowledgments
The authors would like to acknowledge Nasra Haroun, MD, Karin Kristensen, PsyD, Kathy Shafer, BS, Iliana Marks, BS, and Shah Golshan, PhD for their clinical expertise, technical assistance and statistical review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel KM, Jolley S, Hemsley DR, Geyer MA. The influence of schizotypy traits on prepulse inhibition in young healthy controls. Journal of Psychopharmacology. 2004;18(2):181–188. doi: 10.1177/0269881104042617. [DOI] [PubMed] [Google Scholar]
- Addington J, Cadenhead KS, Cannon T, Cornblatt BA, McGlashan T, Perkins D, et al. North American Prodrome Longitudinal Study (NAPLS): A Collaborative Multi-Site Approach to Prodromal Schizophrenia Research. Schizophrenia Bulletin. doi: 10.1093/schbul/sbl075. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The Family History Method Using Diagnostic Criteria: Reliability and Validity. Archives of General Psychiatry. 1977;34(10):1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Bartok E, Berecz R, Glaub T, Degrell I. Cognitive functions in prepsychotic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(4):621–625. doi: 10.1016/j.pnpbp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Zgaljardic DJ. Practice effects during repeated administrations of memory tests with and without alternate forms. J Clin Exp Neuropsychol. 1998;20(3):339–352. doi: 10.1076/jcen.20.3.339.822. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157(4):549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Francey SM, Wood SJ, Jackson HJ, Pantelis C, Phillips LJ, et al. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am J Psychiatry. 2005;162(1):71–78. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, McGorry PD, Francey SM, Phillips LJ, Yung AR, et al. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003;160(10):1790–1794. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, Phillips LJ, Francey SM, Pantelis C, Yung AR, et al. Generalized and Specific Cognitive Performance in Clinical High-Risk Cohorts: A Review Highlighting Potential Vulnerability Markers for Psychosis. Schizophrenia Bulletin. 2006a;32(3):538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, Phillips LJ, Francey SM, Pantelis C, Yung AR, et al. Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophr Bull. 2006b;32(3):538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Tendolkar I, Pukrop R, Schultze-Lutter F, Klosterkotter J, Ruhrmann S. Impaired mismatch negativity generation in prodromal subjects and patients with schizophrenia. Schizophr Res. 2005;73(23):297–310. doi: 10.1016/j.schres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Byrne M, Hodges A, Grant E, Owens DC, Johnstone EC. Neuropsychological assessment of young people at high genetic risk for developing schizophrenia compared with controls: preliminary findings of the Edinburgh High Risk Study (EHRS) Psychol Med. 1999;29(5):1161–1173. doi: 10.1017/s0033291799001002. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Perry W, Shafer K, Braff DL. Cognitive functions in schizotypal personality disordered subjects. Schizophrenia Research. 1999;37:123–132. doi: 10.1016/s0920-9964(98)00147-9. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: Evidence of inhibitory deficits. American Journal of Psychiatry. 2000;157(10):1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioural sciences. 2. Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- Corcoran C, Malaspina D, Hercher L. Prodromal interventions for schizophrenia vulnerability: the risks of being “at risk”. Schizophr Res. 2005;73(23):173–184. doi: 10.1016/j.schres.2004.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt B, Lencz T, Obuchowski M. The schizophrenia prodrome: treatment and high-risk perspectives. Schizophr Res. 2002;54(12):177–186. doi: 10.1016/s0920-9964(01)00365-6. [DOI] [PubMed] [Google Scholar]
- Cornblatt B, Obuchowski M. Update of high-risk research: 1987-1997. International Review of Psychiatry. 1997;9(4):437–447. [Google Scholar]
- Cosway R, Byrne M, Clafferty R, Hodges A, Grant E, Abukmeil SS, et al. Neuropsychological change in young people at high risk for schizophrenia: results from the first two neuropsychological assessments of the Edinburgh High Risk Study. Psychol Med. 2000;30(5):1111–1121. doi: 10.1017/s0033291799002585. [DOI] [PubMed] [Google Scholar]
- Francey SM, Jackson HJ, Phillips LJ, Wood SJ, Yung AR, McGorry PD. Sustained attention in young people at high risk of psychosis does not predict transition to psychosis. Schizophr Res. 2005;79(1):127–136. doi: 10.1016/j.schres.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Franklin GM, Heaton RK, Nelson LM, Filley CM, Seibert C. Correlation of neuropsychological and MRI findings in chronic/progressive multiple sclerosis. Neurology. 1988;38(12):1826–1829. doi: 10.1212/wnl.38.12.1826. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Torrey EF, Gold JM, Bigelow LB, Ragland RD, Taylor E, et al. Genetic risk of neuropsychological impairment in schizophrenia: A study of monozygotic twins discordant and concordant for the disorder. Schizophrenia Research. 1995;17:77–84. doi: 10.1016/0920-9964(95)00032-h. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test. Chicago, IL: Stoelting; 1978. [Google Scholar]
- Gschwandtner U, Aston J, Borgwardt S, Drewe M, Feinendegen C, Lacher D, et al. Neuropsychological and neurophysiological findings in individuals suspected to be at risk for schizophrenia: preliminary results from the Basel early detection of psychosis study - Fruherkennung von Psychosen (FEPSY) Acta Psychiatr Scand. 2003;108(2):152–155. doi: 10.1034/j.1600-0447.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- Hall RC, Parks J. The modified global assessment of functioning scale: addendum. Psychosomatics. 1995;36(4):416–417. doi: 10.1016/S0033-3182(95)71656-5. [DOI] [PubMed] [Google Scholar]
- Hambrecht M, Lammertink M, Klosterkotter J, Matuschek E, Pukrop R. Subjective and objective neuropsychological abnormalities in a psychosis prodrome clinic. Br J Psychiatry Suppl. 2002;43:s30–37. doi: 10.1192/bjp.181.43.s30. [DOI] [PubMed] [Google Scholar]
- Haroun N, Dunn L, Haroun A, Cadenhead KS. Risk and protection in prodromal schizophrenia: ethical implications for clinical practice and future research. Schizophr Bull. 2006;32(1):166–178. doi: 10.1093/schbul/sbj007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins KA, Addington J, Keefe RS, Christensen B, Perkins DO, Zipurksy R, et al. Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophr Res. 2004;67(23):115–122. doi: 10.1016/j.schres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sort Test. Manual. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- Heinrichs R, Zakanis K. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Jonckheere A. A distribution - free K- sample test against ordered alternatives. Biometricko. 1954;41:133–145. [Google Scholar]
- Keefe RS, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophrenia Research. 2006;88:2–35. doi: 10.1016/j.schres.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophrenia Research. 2006;88(13):26–35. doi: 10.1016/j.schres.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Kern RS, Green MF, Cornblatt BA, Owen JR, McQuade RD, Carson WH, et al. The neurocognitive effects of aripiprazole: an open-label comparison with olanzapine. Psychopharmacology. 2006;187(3):312–320. doi: 10.1007/s00213-006-0428-x. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006a;59(9):863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, et al. Generalized and Specific Neurocognitive Deficits in Prodromal Schizophrenia. Biological Psychiatry. 2006b;59(9):863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- McGlashan TH. Psychosis treatment prior to psychosis onset: ethical issues. Schizophr Res. 2001;51(1):47–54. doi: 10.1016/s0920-9964(01)00238-9. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Yung A, Phillips L. Ethics and early intervention in psychosis: keeping up the pace and staying in step. Schizophr Res. 2001;51(1):17–29. doi: 10.1016/s0920-9964(01)00235-3. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophrenia Bulletin. 1999;25(2):233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cannon TD, Ventura J, Cadenhead K, et al. Prodromal assessment using the SIPS and SOPS. Schizophrenia Research. 2004;70(1):74. [Google Scholar]
- Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry. 2005;62(3):254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles-Worsley M, Ord L, Blailes F, Ngiralmau H, Freedman R. P50 sensory gating in adolescents from a pacific island isolate with elevated risk for schizophrenia. Biological Psychiatry. 2004;55(7):663–667. doi: 10.1016/j.biopsych.2003.12.006. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Lenzenweger MF, Holzman PS. Antisaccades and smooth pursuit eye tracking and schizotypy. Archives of General Psychiatry. 1998;55(9):837–843. doi: 10.1001/archpsyc.55.9.837. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. The Structured Interview for DSM-IV Personality (SIDP-IV) Iowa City, Iowa: Department of Psychiatry, University of Iowa; 1995. [Google Scholar]
- Seeber K, Cadenhead KS. How does studying schizotypal personality disorder inform us about the prodrome of schizophrenia? Curr Psychiatry Rep. 2005;7(1):41–50. doi: 10.1007/s11920-005-0024-5. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry. 2004;161(3):398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Kalus OF, Keefe RS. The boundaries of schizophrenia. Psychiatric Clinics of North America. 1993;16(2):217–244. [PubMed] [Google Scholar]
- Tenn CC, Fletcher PJ, Kapur S. A Putative Animal Model of the ‘Prodromal’ State of Schizophrenia. Biological Psychiatry. 2005;57(6):586–593. doi: 10.1016/j.biopsych.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Cassady SL, Adami HM, Medoff DR, Sherr J. Saccadic eye movement abnormalities in relatives of patients with schizophrenia. Schizophr Res. 2000;45(3):235–244. doi: 10.1016/s0920-9964(99)00193-0. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Cassady SL, Adami HM, LaPorte D, Medoff DR, Lahti A. Smooth pursuit eye movements to extraretinal motion signals: Deficits in relatives of patients with schizophrenia. Archives of General Psychiatry. 1998;55:830–836. doi: 10.1001/archpsyc.55.9.830. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98(20):11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twisk J, de Vente W. Attrition in longitudinal studies. How to deal with missing data. J Clin Epidemiol. 2002;55(4):329–337. doi: 10.1016/s0895-4356(01)00476-0. [DOI] [PubMed] [Google Scholar]
- Voglmaier MM, Seidman LJ, Salisbury D, McCarley RW. Neuropsychological dysfunction in schizotypal personality disorder: A profile analysis. Biological Psychiatry. 1997;41:530–540. doi: 10.1016/s0006-3223(96)00056-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Third Edition. San Antonio, Texas: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. Third Edition. San Antonio, Texas: The Psychological Corporation; 1997b. [Google Scholar]
- Wood SJ, Pantelis C, Proffitt T, Phillips LJ, Stuart GW, Buchanan JA, et al. Spatial working memory ability is a marker of risk-for-psychosis. Psychol Med. 2003;33(7):1239–1247. doi: 10.1017/s0033291703008067. [DOI] [PubMed] [Google Scholar]


