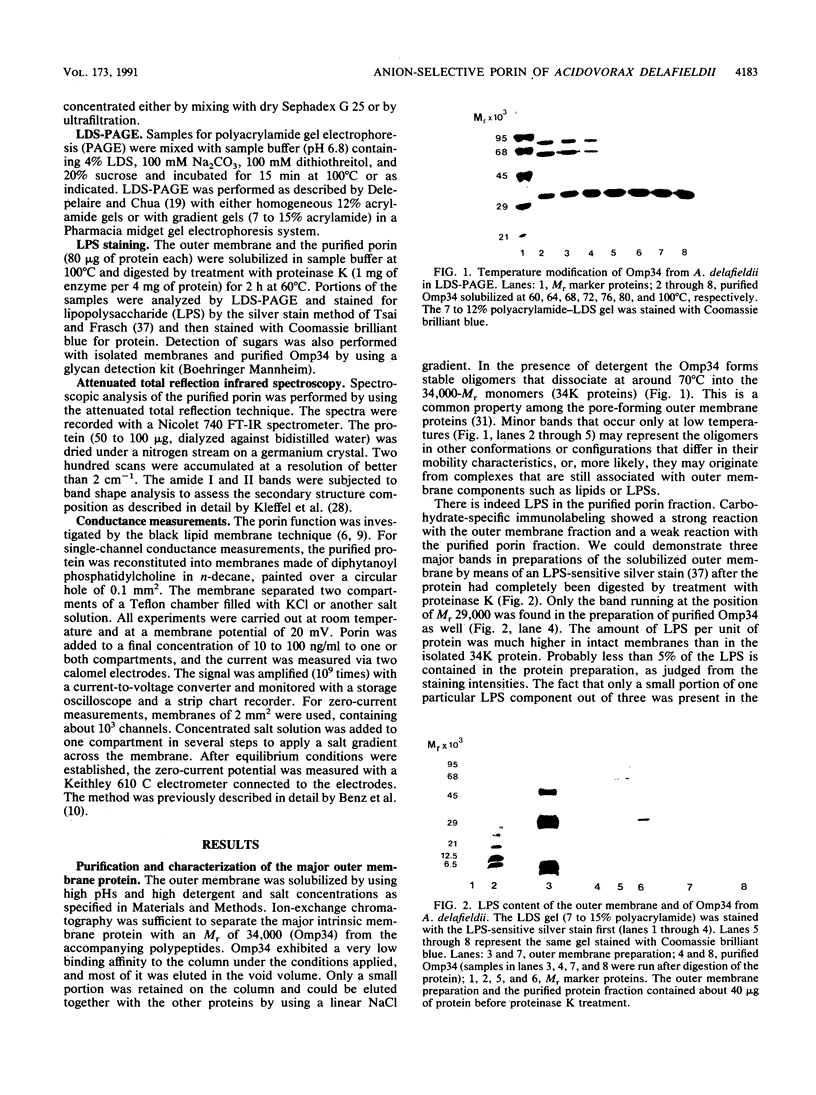
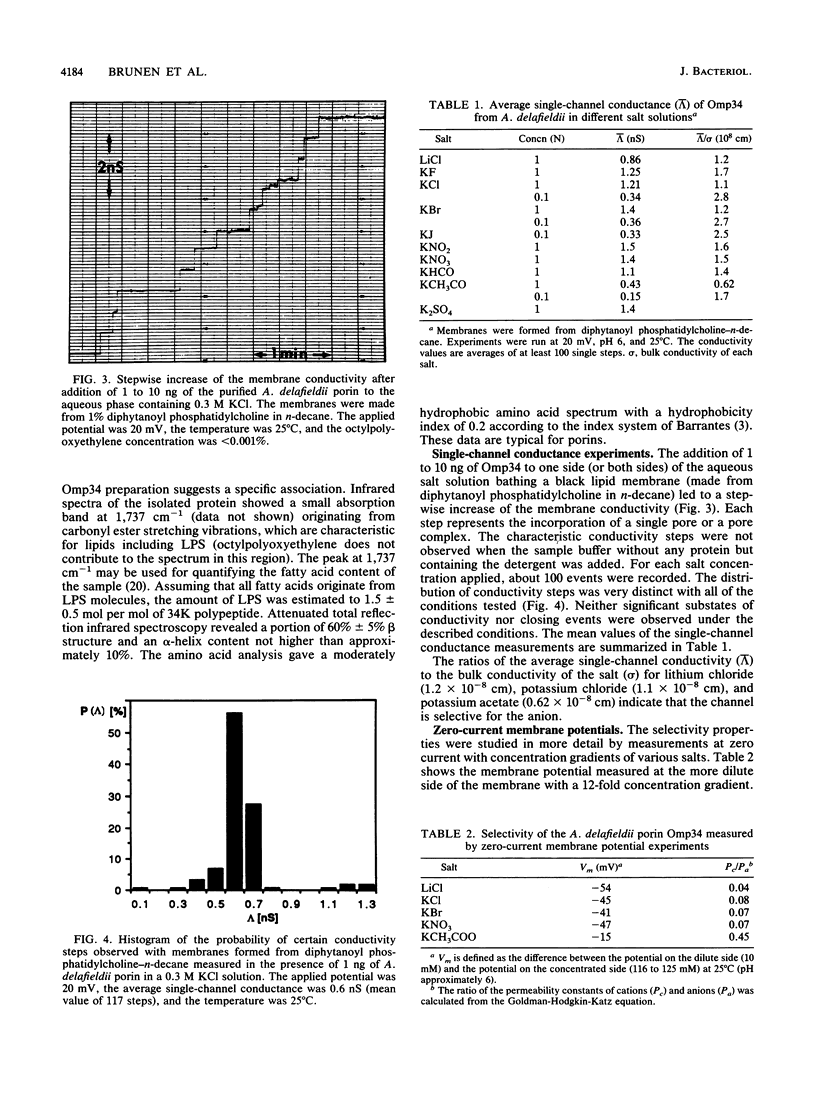
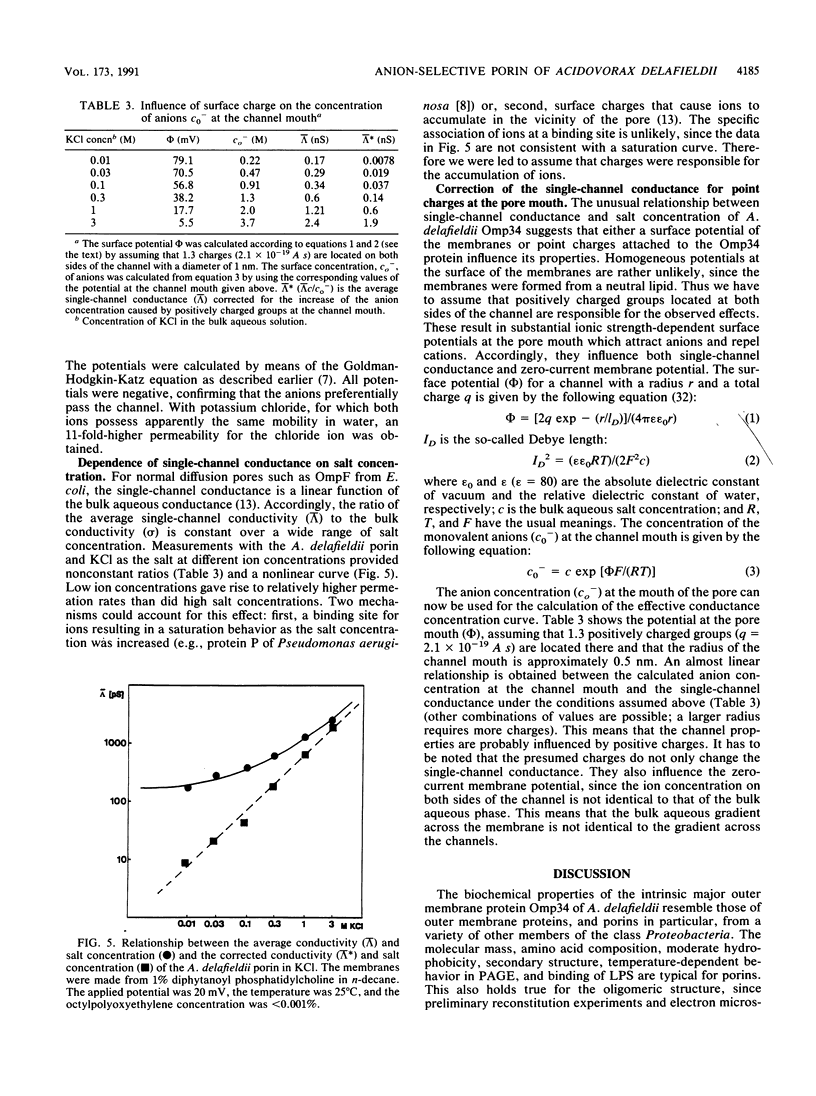
Abstract
The major outer membrane protein (Omp34) of Acidovorax delafieldii (formerly Pseudomonas delafieldii) was purified to homogeneity and was characterized biochemically and functionally. The polypeptide has an apparent molecular weight (Mr) of 34,000, and it forms stable oligomers at pH 9.0 in the presence of 10% octylpolyoxyethylene or 2% lithium dodecyl sulfate below 70 degrees C. The intact protein has a characteristic secondary structure composition, as revealed by Fourier transforming infrared spectroscopy (about 60% beta sheet). These features and the amino acid composition are typical for porins. The purified Omp34 is associated with 1 to 2 mol of lipopolysaccharide per mol of the monomer. Pore-forming activity was demonstrated with lipid bilayer experiments. Single-channel and selectivity measurements showed that the protein forms highly anion-selective channels. The unusual dependence of the single-channel conductance on salt concentration suggests that the porin complexes bear positive surface charges, accumulating negatively charged counterions at the pore mouth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apell H. J., Bamberg E., Läuger P. Effects of surface charge on the conductance of the gramicidin channel. Biochim Biophys Acta. 1979 Apr 19;552(3):369–378. doi: 10.1016/0005-2736(79)90181-0. [DOI] [PubMed] [Google Scholar]
- Armstrong S. K., Parr T. R., Jr, Parker C. D., Hancock R. E. Bordetella pertussis major outer membrane porin protein forms small, anion-selective channels in lipid bilayer membranes. J Bacteriol. 1986 Apr;166(1):212–216. doi: 10.1128/jb.166.1.212-216.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes F. J. The nicotinic cholinergic receptor : different compositions evidenced by statistical analysis. Biochem Biophys Res Commun. 1975 Jan 20;62(2):407–414. doi: 10.1016/s0006-291x(75)80153-7. [DOI] [PubMed] [Google Scholar]
- Bauer K., Struyvé M., Bosch D., Benz R., Tommassen J. One single lysine residue is responsible for the special interaction between polyphosphate and the outer membrane porin PhoE of Escherichia coli. J Biol Chem. 1989 Oct 5;264(28):16393–16398. [PubMed] [Google Scholar]
- Benz R., Bauer K. Permeation of hydrophilic molecules through the outer membrane of gram-negative bacteria. Review on bacterial porins. Eur J Biochem. 1988 Sep 1;176(1):1–19. doi: 10.1111/j.1432-1033.1988.tb14245.x. [DOI] [PubMed] [Google Scholar]
- Benz R., Darveau R. P., Hancock R. E. Outer-membrane protein PhoE from Escherichia coli forms anion-selective pores in lipid-bilayer membranes. Eur J Biochem. 1984 Apr 16;140(2):319–324. doi: 10.1111/j.1432-1033.1984.tb08104.x. [DOI] [PubMed] [Google Scholar]
- Benz R., Hancock R. E. Mechanism of ion transport through the anion-selective channel of the Pseudomonas aeruginosa outer membrane. J Gen Physiol. 1987 Feb;89(2):275–295. doi: 10.1085/jgp.89.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Janko K., Boos W., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;511(3):305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Läuger P. Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1979 Mar 8;551(2):238–247. doi: 10.1016/0005-2736(89)90002-3. [DOI] [PubMed] [Google Scholar]
- Benz R., Schmid A., Hancock R. E. Ion selectivity of gram-negative bacterial porins. J Bacteriol. 1985 May;162(2):722–727. doi: 10.1128/jb.162.2.722-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Schmid A., Vos-Scheperkeuter G. H. Mechanism of sugar transport through the sugar-specific LamB channel of Escherichia coli outer membrane. J Membr Biol. 1987;100(1):21–29. doi: 10.1007/BF02209137. [DOI] [PubMed] [Google Scholar]
- Benz R., Schmid A., Wagner W., Goebel W. Pore formation by the Escherichia coli hemolysin: evidence for an association-dissociation equilibrium of the pore-forming aggregates. Infect Immun. 1989 Mar;57(3):887–895. doi: 10.1128/iai.57.3.887-895.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalcroft J. P., Engelhardt H., Baumeister W. Structure of the porin from a bacterial stalk. FEBS Lett. 1987 Jan 19;211(1):53–58. doi: 10.1016/0014-5793(87)81273-5. [DOI] [PubMed] [Google Scholar]
- Dargent B., Rosenbusch J., Pattus F. Selectivity for maltose and maltodextrins of maltoporin, a pore-forming protein of E. coli outer membrane. FEBS Lett. 1987 Aug 10;220(1):136–142. doi: 10.1016/0014-5793(87)80891-8. [DOI] [PubMed] [Google Scholar]
- Delepelaire P., Chua N. H. Lithium dodecyl sulfate/polyacrylamide gel electrophoresis of thylakoid membranes at 4 degrees C: Characterizations of two additional chlorophyll a-protein complexes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):111–115. doi: 10.1073/pnas.76.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring K. B., Nikaido H. Existence and purification of porin heterotrimers of Escherichia coli K12 OmpC, OmpF, and PhoE proteins. J Biol Chem. 1989 Feb 15;264(5):2810–2815. [PubMed] [Google Scholar]
- Gerbl-Rieger S., Peters J., Kellermann J., Lottspeich F., Baumeister W. Nucleotide and derived amino acid sequences of the major porin of Comamonas acidovorans and comparison of porin primary structures. J Bacteriol. 1991 Apr;173(7):2196–2205. doi: 10.1128/jb.173.7.2196-2205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindahl M. S., Crockford G. W., Hancock R. E. Outer membrane protein NmpC of Escherichia coli: pore-forming properties in black lipid bilayers. J Bacteriol. 1984 Sep;159(3):1053–1055. doi: 10.1128/jb.159.3.1053-1055.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzenburg A., Engel A., Kessler R., Manz H. J., Lustig A., Aebi U. Rapid isolation of OmpF porin-LPS complexes suitable for structure-function studies. Biochemistry. 1989 May 16;28(10):4187–4193. doi: 10.1021/bi00436a010. [DOI] [PubMed] [Google Scholar]
- Jap B. K. Molecular design of PhoE porin and its functional consequences. J Mol Biol. 1989 Jan 20;205(2):407–419. doi: 10.1016/0022-2836(89)90351-3. [DOI] [PubMed] [Google Scholar]
- Kleffel B., Garavito R. M., Baumeister W., Rosenbusch J. P. Secondary structure of a channel-forming protein: porin from E. coli outer membranes. EMBO J. 1985 Jun;4(6):1589–1592. doi: 10.1002/j.1460-2075.1985.tb03821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Nelson A. P., McQuarrie D. A. The effect of discrete charges on the electrical properties of a membrane. I. J Theor Biol. 1975 Nov;55(1):13–27. doi: 10.1016/s0022-5193(75)80106-8. [DOI] [PubMed] [Google Scholar]
- Pressler U., Braun V., Wittmann-Liebold B., Benz R. Structural and functional properties of colicin B. J Biol Chem. 1986 Feb 25;261(6):2654–2659. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Willems A., Falsen E., Pot B., Jantzen E., Hoste B., Vandamme P., Gillis M., Kersters K., De Ley J. Acidovorax, a new genus for Pseudomonas facilis, Pseudomonas delafieldii, E. Falsen (EF) group 13, EF group 16, and several clinical isolates, with the species Acidovorax facilis comb. nov., Acidovorax delafieldii comb. nov., and Acidovorax temperans sp. nov. Int J Syst Bacteriol. 1990 Oct;40(4):384–398. doi: 10.1099/00207713-40-4-384. [DOI] [PubMed] [Google Scholar]
- Young J. D., Blake M., Mauro A., Cohn Z. A. Properties of the major outer membrane protein from Neisseria gonorrhoeae incorporated into model lipid membranes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3831–3835. doi: 10.1073/pnas.80.12.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]





