Abstract
C1q-deficient mice have been shown to develop a lupus-like disease and to display an impaired clearance of apoptotic cells that are enriched in lupus autoantigens. However, the role of C1q in the regulation of autoreactive B cells remains debatable. To explore this we crossed MRL/Mp C1q-deficient mice with knock-in transgenic (Tg) mice expressing an anti-ssDNA antibody (VH3H9R and VH3H9R/VLκ8R). Analysis of the VH3H9R mice showed that in the absence of C1q higher titres of Tg-derived IgM and IgG3 anti-ssDNA antibodies were detectable. In contrast, in the VH3H9R/VLκ8R C1q-deficient animals no increase in Tg antibody levels was observed. In both models the lack of C1q induced a marked reduction of marginal zone B cells and this was paralleled by a significant increase in the percentage of plasmocytes. Thus, one could postulate that in the absence of C1q the failure to clear efficiently dying cells provides an additional stimulus to the autoreactive Tg B cells resulting in their emigration from the marginal zone B cell compartment with subsequent increase in plasmocytes. However, the lack of C1q led to an increased production of Tg IgM and IgG3 antibodies only in VH3H9R mice indicating that additional genetic susceptibility factors are required to break self-tolerance.
Abbreviations: Ab, antibody; AEU, arbitrary ELISA units; FO, follicular; HEL, hen egg lysozyme; MZ, marginal zone; Tg, transgenic; SLE, systematic lupus erythematosus
Keywords: Autoimmunity, Complement, B cells, Rodent, Transgenic
1. Introduction
Hereditary deficiencies of early components of the classical pathway of the complement system are known to predispose to systematic lupus erythematosus (SLE). Among these, C1q deficiency exhibits the strongest association with prevalence greater than 90% suggesting that a physiological activity of the early part of the classical pathway normally protects against the development of SLE (Pickering et al., 2000). Mice with targeted deletion of the C1q gene (C1qa−/−) developed a spontaneous lupus-like disease characterised by the development of anti-nuclear autoimmunity and glomerulonephritis associated with the presence of multiple apoptotic bodies (Botto et al., 1998). Introgression of C1q deficiency onto different genetic backgrounds revealed that in mice C1q operates as a disease modifier. C57BL/6.C1qa−/− mice displayed no increase of IgG autoantibodies (autoAbs) or glomerulonephritis (Mitchell et al., 2002), whilst C1q deficiency backcrossed onto the lupus-prone MRL/Mp background accelerated both the onset and the severity of the autoimmune disease (Mitchell et al., 2002). Consistent with these observations, C1q reconstitution by bone marrow transplant attenuated the autoimmune disease present in MRL/Mp.C1qa−/− mice (Cortes-Hernandez et al., 2004).
Currently, there are two main hypotheses to explain the role of complement in the development of SLE, neither of which is mutually exclusive. The first model, defined as the tolerance hypothesis, proposes a role for complement in determining the activation thresholds of lymphocytes, whereby complement enhances presentation of autoantigens to self-reactive immature B cells resulting in their elimination (Prodeus et al., 1998). The second one, known as the ‘waste disposal’ hypothesis, suggests that in addition to its role in the clearance of immune complexes, complement is involved in the physiological disposal of apoptotic cells (Botto et al., 1998), that have been shown to express lupus autoantigens on their surface (Casciola-Rosen et al., 1994). C1q plays a significant role in the clearance pathway of cellular debris by binding directly or indirectly to apoptotic blebs where it activates complement and mediates phagocytosis by professional and non-professional phagocytes (Korb and Ahearn, 1997; Mevorach et al., 1998; Nauta et al., 2004; Quartier et al., 2005; Taylor et al., 2000). Therefore, improper removal of dying cells in the setting of C1q deficiency could result in the stimulation of autoreactive cells leading to autoimmunity.
Immunoglobulin Tg models have been instrumental in understanding B cell regulation revealing several key mechanisms, including receptor editing, deletion, anergy and ignorance. The hen egg lysozyme (HEL)–anti-HEL (IgHEL) Tg model in particular has been widely used to demonstrate elimination of self-reactive clones by membrane-bound expressed antigen, anergy induction by soluble antigen (sHEL) or ignorance when the amount of antigen is so low that it does not reach the threshold to induce anergy. More recently, it has been shown that intracellular membrane-bound HEL failed to induce tolerance and was instead autoimmunogenic positively selecting IgHEL B1 cells and inducing large numbers of IgM autoantibody-secreting plasma cells (reviewed in Ferry et al., 2006). Taken together, these findings suggest that in this model the fate of self-reactive B cells is determined not only by the abundance, the avidity of the target self-antigen and the affinity of the B cell receptor but also by the location of the “auto”-antigen. The role of complement has been tested in the IgHEL Tg (MD4)–sHEL (ML5) model by crossing the double Tg mice with mice deficient in C1q, C4, C3 or CD21/CD35 (Cutler et al., 2001; Prodeus et al., 1998). IgG anti-HEL Abs remained undetectable in all complement deficient mice, but C4 and CD21/CD35 deficient B cells displayed reduced surface IgM modulation, indicating a lower degree of anergy induction in these mice (Prodeus et al., 1998). However, this was not observed in the C1q deficient mice (Cutler et al., 2001). This discrepancy could either indicate that C4 operates independently from C1q or reflect differences in the genetic background of the mice used (Cutler et al., 2001). Nevertheless sHEL is not the ideal model to study the maintenance of tolerance in SLE as it is neither a natural autoantigen, nor are soluble plasma proteins typically targeted by the SLE autoAbs.
More recently, a model targeting an SLE antigen, DNA, was generated by Weigert and co-workers (Chen et al., 1995). In this model the rearranged variable heavy chain (VH) gene derived from a double stranded DNA-binding hybridoma developed in the autoimmune strain MRL/Mp.lpr/lpr, was inserted at the Igh locus and was referred to as VH3H9R. In contrast to conventional Tg mice, this knock-in model allows the Tg locus to undergo normal editing, isotype switching and somatic mutation. A variety of light chains can combine with the VH3H9 to yield anti-DNA Abs (Radic et al., 1991) but only few light chains are able to “silence” VH3H9R so that it no longer binds to DNA. By virtue of this characteristic the mice expressing only the VH3H9 chain (VH3H9R mice) can generate anti-DNA specificities. However, when the VH3H9R mice were crossed with a Tg knock-in light chain VLκ8 (Prak and Weigert, 1995) to generate monospecific Tg mice (VH3H9R/VLκ8R mice) (Chen et al., 1997a), this combination of heavy and light chain V regions (VH3H9/VLVκ8) bound only ssDNA and not dsDNA (Prak and Weigert, 1995). Previous studies with the VH3H9R mice have shown that the autoreactive Tg B cells accumulated in the splenic marginal zone and were regulated by anergy on non-autoimmune backgrounds such as BALB/c (Chen et al., 1995; Erikson et al., 1991) and C57BL/6 (Fukuyama et al., 2005; Sekiguchi et al., 2002). However, tolerance could be broken in this model if T cell help was provided in the form of a chronic graft versus host disease (Sekiguchi et al., 2002). Consistent with these observations, the double VH3H9R/VLκ8R knock-in Tg B cells were regulated by anergy in non-lupus prone mice (BALB/c), whilst in autoimmune prone MRL/Mp.lpr/lpr animals Tg B cells escaped tolerance induction and underwent class-switching and affinity maturation (Brard et al., 1999). These experiments suggested that the lpr mutation in the MRL background allowed the Tg autoreactive B cells to receive T cell help during a germinal center reaction.
To determine whether C1q is involved in selection of self-reactive B cells, we bred the C1q-deficient mice with the VH3H9R and the VH3H9R/VLκ8R mice and monitored the regulation and activation of anti-DNA Tg B cells over a period of 10 months. The mice in this study were on the autoimmune prone background MRL/Mp expressing the CD95 (Fas) gene. The analysis of these mice revealed that the lack of C1q can influence the levels of IgM and IgG3 Tg-derived antibodies only in the VH3H9R model.
2. Materials and methods
2.1. Mice
MRL/Mp mice were obtained from Harlan Olac, Bichester, UK. MRL/Mp.C1qa−/− deficient mice were generated as previously described (Mitchell et al., 2002). VH3H9R.MRL/Mp (Chen et al., 1995), VLκ8R.MRL/Mp (Prak and Weigert, 1995) and VH3H9R/VLκ8R.MRL/Mp mice were kindly provided by Prof. M. Weigert (Gwen Knapp Center for Lupus and Immunology Research, University of Chicago, Chicago, IL). MRL/Mp.C1qa−/− mice were crossed with the VH3H9R/Vκ8R.MRL/Mp mice and the resulting VH3H9R/VLκ8R.MRL/Mp.C1qa+/− were then crossed either with MRL/Mp or MRL/Mp.C1qa−/− in order to generate littermate animals. Mice were bled every 3 months starting from 2 months of age and at 10 months they were sacrificed. The mice were genotyped by PCR using specific primers. PCR primers were as follow: mC1qA/5′ (5′-GGGGCCTGTGATCCAGACAGG-3′), mC1qA/In2− (5′-TAACCATTGCCTCCAGGATGG-3′) and neo (5′-GGGGATCGGCAATAAAAAGAC-3′) for the C1q genotyping; MW114 (5′-CTGTCAGGAACTGCAGGTAAGG-3′) and MW162 (5′-CATAACATAGGAATATTTACTCCTCGC-3′) for the VH3H9R genotyping (Erikson et al., 1991); MW133 (5′-GGTACCTGTGGGGACATTGTG-3′) and MW157 (5′-AGCACCGAACGTGAGAGG-3′) for the VLκ8R genotyping (Carmack et al., 1991). Animals were kept under specific pathogen-free conditions. All animal care and procedures were conducted according to institutional guidelines and approved by the local ethical committee.
2.2. Flow cytometry
Flow cytometry was performed using a four-color staining of cells and analyzed with a FACSCalibur™ (Becton Dickinson, Mountain View, CA). The following Abs were used: anti-B220 (RA3-6B2), anti-CD5 (53-7.3), anti-CD11b (M1-70), anti-CD19 (1D3), anti-CD23 (B3B4), anti-CD21/CD35 (7G6), anti-CD90.2 (53-2.1), anti-CD138 (281-2), anti-IgM (II/41). All Abs were purchased from BD Biosciences Pharmingen (San Diego, CA) with the exception of the anti-VH3H9 idiotype (1.209), a kind gift from Prof. M. Weigert (Gay et al., 1993). Biotinylated Abs were detected using an allophycocyanin-conjugated streptavidin Ab (BD Biosciences Pharmingen). Staining was performed in the presence of saturating concentration of 24G2 mAb (anti-FcRII/III). Data were analyzed using WinMDI software (Version 2.8; Scripps Institute).
2.3. Serological analyses
Serum levels of IgM and IgG were quantified by ELISA, as described previously (Cortes-Hernandez et al., 2004). Anti-ssDNA Abs, anti-chromatin Abs, anti-histone Abs and anti-dsDNA Abs were measured by ELISA as described previously (Burlingame and Rubin, 1990; Emlen et al., 1990). Microtiter plates were coated with ssDNA prepared from calf thymus DNA (Sigma), chromatin (Lorne Laboratories Ltd., Reading, UK) or histone (Calbiochem, Merck Biosciences, Darmstadt, Germany). For detecting anti-dsDNA Abs, plates were coated with streptavidin (Sigma). ΦX174 double-stranded plasmid DNA (Promega, Southampton, UK) was biotinylated with Photoprobe biotin (Vector Laboratories, Peterborough, UK) and added to the streptavidin. Serum samples were diluted appropriately in PBS 2%BSA, 0.05%Tween-20, 0.02% NaN3. Bound Abs were detected with alkaline phosphatase conjugated goat anti-mouse IgG (γ-chain specific) (Sigma–Aldrich, Dorset, UK) and anti-mouse IgM (Southern Biotechnology Associates, Inc., Birmingham, AL). All autoAb results are expressed in arbitrary ELISA units (AEU) in reference to a standard curve derived from serum pools containing high titers of autoAbs. The idiotype+ Abs were captured with the anti-VH3H9 idiotype (1.209) (Gay et al., 1993) and detected with alkaline phosphatase conjugated goat anti-mouse IgM or IgG subclasses specific Abs (Southern Biotechnology Associates). The standard curve was derived from an IgM VH3H9R/Vκ8R mAb provided by Prof. Weigert or sera containing high IgG subclasses titers of the Tg.
2.4. Renal assessment
Proteinuria was assessed using Haema-combistix (Bayer Diagnostics, Newbury, UK). Kidneys were fixed in Bouin's solution and paraffin embedded, and sections were stained with periodic acid–Schiff reagent. Glomerular histology was graded in a blind fashion scored on a scale of 0–4, as described before (Carlucci et al., 2007).
2.5. Statistics
The data are presented as median, with range of values in brackets, unless otherwise stated. The non-parametric Mann–Whitney U-test was applied throughout with differences being considered significant for p-values < 0.05. Statistics were calculated using GraphPad Prism Version 3.0 (GraphPad Software, San Diego, CA).
3. Results
3.1. Expression of Tg autoantibodies
C1q deficiency has been shown to accelerate the onset and progression of SLE in MRL/Mp mice (Mitchell et al., 2002), but the mechanisms underlying these effects are still uncertain. The VH3H9R/VLκ8R Tg alleles were transferred onto C1q-deficient mice by crossing VH3H9R/VLκ8R.MRL/Mp with MRL/Mp.C1qa−/− and experimental cohorts of littermate mice were generated. Whilst in previous studies the expression of the Tg alleles was monitored by measuring the Tg-specific immunoglobulin allotype (Steeves and Marion, 2004), this analysis could not be applied in these mice as the Tg alleles and the endogenous immunoglobulin allotype were indistinguishable. Nevertheless, we were able to detect the combination of the VH3H9 with the VLVκ8 using an idiotype specific monoclonal Ab named 1.209 (Gay et al., 1993). As the 1.209 mAb can also recognize the VH3H9 paired with other endogenous light chains such as VLκ4 (Gay et al., 1993), this anti-idiotype Ab allowed us not only to assess the VH3H9R/VLκ8R Tg B cells but also some of the VH3H9R Tg B cells. C1q deficiency did not affect the proportion of peripheral blood B cells expressing the Tg in neither of the two models (VH3H9R/VLκ8R.MRL/Mp.C1qa−/−: 89.1 ± 1.1% versus VH3H9R/VLκ8R.MRL/Mp: 90.7 ± 1.5%, p = 0.4118; VH3H9R.MRL/Mp.C1qa−/−: 25.3 ± 1.6% versus VH3H9R.MRL/Mp: 22.3 ± 2.3% p = 0.1669). The low proportion of idiotype+ B cells in the VH3H9R.MRL/Mp mice indicated that most of the B cells in these mice had either paired the VH3H9R Tg allele with a light chain that was not recognised by the anti-idiotype Ab, or edited the VH3H9R allele, or used the endogenous immunoglobulin allele. In the VH3H9R/VLκ8R.MRL/Mp.C1qa−/− mice the B cell receptor was down-modulated to the same extent as in the VH3H9R/VLκ8R.MRL/Mp mice (data not shown).
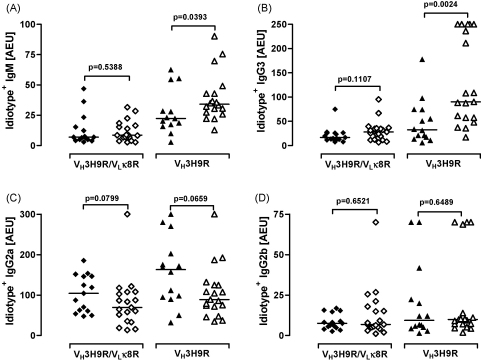
Serological analyses were performed in order to establish whether the Tg autoAbs were expressed. The different cohorts of mice were bled at different time points, and the data presented here are those obtained at 10 month of age when the mice were sacrificed. Substantial levels of idiotype+ IgM, IgG3, IgG2a and IgG2b Abs were detected in the VH3H9R/VLκ8R.MRL/Mp and in VH3H9R.MRL/Mp mice indicating that the anti-DNA knock-in Tg alleles on this background were not regulated by anergy unlike when on the C57BL/6 background (Fig. 1). In the VH3H9R/VLκ8R.MRL/Mp.C1qa−/− mice the levels of idiotype+ Abs were similar to those detected in the strain-matched C1q-sufficient animals. In contrast, the VH3H9R.MRL/Mp.C1qa−/− mice had significantly increased levels of idiotype+ IgM and IgG3 Abs compared to the VH3H9R.MRL/Mp mice (Fig. 1A and B), but similar levels of idiotype+ IgG2a and IgG2b Abs (Fig. 1C and D).
Fig. 1.
Expression of idiotype+ (A) IgM, (B) IgG3, (C) IgG2a and (D) IgG2b immunoglobulins in circulation. The combination of VH3H9R/VLκ8R was assessed using a specific anti-idiotype mAb (1.209). Ten month-old VH3H9R/VLκ8R.MRL/Mp (closed diamonds) and VH3H9R.MRL/Mp (closed triangles) mice displayed detectable levels of idiotype+ IgM, IgG3, IgG2a and IgG2b Abs. IgM and IgG3 levels were markedly increased in VH3H9R.MRL/Mp.C1qa−/− mice. C1q-deficient mice are represented with open symbols. Each symbol represents one mouse, horizontal bars indicate median.
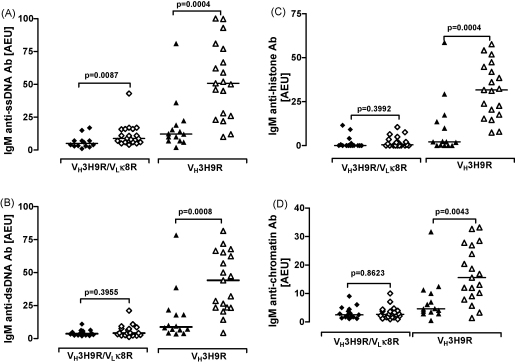
We then measured the levels of IgM and IgG autoAbs. A significant increase in the titre of IgM anti-ssDNA Abs was observed in the VH3H9R/VLκ8R.MRL/Mp.C1qa−/− mice compared to the VH3H9R/VLκ8R.MRL/Mp mice (Fig. 2A). Of note, the VH3H9R/VLκ8R.MRL/Mp.C1qa−/− mice displayed only markedly increased levels of IgM autoAbs directed against ssDNA (the specificity encoded by the Tg alleles) but not against other lupus autoantigens such as dsDNA, histone or chromatin (Fig. 2B–D). In the VH3H9R.MRL/Mp mice the pairing of the VH3H9 with endogenous L chains could yield to more specificities. Consistent with this, VH3H9R.MRL/Mp.C1q−/− mice had significantly increased levels of IgM anti-ssDNA, anti-dsDNA, anti-histone and anti-chromatin Abs (Fig. 2A–D). We then analysed the levels of IgG autoAbs and found that these were similar between the C1q-sufficient and -deficient cohorts (Supporting information Table S1).
Fig. 2.
AutoAb profiles. Serology at 10 months of age in VH3H9R/VLκ8R.MRL/Mp (diamonds) and VH3H9R.MRL/Mp (triangles) mice. C1q-deficient mice are represented with open symbols. Each symbol represents one mouse, horizontal bars indicate median. (A) IgM anti-ssDNA Abs; (B) IgM anti-dsDNA Abs; (C) IgM anti-histone Abs; (D) IgM anti-chromatin Abs measured by ELISA as described in Section 2.
3.2. Renal assessment
At 10 month of age all the animals were sacrificed. VH3H9R/VLκ8R.MRL/Mp and VH3H9R.MRL/Mp animals showed histological evidence of a mild glomerulonephritis (median score 1.0, range: 0.0–3.0 and 1.5, range: 1.0–3.0, respectively) reminiscent of the disease observed in MRL/Mp non-Tg mice (Mitchell et al., 2002). C1q-deficiency in MRL/Mp mice has previously been shown to worsen the kidney pathology (Mitchell et al., 2002). However, in the Tg mice the absence of C1q did not exacerbate the renal disease compared to the strain-matched C1q-sufficient mice (VH3H9R/VLκ8R.MRL/Mp.C1qa−/− mice: median score 1.0, range: 0.0–3.0, p = 0.4287; VH3H9R.MRL/Mp.C1qa−/− mice: median range 2.0, range: 1.0–2.0, p = 0.4564).
3.3. Flow cytometric analysis of splenic and peritoneal B cells
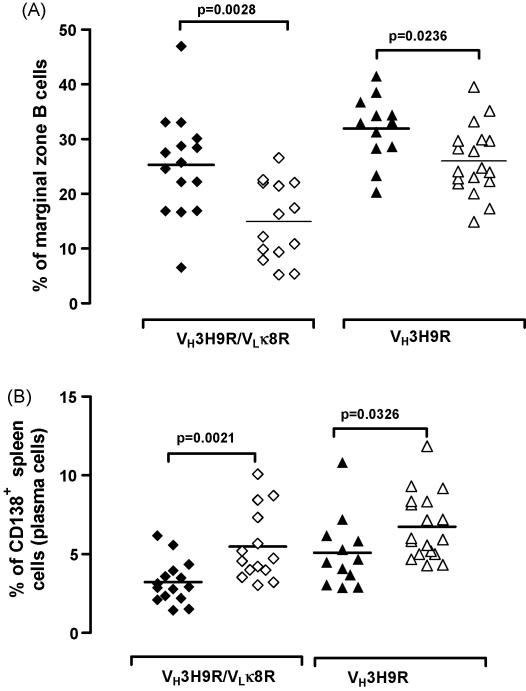
In order to determine if the serological data were accompanied by phenotypic changes in T and B lymphocytes, we performed a comprehensive analysis of the various splenic and peritoneal subpopulations. Cells of at least 7 mice from each cohort were analysed by FACS at the time of the sacrifice using the combinations of markers shown in Table 1. In agreement with the findings in other genetic backgrounds (Li et al., 2002), the VH3H9R.MRL/Mp mice had a larger marginal zone (MZ) B cell population compared to VH3H9R/VLκ8R.MRL/Mp (Fig. 3A) and an increased proportion of idiotype+ B cells in the MZ compartment compared to follicular (FO) compartment (5.9 ± 1.0 versus 4.2 ± 0.6, paired t-test p = 0.0157) (Table 1). More interestingly, in the VH3H9R.MRL/Mp.C1qa−/− mice, which produced higher levels of IgM autoAbs, the MZ B cell population was significantly decreased compared to the VH3H9R.MRL/Mp animals (Fig. 3A) and less idiotype+ B cells were found in the MZ compared to the FO compartment (3.4 ± 0.7 versus 4.8 ± 1.1, paired t-test p = 0.0042) (Table 1). In the VH3H9R/VLκ8R.MRL/Mp mice there was no preferential localization of idiotype+ B cells in the MZ zone (paired t-test p = 0.056) (Table 1). C1q deficiency was associated with a significant reduction in the percentage of MZ B cells (Fig. 3A) and a decrease of idiotype+ B cells in the MZ compared to the FO area (4.2 ± 0.9 versus 17.5 ± 3.2, paired t-test p = 0.0013). These findings indicated that MZ B cells might have been activated in the absence of C1q. Activated MZ B cells have been shown to migrate into the T cell zone and differentiate into plasma cells. Indeed, the percentage of plasma cells was increased in the two C1q-deficient cohorts compared to the respective MRL/Mp controls (Fig. 3B).
Table 1.
Splenic and peritoneum cell populations at 10 months of age
| Cell populations | Staining | VH3H9R/VLκ8R MRL/Mp | VH3H9R/VLκ8R MRL/Mp.C1qa−/− | VH3H9R MRL/Mp | VH3H9R MRL/Mp.C1qa−/− |
|---|---|---|---|---|---|
| n Splenic cells (107) | 11.0 ± 1.3 | 14.9 ± 1.9 | 12.2 ± 1.7 | 14.1 ± 1.8 | |
| n B cells (106) | B220+CD5− | 32.3 ± 3.8 | 29.5 ± 3.4 | 47.5 ± 7.0 | 36.4 ± 5.6 |
| n T cells (106) | CD5+B220− | 52.7 ± 7.1 | 68.3 ± 13.0 | 74.1 ± 11.9 | 80.5 ± 10.7 |
| n B-1 cells (106) | B220+CD5+ | 4.3 ± 0.7 | 4.5 ± 0.7 | 3.6 ± 0.5 | 3.1 ± 0.4 |
| n T1 B cells (106) | CD21/35loCD23lo, CD19 gated | 4.8 ± 0.9 | 3.4 ± 0.4 | 10.5 ± 3.3 | 5.2 ± 0.8 |
| n MZ B cells (106) | CD21/35hiCD23lo, CD19 gated | 8.7 ± 1.8 | 4.1 ± 0.7# | 14.8 ± 2.2 | 9.5 ± 1.5* |
| n FO B cells (106) | CD21/35+CD23hi, CD19 gated | 13.5 ± 1.4 | 17.3 ± 2.2 | 14.3 ± 2.9 | 17.4 ± 3.2 |
| n Plasmocytes (106) | CD138+, CD90.2− CD19 gated | 0.7 ± 0.1 | 1.1 ± 0.1# | 1.3 ± 0.2 | 1.5 ± 0.2 |
| n Idiotype+ MZ B cells (106) | 1.209+, MZ gated | 8.8 ± 2.0 | 4.2 ± 0.9# | 5.9 ± 1.0 | 3.4 ± 0.7* |
| n Idiotype+ FO B cells(106) | 1.209+, FO gated | 12.8 ± 1.6 | 17.5 ± 3.2 | 4.2 ± 0.6 | 4.8 ± 1.1 |
| n Peritoneal cells (106) | 3.1 ± 0.5 | 4.1 ± 0.5 | 3.5 ± 0.5 | 5.8 ± .06* | |
| n B-1a B cells (105) | CD5+CD11blo, CD19+ gated | 1.8 ± 0.3 | 3.8 ± 1.0 | 3.1 ± 1.0 | 7.8 ± 1.9* |
| n B-1b B cells (105) | CD5−CD11b+, CD19+ gated | 10.0 ± 1.6 | 10.6 ± 1.6 | 11.8 ± 1.8 | 18.2 ± 1.4* |
| n B-2 B cells (105) | CD5−CD11b−, CD19+ gated | 8.8 ± 1.5 | 10.0 ± 1.7 | 7.9 ± 1.6 | 13.9 ± 2.0* |
| % Idiotype+ B-1a B cells | 1.209+, B-1a gated | 38.8 ± 4.4 | 26.4 ± 6.9 | 16.2 ± 2.9 | 12.7 ± 2.2 |
| % Idiotype+ B-1b B cells | 1.209+, B-1b gated | 32.1 ± 3.4 | 23.8 ± 4.0 | 20.4 ± 2.3 | 19.3 ± 3.4 |
| % Idiotype+ B-2 B cells | 1.209+, B-2 gated | 53.5 ± 5.4 | 45.3 ± 7.0 | 15.6 ± 1.7 | 12.8 ± 1.6 |
Results are shown as mean ± S.E.M.; MZ: marginal zone; FO: follicular zone.
p-Value < 0.05 VH3H9R.MRL/Mp vs. VH3H9R.MRL/Mp.C1qa−/−.
p-Value < 0.05 VH3H9R/VLκ8R.MRL/Mp vs. VH3H9R/VLκ8R.MRL/Mp.C1qa−/−, n = number.
Fig. 3.
Flow cytometric analysis of splenic and peritoneal cells. In C1q-deficient mice there was a reduction of (A) marginal zone B cells (percentage of CD21highCD23low, CD19+ gated splenic B cells) and this was associated with an increase of (B) plasma cells (percentage of CD138+, B220−CD90− gated splenic cells). Each symbol represents one mouse; VH3H9R/VLκ8R.MRL/Mp mice (diamonds) and VH3H9R.MRL/Mp mice (triangles); C1q-deficient mice are represented with open symbols.
The peritoneal cavity in mice has been shown to be a site where some self-reactive B cells can escape tolerance (Fagarasan et al., 2000) and B-1 cells, one of the major sources of circulating IgM, accumulate. More cells were recovered from the peritoneum of VH3H9R.MRL/Mp.C1qa-/-mice compared to VH3H9R.MRL.Mp mice (p = 0.0051) but not in the VH3H9R/VLκ8R.MRL/Mp.C1qa−/− versus VH3H9R/VLκ8R.MRL/Mp animals (p = 0.1923) (Table 1). When the peritoneal B cell subpopulations were analyzed in more detail, all subsets were increased in the VH3H9R.MRL/Mp.C1qa-/mice compared to the VH3H9R.MRL.Mp mice (Table 1). On the contrary, in the VH3H9R/VLκ8R C1q-deficient animals only B-1a cells were slightly increased compared to their wild type strain-matched controls (Table 1), but this did not reach statistical significance (p = 0.0518). We then analysed the VH3H9/VLκ8 idiotype expression on the different peritoneal B cells. Surprisingly in the peritoneum of the VH3H9R/VLκ8R.MRL.Mp and VH3H9R.MRL.Mp mice the majority of the B cells were not expressing the VH3H9/VLκ8 idiotype (Table 1). Of note the C1q-deficient mice had similar percentage of idiotype+ B cells in the different peritoneal B cell subpopulations compared to C1q-sufficient mice. The reduced proportion of idiotype+ B cells in the peritoneum compared to the peripheral blood and the spleen (data not shown) suggests that in this organ the B cells either preferentially used the endogenous alleles or had been activated and undergone editing.
4. Discussion
C1q deficiency in humans and in mice has been associated with the development of a lupus-like illness. However, the role of C1q in the regulation of autoreactive B cells remains debatable. Here, we explored the regulation of autoreactive B cells by C1q using anti-DNA knock-in Tg models (VH3H9R/VLκ8R and VH3H9R) on the lupus prone MRL/Mp genetic background. The analysis of these mice revealed that the MRL/Mp background was in itself sufficient to allow the expression of anti-DNA Tg autoAbs and that the lack of C1q modified this effect only in the single Tg model (VH3H9R). However, in the absence of C1q the MZ B cell compartment was significantly reduced in both models and this was accompanied by an increase in plasmocytes.
Recently the VH3H9R/VLκ8R anti-DNA knock-in Tg model has been widely used for investigating the mechanisms of regulation of autoAb production in murine models of SLE. There is an increasing evidence that the VH3H9R/VLκ8R and VH3H9R anti-DNA Tg B cells, which have a relatively weak affinity to ssDNA, are regulated by anergy on normal genetic backgrounds such as BALB/c and C57BL/6, but could be induced to lose tolerance when transferred onto lupus models such as (NZB × NZW)F1 or MRL/Mp.lpr/lpr (Brard et al., 1999; Chen et al., 1995; Erikson et al., 1991; Fukuyama et al., 2005; Sekiguchi et al., 2002). In light of these observations it was important for our study to establish whether the autoimmune prone MRL/Mp background was in itself capable of breaking tolerance. MRL/Mp mice are known to develop a mild autoimmune disease which can be accelerated with different disease-modifying genes such as Yaa (Merino et al., 1989), lpr and gld (Cohen and Eisenberg, 1991). The analysis of the idiotype+ (VH3H9R/VLκ8R) Abs revealed that the Tg MRL/Mp mice indeed had in circulation these idiotype+ Abs (IgM, IgG2a, IgG2b, IgG3) indicating that a break of tolerance had spontaneously occurred in these mice. One explanation for this is an intrinsic defect in MRL/Mp B cells and there is some evidence in support of this. MRL/Mp mice have been reported to exhibit a defect in maintaining the developmental arrest of VH3H9/VLλ anti-dsDNA conventional Tg B cells (Mandik-Nayak et al., 1999, 2000) and to have a spontaneous B cell hyperactivity in the absence of Ag in the IgHEL experimental model (Nijnik et al., 2006). However, the VH3H9/VLλ anti-dsDNA autoreactive B cells, despite not being any longer developmentally arrested as in a BALB/c mice, exhibited follicular exclusion and failed to differentiate into plasma cells (Mandik-Nayak et al., 1999). Furthermore Tg MRL/Mp mice expressing IgHEL have been shown to be able to down-regulate their B cell receptor and to be unable to secrete detectable levels of anti-HEL Abs in the presence of sufficient amount of sHEL (Nijnik et al., 2006). Similarly anti-laminin Tg MRL/Mp mice were found to be tolerant (Rudolph et al., 2002). Another potential explanation for the break of the B cell tolerance in the VH3H9R/VLκ8R anti-DNA Tg mice, is that in this model the MRL/Mp background was able to provide sufficient T cell help and the presence of idiotype+ IgG subclasses favour this hypothesis.
We next examined whether C1q could modulate the phenotype of the anti-ssDNA Tg B cells. In the VH3H9R/VLκ8R.MRL/Mp mice the absence of C1q increased significantly the circulating levels of IgM against ssDNA but not against other autoantigens including dsDNA. As the pairing of VH3H9 with VLκ8 prevents the binding of VH3H9 to dsDNA, the elevated amount of IgM anti-ssDNA observed could have been the result of an increased Tg expression. However, the idiotype analysis failed to demonstrate a difference in the levels of IgM idiotype+ Abs between VH3H9R/VLκ8R.MRL/Mp.C1qa−/− and VH3H9R/VLκ8R.MRL/Mp mice, questioning whether the source of the increased levels of IgM anti-ssDNA was indeed the Tg B cells. On the other hand, the VH3H9R.MRL/Mp.C1qa−/− mice displayed significantly higher levels of IgM and IgG3 idiotype+ Abs. As the VH3H9R heavy chain can pair with different endogenous light chains generating a wider range of autoAbs, other specificities were tested. Indeed the absence of C1q increased significantly IgM levels against all the lupus autoantigens analysed.
To gain insight into the mechanisms regulating the autoAb production in the C1q-deficient mice we then carried a detailed analysis of the different B cell populations. Several studies have proposed a possible role for MZ B cells in the development of lupus in mouse models. Although studies in immunoglobulin Tg mice have shown that autoreactive B cells can accumulate in the marginal zone under various experimental situations (Li et al., 2002; Mandik-Nayak et al., 1997, 1999, 2006; Qian et al., 2004; Wen et al., 2005), it remains to be established whether MZ B cells secrete pathogenic autoAbs in any model of lupus. B cells producing potentially pathogenic autoAbs are thought to home to the MZ (Chen et al., 1997b; Li et al., 2002) and sequestration to this site is believed to prevent them from entering into the germinal centres and developing the properties of pathogenic B cells. However, recent studies in lupus-prone mice have reported both enlargements (Grimaldi et al., 2001; Mackay et al., 1999; Wither et al., 2000) and impaired development of the MZ B cell compartment (Amano et al., 2003; Samardzic et al., 2002). Consistent with previous observations in the VH3H9R/VLκ8R model (Wen et al., 2005), the MZ was found to be enlarged in the VH3H9R.MRL/Mp mice but no accumulation of MZ B cells was observed in VH3H9R/VLκ8R.MRL/Mp mice. Importantly C1q deficiency decreased dramatically the proportion of MZ B cells in these mice and this was accompanied by an increase in the percentage of plasma cells. More interestingly, in the C1q-deficient Tg mice we observed a significant disappearance of idiotype+ B cells from the MZ suggesting that these cells had been activated. Marginal zone B cells bearing low affinity self-reactive BCR can react to repetitive Ag and produce natural autoAbs of the IgM isotype upon contact with blood-borne pathogens or self Ag (Oliver et al., 1999). Moreover, MZ B cells can undergo T cell independent switching to IgG, IgA and IgE in response to pathogen associated molecular patterns (PAMP). As C1q has been shown to be involved in the clearance of apoptotic cells, which are enriched in the typical lupus autoantigens, one could postulate that the increased IgM Tg secretion and the reduction of the MZ B cells in the VH3H9R.MRL/Mp.C1qa−/− mice might be related to a failure to clear antigens associated with dying cells. In the absence of C1q the ineffectively cleared autoAgs could stimulate the autoreactive MZ Tg B cells to differentiate into plasmocyte resulting into the decrease of MZ B cells and increase of circulating IgM autoAbs. Similarly, in the spleens of VH3H9R/VLκ8R.MRL/Mp.C1qa−/− mice significantly less idiotype+ B cells were present in the MZ compartment and more plasmocytes were found. However, these cellular changes were not paralleled by an increase of idiotype+ IgM Abs in circulation indicating in this model they were not sufficient to induce autoantibody production.
Another potential source of IgM autoAbs are the B1 cells. B1 cells have long been associated with the secretion of natural Abs against self and foreign pathogens, which can occur without obvious inflammatory response. The importance of B1 cells in the pathogenesis of lupus continues to be debated. A major argument for a role of B1a cells in mouse lupus relates to the expansion of this subset in NZB and (NZB × NZW)F1 mice. Reduction of B1 cells for instance via intraperitoneal injection of H2O delayed disease onset and reduced disease severity in (NZB × NZW)F1 mice (Murakami et al., 1995). However, the expansion of B1a cells was shown not to be critical for the production of IgM or IgG autoAbs in this murine model of SLE (Atencio et al., 2004). The Abs secreted by B1 cells tend to be polyreactive with low-affinity cross-reactivity to a variety of self Ags and these characteristics are very different from the pathogenic IgG Abs produced by lupus mice. In our experimental model, we found an increase in the total number of peritoneal and B1 cells in the VH3H9R.MRL/Mp.C1qa−/− mice compared to their wild type counterparts (Table 1). A similar trend was observed for the B1a cells in the VH3H9R/VLκ8R.MRL/Mp.C1qa−/− mice but this did not reach statistical significance. However, in both models the percentages of idiotype+ B1 cells between C1q-deficient and -sufficient mice were similar. These findings would indicate the lack of C1q may favour the expansion of peritoneal B1 cells and that these cells might have contributed to the increase of serum IgM autoAb levels observed in the VH3H9R.MRL/Mp C1q-deficient mice. Supporting this hypothesis, a recent study showed that C1q deficiency increases the positive selection of B-1 cells and IgM autoAb production by a membrane-bound intracellular auto-Ag (Ferry et al., 2007).
In conclusion, using mice expressing site-directed transgenes for anti-DNA autoAbs we have shown that: (i) the MRL/Mp background was in itself capable of inducing the expression of anti-DNA Tg autoAbs, and (ii) VH3H9R.MRL/Mp.C1qa−/− could influence the production of Tg-derived IgM and IgG3 autoAbs possibly as a result of an impaired disposal of cellular debris. However, we found no evidence of a direct role of C1q in the regulation of self-reactive conventional B cells. Further studies on non-lupus prone genetic backgrounds such as C57BL/6 will be necessary to determine if C1q deficiency can play a more substantial role in shaping the repertoire of the autoreactive B cells.
Acknowledgements
This work was supported by the Arthritis Research Campaign (UK) (grant number 15444) and the Wellcome Trust (grant 071467).
We thank all the staff in the animal facility for their technical assistance, Ms. Margarita Lewis for the processing of the histological specimens and Prof. Richard J. Cornall for his critical and constructive reading of the manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.molimm.2007.06.162.
Appendix A. Supplementary data
References
- Amano H., Amano E., Moll T., Marinkovic D., Ibnou-Zekri N., Martinez-Soria E., Semac I., Wirth T., Nitschke L., Izui S. The Yaa mutation promoting murine lupus causes defective development of marginal zone B cells. J. Immunol. 2003;170:2293–2301. doi: 10.4049/jimmunol.170.5.2293. [DOI] [PubMed] [Google Scholar]
- Atencio S., Amano H., Izui S., Kotzin B.L. Separation of the New Zealand Black genetic contribution to lupus from New Zealand Black determined expansions of marginal zone B and B1a cells. J. Immunol. 2004;172:4159–4166. doi: 10.4049/jimmunol.172.7.4159. [DOI] [PubMed] [Google Scholar]
- Botto M., Dell’Agnola C., Bygrave A.E., Thompson E.M., Cook H.T., Petry F., Loos M., Pandolfi P.P., Walport M.J. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- Brard F., Shannon M., Prak E.L., Litwin S., Weigert M. Somatic mutation and light chain rearrangement generate autoimmunity in anti-single-stranded DNA transgenic MRL/lpr mice. J. Exp. Med. 1999;190:691–704. doi: 10.1084/jem.190.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingame R.W., Rubin R.L. Subnucleosome structures as substrates in enzyme-linked immunosorbent assays. J. Immunol. Methods. 1990;134:187–199. doi: 10.1016/0022-1759(90)90380-e. [DOI] [PubMed] [Google Scholar]
- Carlucci F., Cortes-Hernandez J., Fossati-Jimack L., Bygrave A.E., Walport M.J., Vyse T.J., Cook H.T., Botto M. Genetic dissection of spontaneous autoimmunity driven by 129-derived Chromosome 1 loci when expressed on C57BL/6 mice. J. Immunol. 2007;178:2352–2360. doi: 10.4049/jimmunol.178.4.2352. [DOI] [PubMed] [Google Scholar]
- Carmack C.E., Camper S.A., Mackle J.J., Gerhard W.U., Weigert M.G. Influence of a V kappa 8 L chain transgene on endogenous rearrangements and the immune response to the HA(Sb) determinant on influenza virus. J. Immunol. 1991;147:2024–2033. [PubMed] [Google Scholar]
- Casciola-Rosen L.A., Anhalt G., Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Nagy Z., Prak E.L., Weigert M. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity. 1995;3:747–755. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- Chen C., Prak E.L., Weigert M. Editing disease-associated autoantibodies. Immunity. 1997;6:97–105. doi: 10.1016/s1074-7613(00)80673-1. [DOI] [PubMed] [Google Scholar]
- Chen X., Martin F., Forbush K.A., Perlmutter R.M., Kearney J.F. Evidence for selection of a population of multi-reactive B cells into the splenic marginal zone. Int. Immunol. 1997;9:27–41. doi: 10.1093/intimm/9.1.27. [DOI] [PubMed] [Google Scholar]
- Cohen P.L., Eisenberg R.A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Cortes-Hernandez J., Fossati-Jimack L., Petry F., Loos M., Izui S., Walport M.J., Cook H.T., Botto M. Restoration of C1q levels by bone marrow transplantation attenuates autoimmune disease associated with C1q deficiency in mice. Eur. J. Immunol. 2004;34:3713–3722. doi: 10.1002/eji.200425616. [DOI] [PubMed] [Google Scholar]
- Cutler A.J., Cornall R.J., Ferry H., Manderson A.P., Botto M., Walport M.J. Intact B cell tolerance in the absence of the first component of the classical complement pathway. Eur. J. Immunol. 2001;31:2087–2093. doi: 10.1002/1521-4141(200107)31:7<2087::aid-immu2087>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Emlen W., Jarusiripipat P., Burdick G. A new ELISA for the detection of double-stranded DNA antibodies. J. Immunol. Methods. 1990;132:91–101. doi: 10.1016/0022-1759(90)90402-h. [DOI] [PubMed] [Google Scholar]
- Erikson J., Radic M.Z., Camper S.A., Hardy R.R., Carmack C., Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- Fagarasan S., Watanabe N., Honjo T. Generation, expansion, migration and activation of mouse B1 cells. Immunol. Rev. 2000;176:205–215. doi: 10.1034/j.1600-065x.2000.00604.x. [DOI] [PubMed] [Google Scholar]
- Ferry H., Leung J.C., Lewis G., Nijnik A., Silver K., Lambe T., Cornall R.J. B-cell tolerance. Transplantation. 2006;81:308–315. doi: 10.1097/01.tp.0000203830.79357.39. [DOI] [PubMed] [Google Scholar]
- Ferry H., Potter P.K., Crockford T.L., Nijnik A., Ehrenstein M.R., Walport M.J., Botto M., Cornall R.J. Increased positive selection of B1 cells and reduced B cell tolerance to intracellular antigens in c1q-deficient mice. J. Immunol. 2007;178:2916–2922. doi: 10.4049/jimmunol.178.5.2916. [DOI] [PubMed] [Google Scholar]
- Fukuyama H., Nimmerjahn F., Ravetch J.V. The inhibitory Fcgamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat. Immunol. 2005;6:99–106. doi: 10.1038/ni1151. [DOI] [PubMed] [Google Scholar]
- Gay D., Saunders T., Camper S., Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J. Exp. Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi C.M., Michael D.J., Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J. Immunol. 2001;167:1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- Korb L.C., Ahearn J.M. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J. Immunol. 1997;158:4525–4528. [PubMed] [Google Scholar]
- Li Y., Li H., Weigert M. Autoreactive B cells in the marginal zone that express dual receptors. J. Exp. Med. 2002;195:181–188. doi: 10.1084/jem.20011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F., Woodcock S.A., Lawton P., Ambrose C., Baetscher M., Schneider P., Tschopp J., Browning J.L. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandik-Nayak L., Bui A., Noorchashm H., Eaton A., Erikson J. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. J. Exp. Med. 1997;186:1257–1267. doi: 10.1084/jem.186.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandik-Nayak L., Racz J., Sleckman B.P., Allen P.M. Autoreactive marginal zone B cells are spontaneously activated but lymph node B cells require T cell help. J. Exp. Med. 2006;203:1985–1998. doi: 10.1084/jem.20060701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandik-Nayak L., Seo S., Eaton-Bassiri A., Allman D., Hardy R.R., Erikson J. Functional consequences of the developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J. Immunol. 2000;164:1161–1168. doi: 10.4049/jimmunol.164.3.1161. [DOI] [PubMed] [Google Scholar]
- Mandik-Nayak L., Seo S.J., Sokol C., Potts K.M., Bui A., Erikson J. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J. Exp. Med. 1999;189:1799–1814. doi: 10.1084/jem.189.11.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino R., Shibata T., Kossodo De, Izui S. Differential effect of the autoimmune Yaa and lpr genes on the acceleration of lupus-like syndrome in MRL/MpJ mice. Eur. J. Immunol. 1989;19:2131–2137. doi: 10.1002/eji.1830191124. [DOI] [PubMed] [Google Scholar]
- Mevorach D., Mascarenhas J.O., Gershov D., Elkon K.B. Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.A., Pickering M.C., Warren J., Fossati-Jimack L., Cortes-Hernandez J., Cook H.T., Botto M., Walport M.J. C1q deficiency and autoimmunity: the effects of genetic background on disease expression. J. Immunol. 2002;168:2538–2543. doi: 10.4049/jimmunol.168.5.2538. [DOI] [PubMed] [Google Scholar]
- Murakami M., Yoshioka H., Shirai T., Tsubata T., Honjo T. Prevention of autoimmune symptoms in autoimmune-prone mice by elimination of B-1 cells. Int. Immunol. 1995;7:877–882. doi: 10.1093/intimm/7.5.877. [DOI] [PubMed] [Google Scholar]
- Nauta A.J., Castellano G., Xu W., Woltman A.M., Borrias M.C., Daha M.R., van Kooten C., Roos A. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J. Immunol. 2004;173:3044–3050. doi: 10.4049/jimmunol.173.5.3044. [DOI] [PubMed] [Google Scholar]
- Nijnik A., Ferry H., Lewis G., Rapsomaniki E., Leung J.C., Daser A., Lambe T., Goodnow C.C., Cornall R.J. Spontaneous B cell hyperactivity in autoimmune-prone MRL mice. Int. Immunol. 2006;18:1127–1137. doi: 10.1093/intimm/dxl047. [DOI] [PubMed] [Google Scholar]
- Oliver A.M., Martin F., Kearney J.F. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J. Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- Pickering M.C., Botto M., Taylor P.R., Lachmann P.J., Walport M.J. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv. Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- Prak E.L., Weigert M. Light chain replacement: a new model for antibody gene rearrangement. J. Exp. Med. 1995;182:541–548. doi: 10.1084/jem.182.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodeus A.P., Goerg S., Shen L.M., Pozdnyakova O.O., Chu L., Alicot E.M., Goodnow C.C., Carroll M.C. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9:721–731. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- Qian Y., Wang H., Clarke S.H. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J. Immunol. 2004;172:625–635. doi: 10.4049/jimmunol.172.1.625. [DOI] [PubMed] [Google Scholar]
- Quartier P., Potter P.K., Ehrenstein M.R., Walport M.J., Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur. J. Immunol. 2005;35:252–260. doi: 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]
- Radic M.Z., Mascelli M.A., Erikson J., Shan H., Weigert M. Ig H and L chain contributions to autoimmune specificities. J. Immunol. 1991;146:176–182. [PubMed] [Google Scholar]
- Rudolph E.H., Congdon K.L., Sackey F.N., Fitzsimons M.M., Foster M.H. Humoral autoimmunity to basement membrane antigens is regulated in C57BL/6 and MRL/MpJ mice transgenic for anti-laminin Ig receptors. J. Immunol. 2002;168:5943–5953. doi: 10.4049/jimmunol.168.11.5943. [DOI] [PubMed] [Google Scholar]
- Samardzic T., Marinkovic D., Danzer C.P., Gerlach J., Nitschke L., Wirth T. Reduction of marginal zone B cells in CD22-deficient mice. Eur. J. Immunol. 2002;32:561–567. doi: 10.1002/1521-4141(200202)32:2<561::AID-IMMU561>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Sekiguchi D.R., Jainandunsing S.M., Fields M.L., Maldonado M.A., Madaio M.P., Erikson J., Weigert M., Eisenberg R.A. Chronic graft-versus-host in Ig knockin transgenic mice abrogates B cell tolerance in anti-double-stranded DNA B cells. J. Immunol. 2002;168:4142–4153. doi: 10.4049/jimmunol.168.8.4142. [DOI] [PubMed] [Google Scholar]
- Steeves M.A., Marion T.N. Tolerance to DNA in (NZB × NZW)F1 mice that inherit an anti-DNA V(H) as a conventional micro H chain transgene but not as a V(H) knock-in transgene. J. Immunol. 2004;172:6568–6577. doi: 10.4049/jimmunol.172.11.6568. [DOI] [PubMed] [Google Scholar]
- Taylor P.R., Carugati A., Fadok V.A., Cook H.T., Andrews M., Carroll M.C., Savill J.S., Henson P.M., Botto M., Walport M.J. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J. Exp. Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L., Brill-Dashoff J., Shinton S.A., Asano M., Hardy R.R., Hayakawa K. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 2005;23:297–308. doi: 10.1016/j.immuni.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Wither J.E., Roy V., Brennan L.A. Activated B cells express increased levels of costimulatory molecules in young autoimmune NZB and (NZB × NZW)F(1) mice. Clin. Immunol. 2000;94:51–63. doi: 10.1006/clim.1999.4806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.