Abstract
Background
The prevalence of both childhood and adult obesity is rising in the developed world and there is increasing interest in its underlying causes. A number of studies suggest a positive relationship between birth weight and childhood body mass index, but less is known about specific prenatal environmental influences on more direct measures of obesity. We utilised data from the Southampton Women's Survey to investigate parental influences on neonatal body composition ascertained by dual x-ray absorptiometry.
Methods
Participating mothers were characterised in detail (anthropometry, lifestyle, diet) before and during pregnancy; information was also obtained on their partners. The offspring underwent assessment of fat and lean body mass by dual x-ray absorptiometry (DXA) within 2 weeks of birth. Linear regression methods were used to explore the parental determinants of neonatal body composition.
Results
Complete data were available for 448 mother-offspring pairs. Taller women and those with higher parity had offspring with increased birth weight, fat and lean mass (p<0.05). Mothers who were taller, of greater parity, had greater fat stores or walked more slowly also had offspring with greater proportionate body fat at birth (all p<0.05). There was a weaker trend towards lower percentage fat and greater percentage lean in the offspring of mothers who smoked during pregnancy.
Conclusion
Maternal size, parity, smoking history, walking speed and fat stores are independent determinants of neonatal body composition. If these influences are shown to have persisting effects on body composition through to adulthood, they point to novel public health interventions early in life to prevent later obesity.
Keywords: Epidemiology, osteoporosis, programming, developmental origins
INTRODUCTION
There is increasing evidence to suggest that size at birth and childhood growth are important determinants of body composition and chronic disease in later life (1-6). Although maternal birth weight, smoking, exercise levels and adiposity in pregnancy have all been shown to influence bone mass at birth (5,6), less is known about the prenatal determinants of other aspects of neonatal body composition such as fat and lean mass, and the extent to which these effects are modified by the early postnatal environment. Many epidemiologic studies of obesity have used BMI as the main outcome measure but interpretation of BMI is difficult because it reflects both fat and muscle mass. Dual X-ray absorptiometry (DXA) provides a well-validated, alternative approach to the estimation of each of these two soft-tissue compartments. We report here, the use of this objective measure to assess prenatal influences on body composition (fat and fat-free mass) at birth in a cohort of healthy, normal pregnancies.
METHODS
The Southampton Women's Survey (SWS) includes a prospective cohort of around 12,500 women aged 20-34 years. Characteristics of the cohort have been published previously (7). Assessments of lifestyle, diet (by validated food frequency questionnaire), and anthropometry were performed at study entry and then during early (11 weeks) and late (34 weeks) gestation among those women who became pregnant. Maternal height was measured with a stadiometer, weight with calibrated digital scales, and skin folds (biceps, triceps, supscapular and suprailiac regions) with Harpenden calipers (between-observer variation at all four sites <10%). The mothers were asked to characterise their current walking speed into one of 5 groups (very slow, stroll at an easy pace, normal speed, fairly brisk or fast).
Mothers registered with specific GP practices (to minimise participation load on mothers in sub-studies) were invited to participate in the bone component of the SWS. At birth, the baby was weighed on calibrated digital scales (Seca, UK), and crown-heel length measured using a neonatometer (CMS Ltd, UK). After birth, the mother was asked to agree to her baby undergoing assessment of body composition, within 2 weeks of birth, using a Lunar DPX-L instrument, with specific paediatric software (GE Corporation, Madison, Wisconsin, USA). The instrument underwent daily quality assessment, and was calibrated against a water phantom weekly. At the visit to the scan room, the baby was pacified and fed if necessary, undressed completely, and then swaddled in a standard towel. It was placed on a waterproof sheet in a standard position on the scanner, and was kept in position using rice bags placed over the bottom end of the towel. Short-term and long-term coefficients of variation (CV) for the DXA instrument were 0.8% and 1.4% respectively. Fathers' height and weight were measured when they attended for bone mineral assessment by DXA after the birth of their children.
Statistical analysis
All variables were checked for normality, transformed where necessary (logarithmically or by square root for total and percentage fat), and standardised to SD scores. Correlation and linear regression methods were used to explore the determinants of neonatal body composition, using Stata V8.2 (Statacorp, Texas, USA). Predictors were initially explored in univariate analyses and multivariate models were then constructed. To control for body size, percentage fat and lean mass (DXA-derived fat or lean mass ÷ DXA-derived body mass) were utilised. The study had full ethical approval from the Southampton Local Research Ethics Committee and all participants gave written informed consent.
RESULTS
Characterisation of the cohort
The mean age (SD) of the mothers was 28.4 (3.7) years, and that of the fathers was 34.1 (5.0) years. 24% of mothers declined participation in the DXA component of the study; comparison of the 448 mother offspring pairs entering the study with the remainder of the cohort revealed no differences in maternal age, height, pre-pregnant weight, BMI or tricep skinfold thickness. The neonates included were significantly p<0.05 heavier and had better educated mothers. There were strong, statistically significant (p<0.001) associations between gestational age and neonatal birthweight (r=0.45), lean mass (r=0.41), fat mass (r=0.39), and proportionate lean (r=0.32) and fat mass (r=0.33). After adjustment for gestational age, the boys were slightly longer (0.28 sd, p=0.002) and heavier (0.20 sd, p=0.04) at birth than the girls. Boys also had greater lean mass (0.40 sd, p<0.001) and lower fat mass (0.22 sd, p=0.02). Proportionate body composition varied by gender, such that boys had higher percentage lean (0.37 sd, p<0.001) and lower percentage fat (0.40 sd, p<0.001). Given these differences, all subsequent analyses were performed after adjustment for gestational age, gender, and age at DXA scan.
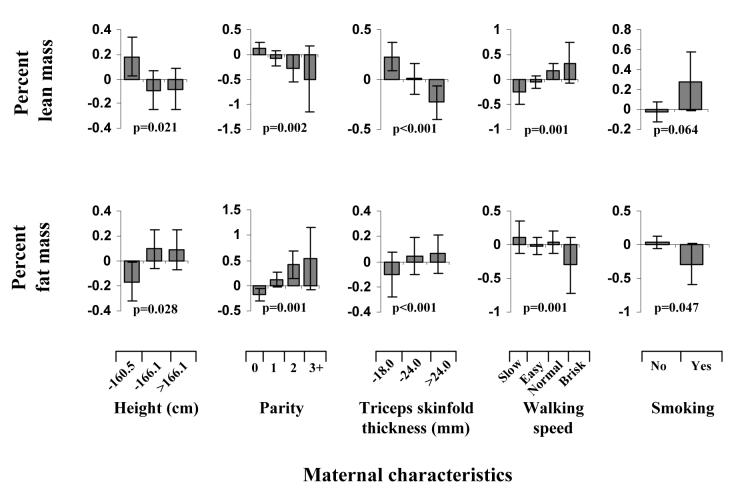
Maternal predictors of neonatal birthweight and body composition (Table and Figure)
Table.
Maternal predictors of neonatal birthweight and body composition (outcomes in SD)
| Birthweight | Percentage Fat Mass | Percentage Lean Mass | |
|---|---|---|---|
| Maternal height (cm) β (95% CI) |
0.041 (0.028, 0.055) | 0.015 (0.0004, 0.029) | −0.016 (−0.030, −0.001) |
| p | <0.001 | 0.044 | 0.032 |
| Parity (no. of children) β (95% CI) |
0.326 (0.212, 0.439) | 0.200 (0.081, 0.320) | −0.185 (−0.306, −0.063) |
| p | <0.001 | 0.001 | 0.003 |
| Walking speed (increasing quarters) β (95% CI) |
−0.167 (−0.277, −0.0584) | −0.164 (−0.279, −0.049) | 0.165 (0.048, 0.281) |
| p | 0.003 | 0.005 | 0.006 |
| Smoking (Yes vs No) β (95% CI) |
−0.611 (−0.908, −0.315) | −0.283 (−0.595, 0.029) | 0.257 (−0.059, 0.573) |
| p | <0.001 | 0.075 | 0.111 |
| Triceps skinfold (SD) β (95% CI) |
0.104 (0.018, 0.190) | 0.145 (0.054, 0.235) | −0.154 (−0.246, −0.063) |
| p | 0.018 | 0.002 | 0.001 |
Birthweight adjusted for gestational age and gender
Measures of body composition adjusted for gender, age at DXA and gestational age
β coefficients presented after mutual adjustment in multiple regression models
Maternal lifestyle and anthropometric factors and neonatal body composition. (Outcome in SDs)
Maternal height and parity were significantly (p<0.05) associated with all outcome measures (birthweight, total lean, total fat and proportionate lean and fat). Additionally, after adjustment for gestational age and gender, the baby's birthweight, total lean and total fat mass were positively associated with maternal triceps skinfold thickness and negatively with maternal walking speed and smoking (all p<0.01, except for lean with triceps and walking speed, which did not reach statistical significance). Mothers who had reduced fat stores, were shorter, or were primiparous, had offspring with decreased percentage fat and increased percentage lean mass (all p<0.05). In contrast, those mothers who smoked during pregnancy or had a faster walking speed had offspring with decreased percentage fat and increased percentage lean mass. For each 1 sd increase in birthweight, there was a 0.66 sd increase in percentage fat and a corresponding decrease in percentage lean mass (p<0.001). The univariate relationships between parity, walking speed, triceps skinfold and each of proportionate fat and lean mass remained significant (p<0.05) after adjusting for multiple comparisons. All these associations were independent of social class. Associations between maternal and neonatal measures were generally stronger for maternal factors assessed in late than in early pregnancy.
The independent predictors of proportionate body composition again included maternal height, parity, triceps skinfold thickness, smoking and walking speed (table 1). Thus mothers who were shorter, primiparous, or had reduced fat stores, had offspring with reduced percentage fat and increased percentage lean mass. In contrast, those mothers who smoked during pregnancy or had a faster walking speed had offspring with decreased percentage lean and increased percentage fat mass, although the associations with smoking were of borderline significance (p=0.075 for fat and 0.111 for lean). Again the associations with triceps skinfold thickness, walking speed and smoking strengthened from before to late pregnancy. Although infants who were breast fed tended to have lower total (p=0.01) and percentage (p=0.009) fat, these associations were markedly attenuated in the multivariate model.
DISCUSSION
We report a prospective mother-offspring cohort study from the general population which aimed to identify the maternal determinants of neonatal body composition. Maternal height, parity, physical activity, and fat stores as assessed by triceps skinfold thickness, were all independent determinants of neonatal percentage fat and lean mass. Maternal smoking in late pregnancy was associated weakly with lower percentage fat and greater percentage lean. Total fat mass was predicted by maternal height, parity, smoking, physical activity and triceps skinfold thickness. In contrast, only maternal height and smoking influenced total lean mass in the neonate. Paternal height was only found to be positively associated with total lean mass, but not total fat mass or proportionate body composition (data not shown). Breast fed babies had lower percentage fat, but this did not confound the prenatal associations.
A large number of epidemiological studies have investigated the association between birth weight and subsequent body mass index (BMI; body weight in kilograms divided by height in metres squared kg/m2). Findings from a comprehensive systematic review confirmed a positive association between birth weight and BMI in both children and adults (8), and it has been proposed that both the prenatal environment (9) and maternal factors (10) may have lifelong consequences for obesity. BMI is frequently used as a marker of obesity because it is widely available and is correlated with direct measures of adiposity in children and younger adults (11). However, BMI also reflects fat-free mass, including muscle mass, and is more difficult to interpret when body composition is changing, for example in childhood and later life.
Findings from the few studies that have examined the relation between birth weight and more direct measures of fat mass, such as skinfold thickness, are less consistent. Two studies showed positive associations between birth weight and both BMI and skinfold thickness in young children (12,13) but a third study in adolescent women showed discordance: the relation between birth weight and skinfold thickness was negative or absent, whereas that between birth weight and BMI was positive (14). A further study using recalled birth weight in young adults showed a negative association between birth weight and skinfold thickness but no relationship between birth weight and BMI (15). A recent study in children and adolescents in which whole body dual energy x-ray absorptiometry, skinfold thickness measurements and bio-impedance analysis were used to measure fat mass, showed that increases in birth weight were associated with increases in fat-free mass but not in fat mass independently of age, sex, height, pubertal stage, socioeconomic status, and physical activity (16).
Our study utilised a well-established prospective cohort, and we had robust characterisation of the mothers before and in early and late pregnancy, allowing us to dissect out the importance of maternal factors at these different time points. Data on infant feeding, but not illness were available. We also had data on the fathers, and measured body composition with an objective technique, which allowed accurate assessment of adiposity. However, whilst DXA is a well-validated tool in adults, there are some problems with neonates, due to their small size and low bone density. We used specific paediatric software, but there was still some error induced by movement of the neonates. This was uniform across the cohort, and it is unlikely that this would have varied significantly by the maternal and paternal factors. Scans showing excessive movement artefact were excluded. Finally, although DXA is an excellent technique for assessing whole body bone and fat, the “lean” that is measured includes not only muscle mass, but also the internal organs.
In conclusion, we have demonstrated that maternal size, parity, smoking history, walking speed and fat stores, as well as paternal size, are independent influences on neonatal body composition. If they have persisting effects through childhood into adulthood, there is potential for novel public health interventions early in life to prevent subsequent obesity.
ACKNOWLEDGEMENTS
This study was supported by the Medical Research Council of Great Britain, the Arthritis Research Campaign, the International Osteoporosis Foundation and the National Osteoporosis Society.
Funding sources: Medical Research Council; Arthritis Research Campaign; National Osteoporosis Society; Wellbeing; Cohen Trust; NE (now North)Thames NHS R&D Directorate.
REFERENCES
- 1.Sayer AA, Syddall HE, Dennison EM, Gilbody HJ, Duggleby SL, Cooper C, et al. Birth weight, weight at one year and body composition in older men: findings from the Hertfordshire Cohort Study. Am J Clin Nut. 2004;80:199–203. doi: 10.1093/ajcn/80.1.199. [DOI] [PubMed] [Google Scholar]
- 2.Law CM, Barker DJP, Osmond C, Fall CHD, Simmonds SJ. Early growth and abdominal fatness in adult life. J Epidemiol Comm Hlth. 1992;46:184–186. doi: 10.1136/jech.46.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fall CH, Stein CE, Kumaran K, Cox F, Osmond C, Barker DJ, Hales CN. Size at birth, maternal weight, and type 2 diabetes in South India. Diabet Med. 1998;15:220–227. doi: 10.1002/(SICI)1096-9136(199803)15:3<220::AID-DIA544>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 4.Osmond C, Barker DJ. Fetal, infant and childhood growth are predictors of coronary heart disease, diabetes and hypertension in adult men and women. Environ Health Perspect. 2000;108(Suppl 3):545–553. doi: 10.1289/ehp.00108s3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baird J, Fisher D, Lucas P, Kleijnon J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and late obesity. BMJ. 2005;331:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godfrey K, Walker-Bone K, Robinson S, et al. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res. 2001;16:1694–1703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- 7.Inskip H, Godfrey KM, Robinson SM, Law CM, Barker DJP, Cooper C, the SWS Study Group Cohort Profile: The Southampton Women's Survey. Int J Epidemiol. 2006;35:42–48. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord. 1999;23(Suppl 8):S1–107. [PubMed] [Google Scholar]
- 9.Oken E, Gillman MW. Fetal origins of obesity. Obes.Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 10.Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. BMJ. 2001;323:1331–5. doi: 10.1136/bmj.323.7325.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roche AF, Sievogel RM, Chumlea WC, Webb P. Grading body fatness from limited anthropometric data. Am J Clin Nutr. 1981;34:2831–8. doi: 10.1093/ajcn/34.12.2831. [DOI] [PubMed] [Google Scholar]
- 12.Kramer MS, Barr RG, Pless IB, Bolisjoyly C, McVey-White L, Leduc DG. Determinants of weight and adiposity in early childhood. Can.J Public Health. 1986;77(Suppl 1):98–103. 98-103. [PubMed] [Google Scholar]
- 13.Zive MM, McKay H, Frank-Spohrer GC, Broyles SL, Nelson JA, Nader PR. Infant-feeding practices and adiposity in 4-y-old Anglo- and Mexican-Americans. Am J Clin Nutr. 1992;55:1104–8. doi: 10.1093/ajcn/55.6.1104. [DOI] [PubMed] [Google Scholar]
- 14.Barker M, Robinson S, Osmond C, Barker DJ. Birth weight and body fat distribution in adolescent girls. Arch Dis Child. 1997;77:381–383. doi: 10.1136/adc.77.5.381. [DOI] [PubMed] [Google Scholar]
- 15.Te Velde SJ, Twisk JW, Van Mechelen W, Kemper HC. Birth weight, adult body composition, and subcutaneous fat distribution. Obes Res. 2003;11:202–8. doi: 10.1038/oby.2003.32. [DOI] [PubMed] [Google Scholar]
- 16.Singhal A, Wells J, Cole TJ, Fewtrell M, Lucas A. Programming of lean body mass: a link between birth weight, obesity, and cardiovascular disease? Am J Clin Nutr. 2003;77:726–30. doi: 10.1093/ajcn/77.3.726. [DOI] [PubMed] [Google Scholar]