Abstract
Background
Body mass index (BMI) and bone mineral density (BMD) are positively correlated in several studies, but few data are available relating bone density, lipid profile and anthropometric measures. We addressed these relationships in a large well-characterised cohort of men and women (The Hertfordshire Cohort Study, UK).
Methods
Four hundred and sixty five men and 448 women from Hertfordshire, UK were recruited. Information was available on demographic and lifestyle factors, anthropometric measurements, body fat percentage, fasting triglycerides, cholesterol (total, HDL, LDL), apolipoprotein (a) and apolipoprotein (b); bone mineral density (BMD) was recorded at the lumbar spine and total femur.
Results
BMD at the lumbar spine (males r=0.15, p=0.001; females r=0.14 p=0.003) and total femoral region (males r=0.18, p=0.0001; females r==0.16, p=0.0008) was related to serum triglyceride level, even after adjustment for waist hip ratio, age, social class and lifestyle factors, but not if body fat percentage was substituted for waist-hip ratio in the regression model. Fasting HDL cholesterol level was related to lumbar spine BMD in women (r=−0.15, p=0.001) and total femoral BMD (males r=−0.15, p=0.002; females r=−0.23, p<0.0001); these relationships were also attenuated by adjustment for body fat percentage but not waist hip ratio. No relationships were seen between total or LDL cholesterol with BMD.
Conclusions
We have demonstrated relationships between lipid profile and BMD that are robust to adjustment for one measure of central obesity (waist-hip ratio) but not total body fat.
Keywords: lipid, bone, cohort, anthropometry, obesity
Introduction
Osteoporosis is a major public health problem through its association with age related fractures. Bone mineral density (BMD) is an important determinant of fracture risk, with an approximate doubling of fracture risk for every standard deviation reduction in BMD [1]. Low bone mineral density is associated with an increased risk of cardiovascular mortality in postmenopausal women [2] and several drugs, notably the statins [3], Hormone Replacement Therapy (HRT) [4] and bisphosphonates [5] are known to influence both bone density and lipid synthesis. These observations, and the knowledge that osteoblasts and adipocytes share a common progenitor from stromal cells in bone marrow, has led investigators to study the relationship between cholesterol concentration and bone mass and postulate that a relationship between hyperlipidaemia and BMD is the missing link between atherosclerosis and osteoporosis [6-19]. However, studies performed to date have given conflicting results, perhaps because of the limited lipid data available, variable anthropometric data collected and small numbers (particularly of men) studied. A recent and very large study that utilised the US NHANES data set found relationships between BMD and total, LDL and HDL cholesterol that were removed after adjustment for BMI [19]. The authors concluded that one reason for the discrepancy among studies performed in the US and elsewhere may be modification of the potential relationship between lipids and BMD by genetic heritage. We therefore sought to examine the same relationships in the Hertfordshire (UK) Cohort Study, where a group of 1000 men and women have been extensively characterised with regard to lifestyle factors, anthropometry, fasting lipid profile and bone mass.
Materials & Methods
This study utilised a birth cohort that has been studied to examine the relationship between early growth and the risk of adult diseases such as osteoporosis. As previously described [20] in Hertfordshire, UK from 1911 to 1948 each birth was notified by the attending midwife and the birth-weight recorded. Subsequently, health visitors who saw each child during infancy recorded its weight at the age of one year. We used the National Health Service Central Registry at Southport to trace 1760 men and 1447 women born in Hertfordshire between 1931 and 1939 who were singleton births and had both birth and infant weights recorded and were still resident in East Hertfordshire in the late 1990's. Permission to contact 1397 men and 1364 women was obtained from the general practitioners. Of these subjects 768 (55%) of the men and 714 (52%) of the women agreed to take part in a home interview in which trained nurses administered a lifestyle questionnaire. This included information on socioeconomic status, medical history (including the Rose chest pain questionnaire) [21], cigarette smoking, alcohol consumption, dietary calcium intake, physical activity and reproductive variables in women. The subject was then invited to attend a local morning clinic after an overnight fast.
At this clinic visit, height was measured to the nearest 0.1cm using a Harpenden pocket stadiometer (Chasmors Ltd, London, UK) and weight to the nearest 0.1kg on a SECA floor scale (Chasmors Ltd, London, UK). Body mass index (BMI) was calculated as weight divided by height2 (kg/m2). Waist (mid way between the costal margin and the iliac crest in the mid axillary line) and hip (greatest diameter around the gluteal region) circumferences were measured with steel tape and skinfold thickness was determined at four sites (biceps, triceps, subscapular and suprailiac) using a Harpenden skinfold calliper (Chasmors Ltd, London, UK). Body fat percentage was calculated according to the methods of Durmin and Womesley [22]. An electrocardiogram (ECG) was recorded. Fasting blood samples were obtained, and the samples analysed for measurement of total cholesterol, HDL cholesterol, triglycerides, apolipoprotein (a) and (b). LDL cholesterol concentrations were calculated using the Friedwald-Fredrickson method [23]. While cholesterol, HDL and triglycerides were all measured by standard enzymatic methods, apolipoprotein (a) and (b) were measured by immunoturbimetric assays using specific antibodies.
Bone mineral content (BMC), bone area and BMD were measured in a subgroup of 498 men and 468 women by dual energy X-ray absorptiometry at the lumbar spine and total proximal femur using a Hologic QDR 4500 instrument. Measurement precision error, expressed as coefficient of variation, was 1.55% for lumbar spine BMD, 1.45% for total femur and 1.83% for femoral neck BMD for the Hologic QDR 4500; these figures were obtained by twenty five volunteers who were not part of the study undergoing 2 scans on the same day, getting on and off the table between examinations. Short-term (2 month) precision error for the QDR 4500 was less than 1% for both sites (manufacturers figures). Individuals taking drugs known to alter bone metabolism (such as bisphosphonates and statin therapy) were excluded from this part of the study, although women taking Hormone Replacement Therapy (HRT) were allowed to participate, and statistical analyses adjusted appropriately. There were no other exclusion criteria to this part of the study, and subjects were approached for consent as they attended clinic. Analyses were performed including and excluding subjects with diabetes mellitus.
This research was conducted in accordance with the guidelines in The Declaration of Helsinki; ethical approval for the study was obtained from the Hertfordshire Local Research Ethics Committee and each person gave written, informed consent.
Normality of variables was assessed and variables transformed as required. The STATA, release 8 statistical software package was used for the analyses. Pearson correlations were used to analyse the relationship between lipid profile and bone mass, followed by multiple linear regression models to adjust for lifestyle and anthropometric factors. Data for at least one of total cholesterol, HDL cholesterol, triglycerides, apolipoprotein (a) or (b) were available for 465 (93%) of the men, and 448 (96%) of the women, who participated in the DXA study; these men and women comprised the study group for this manuscript.
Results
The characteristics of the study population at baseline are displayed in Table 1. The mean age of the men and women studied was 64.9 years. Thirty four percent of the men and 62 percent of the women had never smoked, while 52% of the men (28% of the women) and 15% of the men (10% of the women) were ex-smokers and current smokers respectively. Four percent of men and 18 percent of women were non-drinkers, while 22% of men and 12% of women were moderate drinkers (i.e. 11-21 units per week for men, 8-14 units per week for women). Twenty five percent of men and 3% women consumed greater that the recommended number of units of alcohol per week (i.e. > 21 units per week for men, >14 units per week for women). Two hundred and fifty eight (58%) women in this cohort had never used HRT, 85 (19%) had discontinued use more that 5 years before they were seen as part of this study, 29 (6%) had stopped within the last 5 years, and 76 (17%) were still taking HRT at the time of their clinic visit. Among women, the frequency distribution of years since the menopause was as follows: <5yrs, 13 (3%); 5-9yrs, 47 (11%); 10-14yrs, 118 (26%); 15-19yrs, 89 (20%); 20+yrs, 69 (15%); hysterectomy, 109 (24%). Subjects who participated in the DXA sub-study did not significantly vary from the clinic population as a whole with regard to age, social class, cigarette and alcohol consumption, height, weight or body mass index.
Table I.
Summary characteristics of study participants
| Characteristic | Males (n=465) | Females (n=448) |
|---|---|---|
| Mean (SD) unless stated otherwise | ||
| Age (yrs) | 64.3 (2.5) | 65.6 (2.5) |
| BMI (kg/m2) a | 26.5 (1.1) | 26.8 (1.2) |
| Height (cm) | 174.2 (6.8) | 160.8 (5.9) |
| Weight (kg)a | 80.3 (1.2) | 69.1 (1.2) |
| Body fat (%)b | 28.4 (5.1) | 40.1 (4.6) |
| Waist circumference (cm)b | 98.9 (9.8) | 91.0 (12.8) |
| Hip circumference (cm)b | 103.8 (6.7) | 107.4 (10.8) |
| Waist hip ratiob | 0.95 (0.05) | 0.85 (0.07) |
| Daily calcium intake (mg)a | 1221 (1.3) | 1083 (1.3) |
| Alcohol consumption (units per week)c | 10.3 (3.1, 22.5) | 3.0 (0.5, 7.0) |
| Habitual activity (%)d | 64.3 (14.9) | 61.5 (14.8) |
| N(%) Current manual social class (IIIM-V)e | 181 (38.9) | 174 (38.8) |
| N(%) Current non-manual social class (I-IIIN) e | 258 (55.5) | 274 (61.2) |
| Lumbar spine BMD (g/cm2)f | 1.08 (0.16) | 0.96 (0.17) |
| Femoral neck BMD (g/cm2) f | 0.85 (0.12) | 0.76 (0.12) |
| Total femoral BMD (g/cm2) f | 1.04 (0.13) | 0.89 (0.13) |
Geometric mean and SD
No data was available for one woman
Median and IQR among drinkers. 18 men and 80women stated that they do not drink alcohol.
Standardised score ranging 0-100 derived from frequency of gardening, housework, climbing stairs and carrying loads in a typical week. Higher scores indicate greater level of activity.
Social class was unclassified for 26 men. I-IIIN and IIIM-V denote classes one to three (non-manual), and three (manual) to five, of the 1990 OPCS Standard Occupational Classification scheme for occupation and social class. Social class was identified on the basis of own current or most recent full time occupation for men and never-married women, but on the basis of the husband's occupation for ever-married women.
1 man was not scanned at the lumbar spine. 3 men and 1 woman were not scanned at the hip.
In this cohort, BMD at all sites, particularly at the femur region, was strongly associated with adult anthropometric measures, including height (p<0.03), weight (p<0.001), waist-hip ratio (p<0.01), body fat percentage (p<0.001) and all skin fold measurements (p<0.005). Different measures of adiposity (such as percentage body fat and waist-hip ratio) were highly correlated, as anticipated (r=0.42-0.54, p=0.000); relationships between body build and lipid profile are given in Table 2. Adjustment for age, cigarette and alcohol consumption, physical activity, social class (and HRT use and years since menopause in women) in a multiple regression model made little difference to our results.
Table 2.
Correlation coefficients between measures of adiposity and lipid concentration among participants from the Hertfordshire Cohort Study
| MEN | WOMEN | |||
|---|---|---|---|---|
| Waist-hip ratio | % Body fat | Waist-hip ratio | % Body fat | |
| Triglycerides | 0.35*** | 0.33*** | 0.38*** | 0.32*** |
| Total cholesterol | 0.09 | 0.12* | 0.05 | 0.006 |
| HDL cholesterol | −0.22*** | −0.26*** | −0.36*** | −0.32*** |
| LDL cholesterol | 0.05 | 0.10 | 0.08 | 0.05 |
| Apolipoprotein (a) | −0.09 | −0.19*** | −0.17*** | −0.18*** |
| Apolipoprotein (b) | 0.14** | 0.18*** | 0.16 | 0.14** |
p<0.05
p<0.01
p<0.001
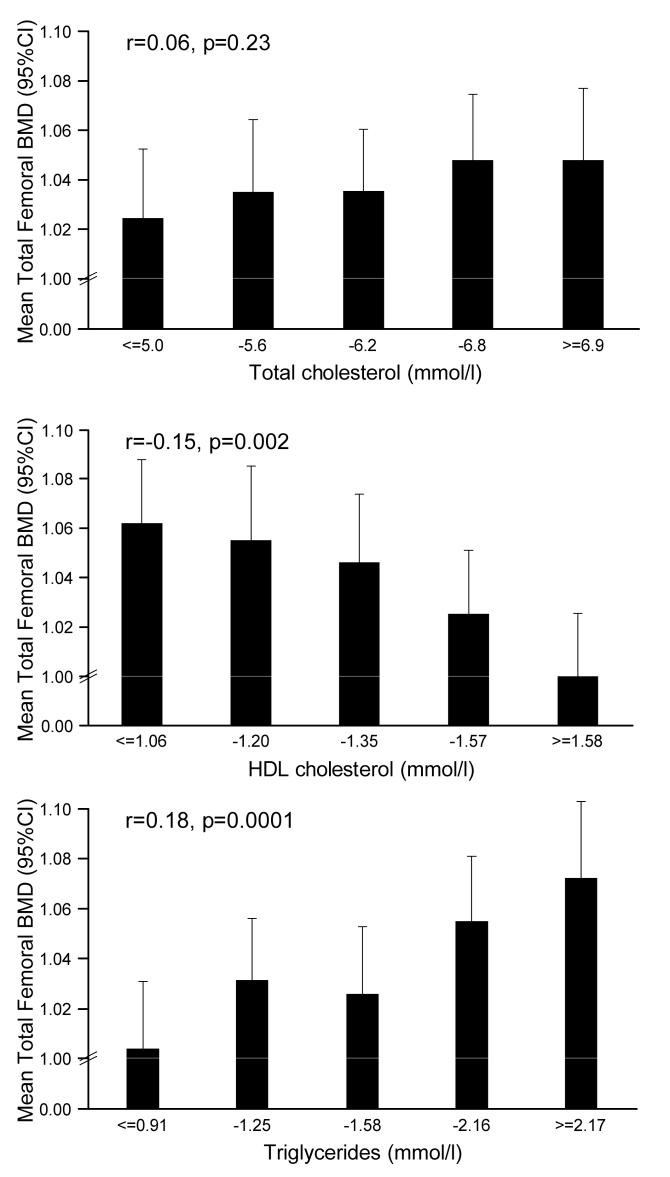
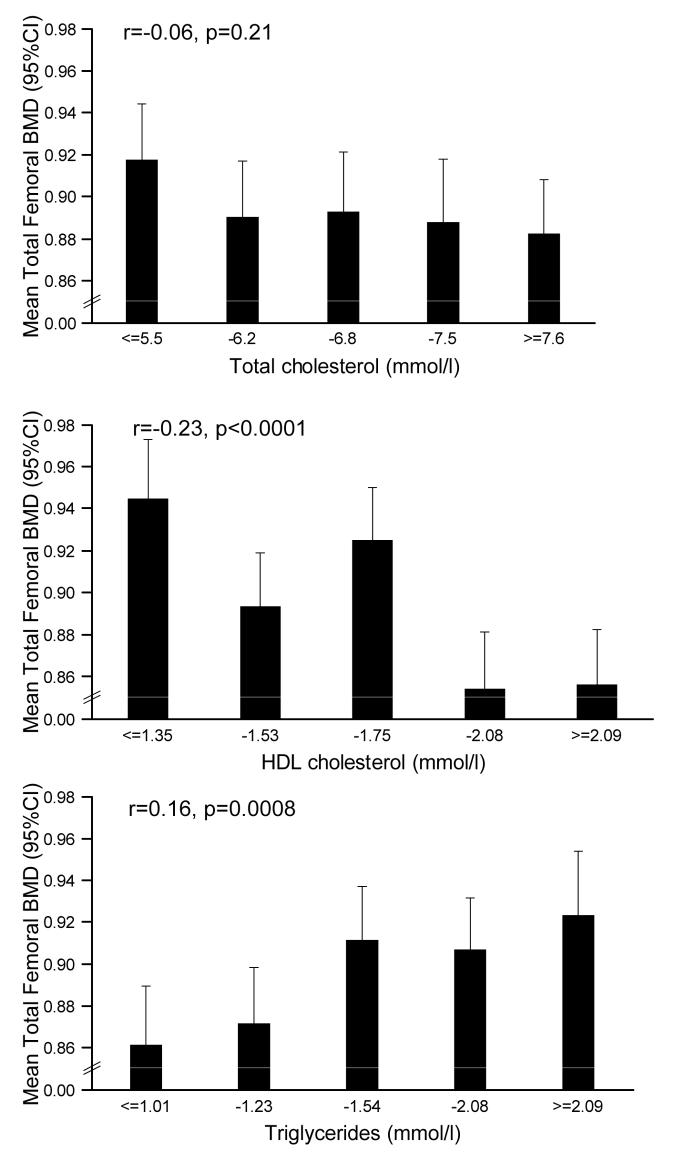
BMD at the lumbar spine (males r=0.15, p=0.001; females r=0.14 p=0.003) and total femoral region (males r=0.18, p=0.0001; females r==0.16, p=0.0008) was strongly related to serum triglyceride level, and these relationships remained significant after adjustment for waist hip ratio, age, social class and lifestyle factors, but not if body fat percentage was substituted for waist-hip ratio in the regression model. Relationships were also observed between fasting HDL cholesterol level and BMD at the lumbar spine in women (r=−0.15, p=0.001) and total femoral region in both sexes (males r=−0.15, p=0.002; females r=−0.23, p<0.0001); these relationships were also attenuated by adjustment for body fat percentage only. No relationships were seen between total or LDL cholesterol with BMD (figures 1 and 2). Relationships were seen between total femoral BMD and HDL/LDL cholesterol (men: r=−0.12, p=0.01; women: r=−0.12, p=0.01); these relations persisted in women after adjustment for any measure of adiposity including waist/hip ratio (men; p=0.09, women; p=0.008). Relationships between lumbar spine BMD and HDL/LDL cholesterol were non-significant. Finally, total femoral BMD was associated with apolipoprotein (a) (men r=−0.11, p=0.03; women r=−0.13, p=0.005) and apolipoprotein (b) (r=0.13, p=0.01), but relationships were attenuated by adjustment for waist-hip ratio.
Figure 1.
Relationships between total hip BMD and fasting cholesterol in men
Figure 2.
Relationships between total hip BMD and fasting cholesterol in women
We also calculated the proportion of variance in the dependent variable (BMD) explained by all the specific independent variables. Hence among men, the proportion of variance in total femoral BMD explained by percentage body fat, age, smoking, alcohol consumption, social class and physical activity and lipid measure ranged from 11-13%; in women, the corresponding figure (in a model including years since menopause and HRT use) was greater (22-23%). Similar calculations for the proportion of variance in lumbar spine BMD explained by percentage body fat, age, smoking, alcohol consumption, social class and physical activity and lipid measure ranged from 3-6% in men and 15-16% in women.
Eighty subjects (forty men and forty women) were taking fibrate or statin medication. Exclusion of this group made little difference to the regression coefficients in the analyses above. Similarly, 65 subjects (33 men and 32 women) received a diagnosis of diabetes as a result of tests performed at the study clinic. Again, exclusion of this group made little difference to the regression coefficients obtained.
Forty-eight men and 40 women had ischaemic heart disease in our cohort, defined according to a history of typical angina according to the Rose chest-pain questionnaire, previous coronary artery bypass grafting (CABG), or the presence of major-q waves on an electrocardiograph (ECG). There were no relationships between ischaemic heart disease and BMD at either of the 2 sites measured, before or after adjusting for confounding factors (age, social class, physical activity, cigarette and alcohol consumption, body mass index, statin use, and HRT use and menopausal status in women).
Discussion
In this study we sought to reproduce the findings of a large US study relating lipid profile to BMD in a UK population, and in particular to explore the possibility that the relationship between lipid concentration and bone mass was confounded by adiposity. We have demonstrated relationships between lipid profile and BMD that are largely robust to adjustment for measurement of central obesity (the pattern observed as part of the metabolic syndrome) but not total body fat. While interpretation is difficult due to the highly correlated measures of obesity, our findings would concur with the US study previously described [19]. These associations found did not translate into a significant relationship between BMD and ischaemic heart disease.
Our study has a number of weaknesses. The individuals recruited were selected because they had been born in Hertfordshire, UK and continued to live there at the age of 60-75 years, as in previous studies. However, we have previously demonstrated that the Hertfordshire populations studied have similar smoking characteristics and bone density to national figures [24], suggesting that selection bias is minimal. We also confirmed that there were no significant differences in anthropometric measures, cigarette or alcohol consumption or physical activity levels between those individuals who did or did not elect to undergo a bone density test. Regrettably, the bone density data available does not include body composition. Nor are oestrogen levels currently available in this population. While we failed to demonstrate relationships between BMD and ischaemic heart disease in this population, this may reflect limited power to uncover such relations. Finally, the relationships we describe relate lipid profile to areal bone density BMD, rather than any measure of skeletal size such as bone mineral concentration (BMC). We elected to present this outcome measure as we felt that biologically we might expect relationships to be greater with an areal density measure than a size measure, and it made comparison with similar studies easier.
A number of studies have suggested a positive relationship between BMD and triglyceride level, in concordance with our own findings [10,13], while the literature concerning relationships between HDL cholesterol levels and BMD is conflicting [12, 13,15,15, 18,19]. While D'Amelio et al [12] found an inverse relationship similar to our own results, Yamaguchi et al [14] described a positive relationship, and Cui et al [13] and Poli et al [15] described no relationship. Aside from genetic differences, one possible explanation may be the importance of estrogen concentration in these groups; most studies have explored relationships in post-menopausal women, but time since menopause varies considerably in different groups, and different studies have taken varying approaches to HRT, some excluding any women on such medication from the analysis while others have adjusted for drug history in the final analyses. One large study [13] that did explore relationships in pre-and post-menopausal women found very different relationships in the two groups supporting this hypothesis. Finally, most studies have failed to find an association between total cholesterol concentration and BMD [7,9,16,17] in agreement with our results. This included by far the largest investigation to date, which utilised the US NHANES data set of over 13,000 participants [19]. In this group, crude analyses revealed associations between total, HDL and LDL cholesterol that were removed after adjustment for body mass index. The participants of this study were sampled so as to reflect the distribution of racial/ethnic mix of the United States, and the authors comment that differences in this study compared to much smaller European studies may reflect modification of the potential relationship between lipids and BMD by genetic heritage.
There are numerous biological reasons to seek evidence of an association between bone density and lipid profile. Bone loss is associated with an expansion of adipose tissue in the marrow [25], and osteoblasts and adipocytes share a common progenitor arising from the stromal cells in the marrow [26]. Products of lipoprotein oxidation and an atherogenic diet inhibit preosteoblast differentiation [27] and result in reduced bone mineralisation [28]. Mutations in the low-density lipoprotein (LDL) receptor-related protein 5 (LRP5) [29] and apolipoprotein E genotype [30] are associated with alteration in bone mineralisation. Numerous drugs, including the aminobisphosphonates [5] and hormone replacement therapy [4] have been shown to influence both bone mass and lipid profile. Bone matrix proteins, including osteopontin [31], osteocalcin [32] and bone morphogenetic protein [33] have been found in atherosclerotic plaques.
Older women with low BMD have an increased risk of fracture, cardiovascular disease and mortality, and it has been suggested that estrogen deficiency may underlie this association. A recent paper from the Study of Osteoporotic Fractures found that a higher bone loss rate at the hip was associated with increased mortality from cardiovascular disease [34]. Hence our failure to find an association between baseline BMD and cardiovascular disease may reflect the cross-sectional nature of our study, and the lower age of the participants studied.
In conclusion, this study has found relationships between circulating lipid profile and adult bone mass in both sexes. These relationships were greatly attenuated by adjustment for total fat mass, supporting the idea that adiposity may confound the relationship between lipids and bone mass. These findings would be broadly in accord with US data recently published.
Acknowledgements
We thank the men and women who participated in the study, the General Practitioners who allowed their patients to be approached, and the nurses and radiology staff who administered the bone density measurements. Computing support was provided by Vanessa Cox. The study was funded by grants from the Medical Research Council and the Arthritis Rheumatism Campaign.
References
- 1.Johnell O, Kanis JA, Oden A, Johansson H, DeLaet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ, III, O'Neill T, Pols H, Reeve J. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–94. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 2.Von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106:273–278. doi: 10.1016/s0002-9343(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang PS, Solomon DH, Mogan H, Avorn J. HMG-CoA reductase inhibitors and the risk of hip fractures in elderly patients. JAMA. 2000;283:3211–3216. doi: 10.1001/jama.283.24.3211. [DOI] [PubMed] [Google Scholar]
- 4.Rossouw JW. Hormone replacement therapy and cardiovascular disease. Curr Opin Lipidol. 1999;10:429–434. doi: 10.1097/00041433-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Adami S, Braga V, Guidi GC, Gatti D, Gerardi D, Fracassi E. Chronic intravenous aminobisphosphonate therapy increases high-density lipoprotein cholesterol and decreases low-density lipoprotein cholesterol. J Bone Miner Res. 2000;15:559–604. doi: 10.1359/jbmr.2000.15.3.599. [DOI] [PubMed] [Google Scholar]
- 6.Adami S, Braga V, Zamboni M, et al. Relationship between lipids and bone mass in 2 cohorts of healthy men and women. Calcif Tissue Int. 2004;74:136–142. doi: 10.1007/s00223-003-0050-4. [DOI] [PubMed] [Google Scholar]
- 7.Samelson EJ, Cupples LA, Hannan MT, et al. Long-term effects of serum cholesterol on bone mineral density in women and men: the Framingham Osteoporosis Study. Bone. 2004;34:557–561. doi: 10.1016/j.bone.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Orozco P. Atherogenic lipid profile and elevated lipoprotein (a) are associated with lower bone mineral density in early postmenopausal overweight women. Eur J Epidemiol. 2004;19:1105–12. doi: 10.1007/s10654-004-1706-8. [DOI] [PubMed] [Google Scholar]
- 9.Wu LY, Yang TC, Kuo SW, et al. Correlation between bone mineral density and plasma lipids in Taiwan. Endocr Res. 2003;29:317–25. doi: 10.1081/erc-120025039. [DOI] [PubMed] [Google Scholar]
- 10.Adami S, Braga V, Gatti D. Association between bone mineral density and serum lipid in men. JAMA. 2001;286:791–792. doi: 10.1001/jama.286.7.791. [DOI] [PubMed] [Google Scholar]
- 11.Zabaglia SF, Pedro AO, Pinto Neto AM, Guarisi T, Paiva LH, Lane E. An exploratory study of the association between lipid profile and bone mineral density in menopausal women in a campinas reference hospital. Cad Saude Publica. 1998;14:779–786. doi: 10.1590/s0102-311x1998000400019. [DOI] [PubMed] [Google Scholar]
- 12.D'Amelio P, Pescarmona GP, Gariboldi A, Isaia GC. High density lipoproteins (HDL) in women with postmenopausal osteoporosis: a preliminary study. Menopause. 2001;8:429–432. doi: 10.1097/00042192-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Cui LH, Shin MH, Chung EK, et al. Association between bone mineral densities and serum lipid profiles of pre- and post-menopausal rural women in South Korea. Osteoporosis Int. 2005;16:1975–1981. doi: 10.1007/s00198-005-1977-2. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T, Sugimoto T, Yano S, et al. Plasma lipids and osteoporosis in postmenopausal women. Endocr J. 2002;49:211–217. doi: 10.1507/endocrj.49.211. [DOI] [PubMed] [Google Scholar]
- 15.Poli A, Bruschi F, Cesana B, Rossi M, Paoletti R, Crosignani PG. Plasma low-density lipoprotein cholesterol and bone mass densitometry in postmenopausal women. Obstet Gynecol. 2003;102:922–926. doi: 10.1016/j.obstetgynecol.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Castrillon JL, De Luis D, Martin-Escudero JC, Asensio T, del Amo R, Izaola O. Non-insulin-dependent diabetes, bone mineral density and cardiovascular risk factors. J Diabetes Complications. 2004;18:317–21. doi: 10.1016/S1056-8727(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 17.Tanko LB, Bagger YZ, Nielsen SB, Christiansen C. Does serum cholesterol contribute to vertebral bone loss in post-menopausal women? Bone. 2003;32:8–14. doi: 10.1016/s8756-3282(02)00918-3. [DOI] [PubMed] [Google Scholar]
- 18.Brownbill RA, Ilich JZ. Lipid profile and bone paradox: higher serum lipids are associated with higher bone mineral density in postmenopausal women. J Womens Health. 2006;15:261–70. doi: 10.1089/jwh.2006.15.261. [DOI] [PubMed] [Google Scholar]
- 19.Solomon DH, Avorn J, Canning CF, Wang PS. Lipid levels and bone mineral density. Am J Med. 2005;118:1414.e1–1414.e5. doi: 10.1016/j.amjmed.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Dennison EM, Syddall HE, Aihie Sayer A, Craighead S, Phillips DIW, Cooper C. Type 2 diabetes is associated with increased axial bone density in men and women from the Hertfordshire Cohort Study: evidence for an indirect effect of insulin secretion? Diabetologia. 2004;47:1963–1968. doi: 10.1007/s00125-004-1560-y. [DOI] [PubMed] [Google Scholar]
- 21.Heyden S, Bartel AG, Tabesh E, et al. Angina pectoris and the Rose questionnaire. Arch Intern Med. 1971;128:961–4. [PubMed] [Google Scholar]
- 22.Womersley J, Durnin JV. An experimental study on variability of measurement of skinfold thickness on young adults. Hum Biol. 1973;45:281–92. [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Sydall HE, Aihie Sayer A, Dennison EM, Martin HJ, Barker DJP, Cooper C. Cohort profile: the Hertfordshire Cohort Study. International Journal of Epidemiology. 2005 doi: 10.1093/ije/dyi127. (in press) [DOI] [PubMed] [Google Scholar]
- 25.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue: a quantitative study of 84 iliac bone biopsies. Clin Orthop. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Simmons DJ. The in vivo role of bone marrow fibroblast-like stromal cells. Calcif Tissue Int. 1996;58:129–132. doi: 10.1007/BF02526876. [DOI] [PubMed] [Google Scholar]
- 27.Diascro DD, Jr, Vogel RL, Johnson TE, et al. High fatty acid content in rabbit serum is responsible for the differentiation of osteoblasts into adipocyte-like cells. J Bone Miner Res. 1998;13:96–106. doi: 10.1359/jbmr.1998.13.1.96. [DOI] [PubMed] [Google Scholar]
- 28.Parhami F, Mody N, Gharavi N, Ballard AJ, Tintut Y, Demer LL. Role of the cholesterol biosynthetic pathway in osteoblastic differentiation of marrow stromal cells. J Bone Miner Res. 2002;17:1997–2003. doi: 10.1359/jbmr.2002.17.11.1997. [DOI] [PubMed] [Google Scholar]
- 29.Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerdes LU, Vestergaard P, Hermann AP, Mosekilde L. Regional and hormone-dependent effects of apolipoprotein E genotype on changes in bone mineral in perimenopausal women. J Bone Miner Res. 2001;10:1906–1916. doi: 10.1359/jbmr.2001.16.10.1906. [DOI] [PubMed] [Google Scholar]
- 31.Giachelli CM, Liaw L, Murry CE, Schwartz SM, Almeida M. Osteopontin expression in cardiovascular diseases. Ann N Y Acad Sci. 1995;760:109–26. doi: 10.1111/j.1749-6632.1995.tb44624.x. [DOI] [PubMed] [Google Scholar]
- 32.Fleet J, Hock J. Identification of osteocalcin in mRNA in nonosteoid tissue of rats and human by reverse transcription-polymerase chain reaction. J Bone Miner Res. 1994;9:1565–73. doi: 10.1002/jbmr.5650091009. [DOI] [PubMed] [Google Scholar]
- 33.Bostrom K. Insights into the mechanism of vascular calcification. Am J cardiol. 2001;88(2A):20E. doi: 10.1016/s0002-9149(01)01718-0. [DOI] [PubMed] [Google Scholar]
- 34.Kado DM, Browner WS, Blackwell T, Gore R, Cummings SR. rate of bone loss is associated with mortality in older women: a prospective study. J Bone Miner Res. 2000;15:1974–80. doi: 10.1359/jbmr.2000.15.10.1974. [DOI] [PubMed] [Google Scholar]