Abstract
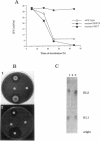
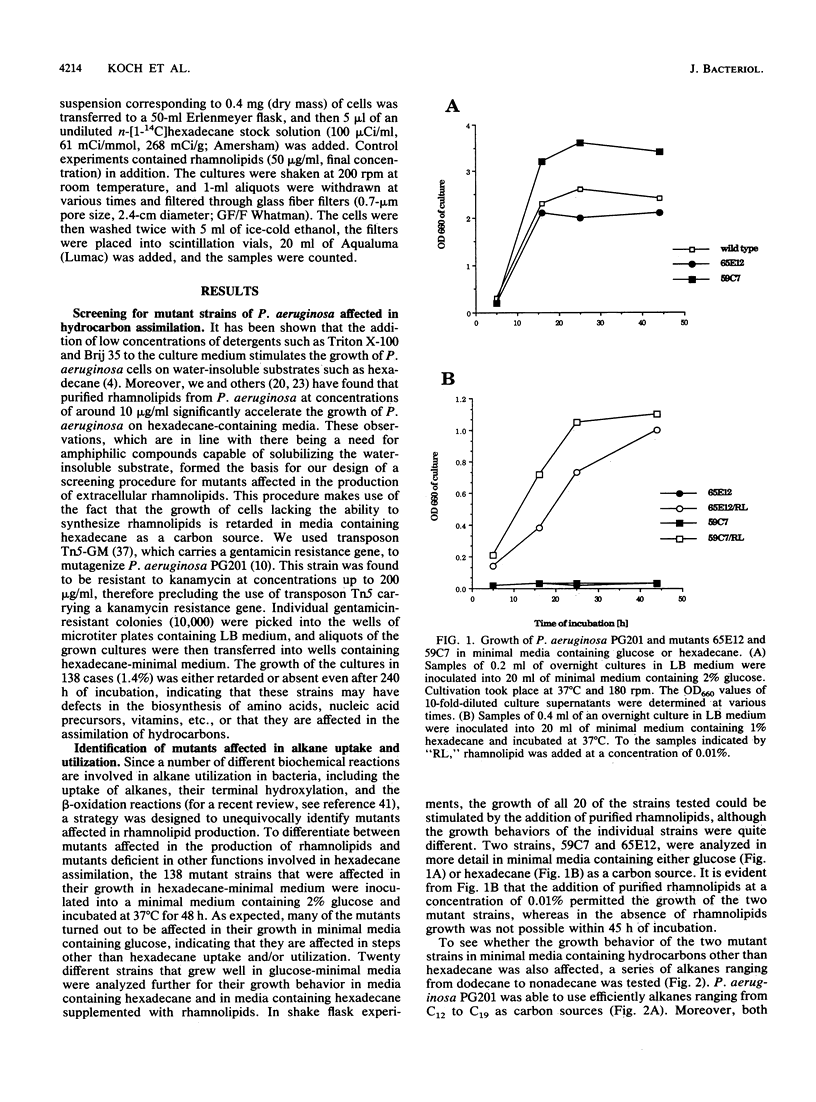
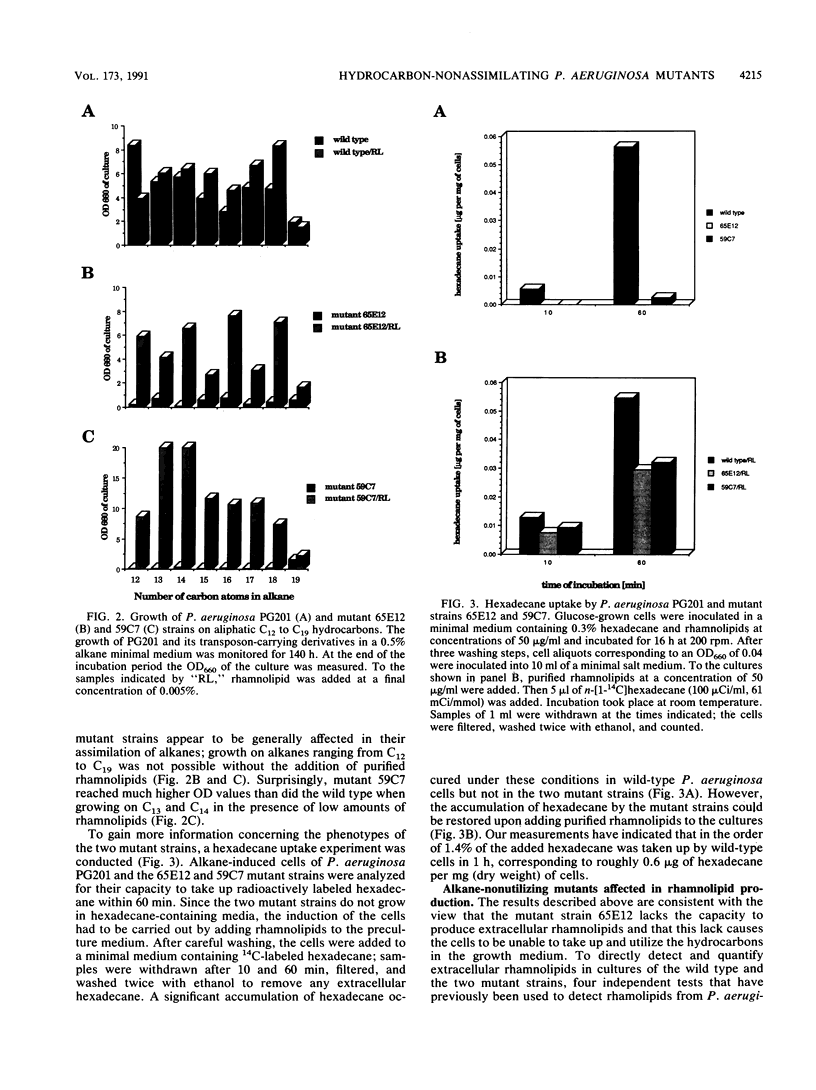
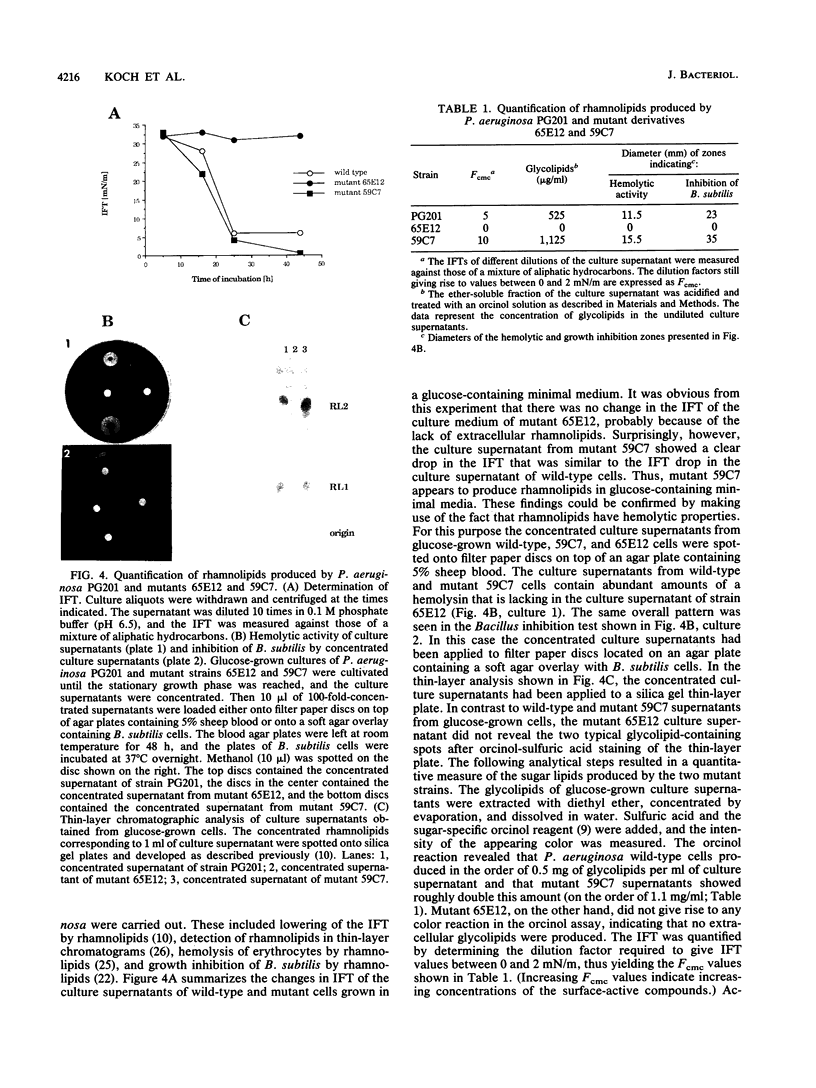
We isolated transposon Tn5-GM-induced mutants of Pseudomonas aeruginosa PG201 that were unable to grow in minimal media containing hexadecane as a carbon source. Some of these mutants lacked extracellular rhamnolipids, as shown by measuring the surface and interfacial tensions of the cell culture supernatants. Furthermore, the concentrated culture media of the mutant strains were tested for the presence of rhamnolipids by thin-layer chromatography and for rhamnolipid activities, including hemolysis and growth inhibition of Bacillus subtilis. Mutant 65E12 was unable to produce extracellular rhamnolipids under any of the conditions tested, lacked the capacity to take up 14C-labeled hexadecane, and did not grow in media containing individual alkanes with chain lengths ranging from C12 to C19. However, growth on these alkanes and uptake of [14C]hexadecane were restored when small amounts of purified rhamnolipids were added to the cultures. Mutant 59C7 was unable to grow in media containing hexadecane, nor was it able to take up [14C]hexadecane. The addition of small amounts of rhamnolipids restored growth on alkanes and [14C]hexadecane uptake. In glucose-containing media, however, mutant 59C7 produced rhamnolipids at levels about twice as high as those of the wild-type strain. These results show that rhamnolipids play a major role in hexadecane uptake and utilization by P. aeruginosa.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGER M. M., GLASER L., BURTON R. M. THE ENZYMATIC SYNTHESIS OF A RHAMNOSE-CONTAINING GLYCOLIPID BY EXTRACTS OF PSEUDOMONAS AERUGINOSA. J Biol Chem. 1963 Aug;238:2595–2602. [PubMed] [Google Scholar]
- Bar-Ness R., Avrahamy N., Matsuyama T., Rosenberg M. Increased cell surface hydrophobicity of a Serratia marcescens NS 38 mutant lacking wetting activity. J Bacteriol. 1988 Sep;170(9):4361–4364. doi: 10.1128/jb.170.9.4361-4364.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berka R. M., Gray G. L., Vasil M. L. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect Immun. 1981 Dec;34(3):1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuil C., Kushner D. J. Effects of lipids, fatty acids, and other detergents on bacterial utilization of hexadecane. Can J Microbiol. 1980 Feb;26(2):223–-31. doi: 10.1139/m80-034. [DOI] [PubMed] [Google Scholar]
- Bunster L., Fokkema N. J., Schippers B. Effect of Surface-Active Pseudomonas spp. on Leaf Wettability. Appl Environ Microbiol. 1989 Jun;55(6):1340–1345. doi: 10.1128/aem.55.6.1340-1345.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Santos L., Käppeli O., Fiechter A. Pseudomonas aeruginosa biosurfactant production in continuous culture with glucose as carbon source. Appl Environ Microbiol. 1984 Aug;48(2):301–305. doi: 10.1128/aem.48.2.301-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSER G., KARNOVSKY M. L. Rhamnose and rhamnolipide biosynthesis by Pseudomonas aeruginosa. J Biol Chem. 1957 Jan;224(1):91–105. [PubMed] [Google Scholar]
- HAUSER G., KARNOVSKY M. L. Studies on the biosynthesis of L-rhammose. J Biol Chem. 1958 Aug;233(2):287–291. [PubMed] [Google Scholar]
- HAUSER G., KARNOVSKY M. L. Studies on the production of glycolipide by Pseudomonas aeruginosa. J Bacteriol. 1954 Dec;68(6):645–654. doi: 10.1128/jb.68.6.645-654.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S., Elashvili I., Valdes J. J., Kamely D., Chakrabarty A. M. Enhanced removal of Exxon Valdez spilled oil from Alaskan gravel by a microbial surfactant. Biotechnology (N Y) 1990 Mar;8(3):228–230. doi: 10.1038/nbt0390-228. [DOI] [PubMed] [Google Scholar]
- Ito S., Honda H., Tomita F., Suzuki T. Rhamnolipids produced by Pseudomonas aeruginosa grown on n-paraffin (mixture of C 12 , C 13 and C 14 fractions). J Antibiot (Tokyo) 1971 Dec;24(12):855–859. doi: 10.7164/antibiotics.24.855. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Boese-Marrazzo D. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect Immun. 1980 Sep;29(3):1028–1033. doi: 10.1128/iai.29.3.1028-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Sogawa M., Yano I. Direct Colony Thin-Layer Chromatography and Rapid Characterization of Serratia marcescens Mutants Defective in Production of Wetting Agents. Appl Environ Microbiol. 1987 May;53(5):1186–1188. doi: 10.1128/aem.53.5.1186-1188.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan C. N., Gibbs B. F. Correlation of nitrogen metabolism with biosurfactant production by Pseudomonas aeruginosa. Appl Environ Microbiol. 1989 Nov;55(11):3016–3019. doi: 10.1128/aem.55.11.3016-3019.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Marahiel M. A., Zuber P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J Bacteriol. 1988 Dec;170(12):5662–5668. doi: 10.1128/jb.170.12.5662-5668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAZAKI R., OKAZAKIT, STROMINGER J. L., MICHELSON A. M. Thymidine diphosphate 4-keto-6-deoxy-d-glucose, an intermediate in thymidine diphosphate L-rhamnose synthesis in Escherichia coli strains. J Biol Chem. 1962 Oct;237:3014–3026. [PubMed] [Google Scholar]
- Reiling H. E., Thanei-Wyss U., Guerra-Santos L. H., Hirt R., Käppeli O., Fiechter A. Pilot plant production of rhamnolipid biosurfactant by Pseudomonas aeruginosa. Appl Environ Microbiol. 1986 May;51(5):985–989. doi: 10.1128/aem.51.5.985-989.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M. Bacterial adherence to polystyrene: a replica method of screening for bacterial hydrophobicity. Appl Environ Microbiol. 1981 Aug;42(2):375–377. doi: 10.1128/aem.42.2.375-377.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Rosenberg E. Role of adherence in growth of Acinetobacter calcoaceticus RAG-1 on hexadecane. J Bacteriol. 1981 Oct;148(1):51–57. doi: 10.1128/jb.148.1.51-57.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Yoshikawa M. A series of Tn5 variants with various drug-resistance markers and suicide vector for transposon mutagenesis. Gene. 1987;56(2-3):283–288. doi: 10.1016/0378-1119(87)90145-4. [DOI] [PubMed] [Google Scholar]
- Syldatk C., Lang S., Wagner F., Wray V., Witte L. Chemical and physical characterization of four interfacial-active rhamnolipids from Pseudomonas spec. DSM 2874 grown on n-alkanes. Z Naturforsch C. 1985 Jan-Feb;40(1-2):51–60. doi: 10.1515/znc-1985-1-212. [DOI] [PubMed] [Google Scholar]
- Walter M. V., Porteous A., Seidler R. J. Measuring genetic stability in bacteria of potential use in genetic engineering. Appl Environ Microbiol. 1987 Jan;53(1):105–109. doi: 10.1128/aem.53.1.105-109.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witholt B., de Smet M. J., Kingma J., van Beilen J. B., Kok M., Lageveen R. G., Eggink G. Bioconversions of aliphatic compounds by Pseudomonas oleovorans in multiphase bioreactors: background and economic potential. Trends Biotechnol. 1990 Feb;8(2):46–52. doi: 10.1016/0167-7799(90)90133-i. [DOI] [PubMed] [Google Scholar]