1. Introduction
1.1 Background
Patients with cough frequently present to clinicians working in both primary and secondary care.1,2 Acute cough, which often follows an upper respiratory tract infection, may be initially disruptive but is usually self‐limiting and rarely needs significant medical intervention. Chronic cough is often the key symptom of many important chronic respiratory diseases but may be the sole presenting feature of a number of extrapulmonary conditions, in particular upper airway and gastrointestinal disease. Even with a clear diagnosis, cough can be difficult to control and, for the patient, can be associated with impaired quality of life.3,4 Sessions dedicated to cough at respiratory meetings are popular, suggesting that the pathophysiology, evaluation, and successful treatment of cough remain topics of keen interest to many medical practitioners.
1.2 Need and purpose of BTS recommendations on the management of cough
The American College of Chest Physicians (ACCP) and the European Respiratory Society (ERS)5,6 have each endorsed their own set of guidelines on the management of cough; however, criticism7 of their content and breadth suggest the need for further concise recommendations. The British Thoracic Society guidelines cover not only chronic cough but also acute cough and the organisational issues of cough clinics. International differences in delivery of respiratory health care and management strategies support the notion that such guidelines would be desirable. The British Thoracic Society Standards of Care Committee agreed to the development of a Working Group tasked with the job of producing a set of guidelines for the management of cough with the following key objectives:
To produce guidelines that are relevant to the clinical management of cough in both primary and secondary care.
To produce a critical review of the available literature.
To highlight cough as a clinical and research area of considerable importance.
To encourage extended cooperation between clinicians, scientists, and the pharmaceutical industry with the core aim of developing effective cough therapies.
1.3 Structure of the guidelines
The guidelines are prefaced with the key points and recommendations summarised as a table of abstracted bullet points. The subsequent section begins with concise definitions for the key terms: cough, acute cough and chronic cough. Individual sections detailing guidelines for the management of acute and chronic cough with additional recommendations for specialist cough clinics follow. Each of these sections includes separate recommendations for management of cough in adults. The final section contains appendices which include a recommended cough management algorithm for adults (available online only at http://www.thoraxjnl.com/supplemental), together with a patient information sheet designed for primary care.
1.4 Methodology for generation of the guidelines
The members of the guideline group initially met to discuss content, format and purpose of the document and to consider the most appropriate methodology for the critical review of available literature and the generation of recommendations. Consensus was obtained on these points and members of the Guideline Group were allocated to one of three subgroups concerned with acute cough, chronic cough, or specialist cough clinics. These three clinical areas were further divided into sections and individuals were identified to conduct an independent literature search for each of these and to produce a discussion document based on their literature appraisal. The search engines recommended were Medline (1966 onwards), EMBASE, and the Cochrane Library database. These were applied to locate all English language studies relevant to the aetiology, diagnosis, severity staging, investigation, prognosis, complications, or treatment of chronic cough in adults over 16 years.
At a subsequent meeting of the Guideline Group these documents were presented, discussed, and recommendations agreed upon. The existing lack of evidence made the formulation of evidence based guidelines difficult. A striking example of this is that a search of the Cochrane Library database to 2005 for systematic reviews of treatment of cough in adults generated one article. Consequently, recommendations have been made based on the available reliability of evidence and, where indicated, on the clinical experience of the members of the Guideline Group.
Because of the generally poor level of evidence and the consequent arbitrary nature of the recommendations, a grading system was thought to be inappropriate.
Once the individual sections were complete, an initial document was drafted which was then circulated to the BTS Standards of Care Committee.
Summary of key points and recommendations
Introduction
Key points
Cough is a forced expulsive manoeuvre, usually against a closed glottis and which is associated with a characteristic sound.
Cough frequently presents as a troublesome symptom to clinicians working in both primary and secondary care.
Acute cough
Key points
Acute cough is defined as one lasting less than 3 weeks.
Acute cough is the commonest new presentation in primary care and is most commonly associated with viral upper respiratory tract infection.
In the absence of significant co‐morbidity, an acute cough is normally benign and self‐limiting.
It is the commonest symptom associated with acute exacerbations and hospitalisations with asthma and COPD.
The cost of acute cough to the UK economy is estimated to be at least £979 million. This comprises £875 million to loss of productivity and £104 million cost to the healthcare system and the purchase of non‐prescription medicines.
Recommendations
Indications for further investigation include haemoptysis, prominent systemic illness, suspicion of inhaled foreign body, suspicion of lung cancer.
Patients report benefit from various over‐the‐counter preparations; there is little evidence of a specific pharmacological effect.
Chronic cough
Key points
Chronic cough is defined as one lasting more than 8 weeks.
It is reported by 10–20% of adults, commoner in females and obese.
Cough accounts for 10% of respiratory referrals to secondary care.
Most patients present with a dry or minimally productive cough.
Decrement in quality of life is comparable with severe COPD.
The presence of significant sputum production usually indicates primary lung pathology.
In chronic cough a heightened cough reflex is the primary abnormality.
Clinical evaluation of chronic cough
Recommendations
A detailed history including a thorough occupational history should be performed in all patients.
Physical examination should concentrate on the afferent sites identified as most commonly associated with cough.
The evaluation of patients with chronic cough should include an assessment of health status and cough severity. Cough visual analogue scores are an alternative to cough specific quality of life questionnaires but are less well validated. (Audit)
Chest radiograph and spirometry are mandatory. (Audit)
Bronchial provocation testing should be performed in patients without a clinically obvious aetiology referred to a respiratory physician with chronic cough and normal spirometry.
Bronchoscopy should be undertaken in all patients with chronic cough in whom inhalation of a foreign body is suspected.
High resolution computed tomography may be of use in patients with chronic cough in whom other more targeted investigations are normal.
Optimal management should comprise a combination of diagnostic testing and treatment trials based on the most probable aggravant(s).
Treatment effects should be formally quantified. (Audit)
A recommended diagnostic algorithm for the evaluation of an adult with chronic cough is displayed in Appendix 2 (Parts 1 and 2) available online at http://www.thoraxjnl.com/supplemental.
Management of specific aggravants
Key point
Most cases of troublesome cough reflect the presence of an aggravant (asthma, drugs, environmental, gastro‐oesophageal reflux, upper airway pathology) in a susceptible individual.
Asthma/eosinophic bronchitis
Key points
Cough may be the only manifestation of these syndromes.
No currently available tests of airway function can reliably exclude a corticosteroid responsive cough.
Recommendation
Cough is unlikely to be due to eosinophilic airway inflammation if there is no response to a two week oral steroid trial. (Audit)
Drugs
Recommendation
No patient with a troublesome cough should continue on ACE inhibitors.
Environment
Key point
One of the commonest causes of persistent cough is smoking and appears to be dose related.
Recommendation
Smoking cessation should be encouraged as it is accompanied by significant remission in cough symptoms.
Gastro‐oesophageal reflux disease (GORD)
Key points
Failure to consider GORD as a cause for cough is a common reason for treatment failure.
Reflux associated cough may occur in the absence of gastrointestinal symptoms.
Recommendations
Intensive acid suppression with proton pump inhibitors and alginates should be undertaken for a minimum of 3 months. (Audit)
Antireflux therapy may be effective in treating cough in carefully selected cases.
Upper airway pathology
Key points
Rhinosinusitis is commonly associated with chronic cough.
There is an association between upper airway disease and cough but a poor association between the various symptoms and cough.
There is disparity in the reported efficacy of antihistamines.
Recommendations
In the presence of prominent upper airway symptoms a trial of topical corticosteroid is recommended.
Undiagnosed or idiopathic cough
Key points
Chronic cough should only be considered idiopathic following thorough assessment at a specialist cough clinic.
The clinical history of reflux cough is often present in patients with idiopathic cough.
A typical lymphocytic airways inflammation is seen in idiopathic cough.
Treatment of cough due to other common respiratory diseases
Key point
Cough can be a debilitating symptom in many common acute and chronic respiratory diseases.
Recommendation
Suppression may be relatively contraindicated especially when cough clearance is important.
Specialist cough clinics
Key points
A systematic approach to diagnosis and treatment remains the most effective way to manage chronic cough.
Important questions remain as to the complexity and cost effectiveness of existing diagnostic algorithms.
Recommendations
No single existing diagnostic protocol can be recommended.
A combination of selected diagnostic testing and empirical trials of treatment is likely to be most cost effective.
Referral to a specialist cough clinic should be encouraged and a directory of specialist centres should be made available.
Specialist investigations
Key point
Debate remains as to the interpretation and clinical utility of more complex investigations.
Bronchial provocation testing
Recommendations
Bronchial provocation testing should be performed in patients without a clinically obvious aetiology referred to a respiratory physician with chronic cough and normal spirometry.
A negative test excludes asthma but does not rule out a steroid responsive cough.
Oesophageal studies
Recommendations
Empirical treatment should be offered to patients with cough and typical reflux symptoms before oesophageal testing.
No current test of oesophageal function predicts treatment response.
Upper airway investigations
Recommendations
Examination of ear, nose and throat should be performed in preference to sinus imaging in patients suspected of having rhinosinusitis, but with persisting cough despite an adequate trial of treatment directed at the upper airway.
Specialist cough clinics should have access to fibreoptic laryngoscopy, preferably within the clinic setting.
Cough provocation testing
Recommendations
There is no current evidence to support the routine use of cough challenge testing in the management of chronic cough.
For research purposes, standardisation of methodology is required and accurate data on the distribution of cough responsiveness within the population are needed.
Measurement and monitoring of cough
Recommendations
Accurate measurement of cough helps determine cough severity, assess treatment efficacy, and may provide diagnostic information.
Ambulatory cough recording currently offers most promise in the objective assessment of cough, although further technical refinement is required if it is to be broadly accessible to physicians.
Assessing airway inflammation
Recommendations
The demonstration of sputum eosinophilia has important treatment implications and should be available in cough clinics.
Induced sputum should be requested after exclusion of the other common causes.
There is insufficient evidence to recommend the routine use of exhaled breath measurements in the clinical evaluation of chronic cough.
Potential new treatments for cough
Key point
There are no effective treatments controlling the cough response per se with an acceptable therapeutic ratio.
Recommendation
There is a need for multicentre clinical trials on new drugs carried out across specialist centres using objective methods of cough counting as well as subjective quality of life and symptom indexes.
1.5 Updating of recommendations
It is envisaged that the Executive Committee of the Guideline Group will meet every two years to review any new published evidence obtained from a subsequent structured literature search. An additional purpose of these update meetings will be to formulate key clinical and research priorities.
1.6 Audit
A number of quality indicators were chosen from recommendations made in this document against which the quality of management of cough could be measured. The key indicators were:
Chest radiography and spirometry are mandatory in the evaluation of chronic cough.
The severity of the cough should be quantified.
Treatment effects should be formally quantified.
Intensive acid suppression with proton pump inhibitors should be undertaken for a minimum of 2 months.
Decision to continue steroids made on the basis of a 2 week trial of oral corticosteroids.
2. Definitions
2.1. Cough
Debate exists as to the most appropriate clinical definition of a cough event.8 For the purposes of this document, the members of the Task Force agreed the following definition: “Cough is a forced expulsive manoeuvre, usually against a closed glottis and which is associated with a characteristic sound.”
2.2 Acute and chronic cough
Recommendations
Acute cough is defined as one lasting less than 3 weeks.
Chronic cough is defined as one lasting more than 8 weeks.
Classification of cough based on symptom duration is somewhat arbitrary. A cough lasting less than 3 weeks is termed acute and one lasting longer than 8 weeks is defined as chronic. Acute cough is usually a result of a viral upper respiratory tract infection as almost all such coughs resolve within this time period.9 A post‐infective cough may, however, persist for a considerable period of time. An upper respiratory tract infection (URTI) cough lingering for more than 3 weeks is usually termed “post‐viral cough”. The grey area between 3 and 8 weeks of cough is difficult to define aetiologically since all chronic cough will have started as an acute cough, but the clear diagnostic groups of chronic cough are diluted by those patients with post‐viral cough.
3. Acute cough
3.1 Epidemiology
Key points
Acute cough is the commonest new presentation in primary care.
It is most commonly associated with viral upper respiratory tract infection.
In the absence of significant co‐morbidity, it is normally benign and self‐limiting.
It is one of the commonest symptoms associated with acute exacerbations and hospitalisations with asthma and chronic obstructive pulmonary disease (COPD).
Acute cough is usually caused by a viral URTI but may arise from other aetiologies such as pneumonia or aspiration of a foreign body. The duration of a single episode of URTI associated cough varies but is rarely more than 2 weeks. A cut off of 2 months for chronic cough has been arbitrarily agreed in both American10 and European guidelines.6 The economic impact of acute cough may be usefully thought of in terms of a series of patient thresholds that trigger interventions such as the purchase of a cough medicine or consultation with a general practitioner (GP).
3.1.1 Incidence of URTI
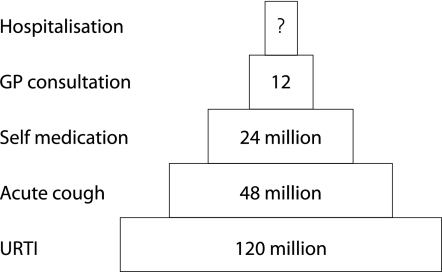
Symptomatic URTI occur at rates of 2–5 per adult person per year, with school children suffering 7–10 episodes per year.11 If one accepts the lowest rate of URTI of two episodes per person per year, then this translates into a conservative estimate of an incidence of 120 million episodes of URTI per year in the UK (fig 1).
Figure 1 Pyramid of incidence of acute cough. The base represents the population with an upper respiratory tract infection (URTI), some of whom will suffer from acute cough. Level 2 represents all those suffering from acute cough. Level 3 is the proportion of those suffering from acute cough who reach the threshold of severity of cough to trigger the purchase of a cough medicine. Level 4 is the proportion of those suffering from acute cough who reach the threshold of severity of cough to trigger a GP consultation. Level 5 is the proportion of those suffering from acute cough who are admitted to hospital. It is not possible to estimate the number of this latter group (see text).
3.1.2 Incidence of acute cough
Only a proportion of cases of URTI are associated with cough as a symptom. In naturally acquired URTI, cough was present in 40–50% of patients.12,13 This translates into an incidence of approximately 48 million cases of acute cough per year in the UK. The severity and duration of acute cough will vary widely but many will reach a threshold of severity that precipitates self‐medication.
3.1.3 Incidence of self‐medication
The sale of non‐prescription liquid cough medicines grossed £96.5 millions in 2001 in the UK.14 This sales figure is an underestimate of total sales as it is for sales from pharmacy and grocery outlets only, and does not include sales from outlets such as supermarkets and convenience stores. With cough medicines averaging £3–4 per unit, this represents at least 24 million episodes per year in the UK.
3.1.4 Consultations with a general practitioner
Morbidity statistics from general practice for the period 1991–2 reported that more people consulted for respiratory illnesses (31%) than for any other single disease category.15 With 20% of patients consulting for URTI, this translates into 12 million consultations per year, with acute cough representing “the largest single cause of consultation in primary care”.16
3.1.5 Hospital admissions
In normal subjects acute cough associated with URTI is not usually a cause of hospital admission. However, in patients with co‐morbidity such as asthma11 and COPD,17 viral URTI is the commonest cause of admission. Cough is a common symptom in this group of patients as well as those admitted to hospital for complications associated with infection with influenza or respiratory syncytial virus (RSV).
3.1.6 Sex differences
Between 16 and 64 years of age women are almost twice as likely as men to consult their GP for URTI,15 and this may relate to a sex difference in the sensitivity of the cough reflex.
3.1.7 Age
The incidence of URTI is much greater in infants and children than in adults. The GP consultation rate for URTI for the age group 0–4 years is about four times greater than the adult rate of consultation.15
3.1.8 Seasonality
Acute viral URTIs exhibit seasonality, and this causes seasonality in the incidence of acute cough and sales of cough medicines18 as well as hospital admissions with co‐morbidity. Cough is a common symptom associated with influenza and influenza‐like illness, with 93% of patients suffering from influenza having cough as a symptom.19 Influenza activity in the population shows a clear seasonality that usually peaks at the turn of the year around week 52.20 The seasonality of influenza‐like illness will contribute to seasonality of cough as a common seasonal symptom in the general population.
3.2 Economic impact of acute cough
Key point
The cost of acute cough to the UK economy is estimated to be at least £979 million. This comprises £875 million in loss of productivity and £104 million cost to the healthcare system and the purchase of non‐prescription medicines. More accurate estimates specific to the UK are required.
The economic cost of cough is a combination of at least the following six factors:
“on‐the‐job” productivity reduction;
absenteeism from work;
absenteeism due to care giving for others (mainly children) with URTI;
physician consultation cost;
prescription medication cost;
non‐prescription medication cost.
The economic burden of acute cough in the UK is not well characterised, so the figures quoted are extrapolations from US data where this subject appears to receive more attention.21,22 In the US it is estimated that $25 000 million is lost due to the common cold (excluding influenza‐related URTIs), of which $16 600 million is “on‐the‐job” productivity loss, $8000 million is due to absenteeism, and $230 million is due to caregiver absenteeism.
Assuming that the rate of viral URTI is the same in the UK as the US, adjustment for population differences (UK population estimates (2001) from www.statistics.gov.uk/census2000/profiles/uk.asp), US population estimates (2000) from www.census.gov) suggests a total loss of £3500 million, of which £2300 million is due to on‐the‐job productivity loss, £1100 million is due to absenteeism, and £32 million is due to care giving. Using UK figures which suggest up to 25% of URTI sufferers report cough as the main reason they consulted a healthcare professional, this translates into a loss of productivity of £875 million due to URTI associated cough.
The cost of medical consultation and non‐prescription treatment for acute cough is estimated to be at least £104 million.14
3.3 Management of acute cough
Recommendation
Indications for further investigation include haemoptysis, prominent systemic illness, suspicion of inhaled foreign body, suspicion of lung cancer.
3.3.1 General
In the large majority of cases, acute cough is unlikely to need any investigation. General advice may be sufficient and a patient information sheet (see Appendix 1) may be helpful.
3.3.2 Taking a history
At risk groups and danger signs
Although cough is very common and usually self‐limiting, it is sometimes the first indication of a serious condition (table 1).
Table 1 Common serious conditions presenting with isolated cough.
| • Neoplasm |
| • Infection, e.g. tuberculosis |
| • Foreign body inhalation |
| • Acute allergy – anaphylaxis |
| • Interstitial lung disease |
For most of these patients cough is not the only symptom and the presence of a number of others should prompt a chest radiograph (see tables 2 and 3). These features—as well as a history of foreign body inhalation—should always be ruled out by direct questions.
Table 2 Symptoms associated with acute cough prompting a chest radiograph.
| • Haemoptysis |
| • Breathlessness |
| • Fever |
| • Chest pain |
| • Weight loss |
Table 3 Causes of acute cough with a normal chest radiograph.
| • Viral respiratory tract infection |
| Respiratory syncytial virus |
| Rhinovirus |
| Influenza |
| Parainfluenza |
| Adenovirus |
| Respiratory corona virus |
| Metapneumovirus |
| • Bacterial infection (acute bronchitis) |
| • Inhaled foreign body |
| • Inhaled toxic fume |
Specialist referral for consideration of bronchoscopy is mandatory when there is a history of significant haemoptysis or possible foreign body inhalation. A change in the voice may indicate vocal cord palsy.
Acute cough with increasing breathlessness—while usually due to acute bronchitis—should be assessed for asthma or anaphylaxis and treated appropriately.
Acute cough with fever, malaise, purulent sputum, or history of recent infection should be assessed for possible serious acute lung infection.
3.3.3 Physical examination
At the outset of the common cold there may be clinical evidence of a rhinitis and pharyngitis with inflamed nasal mucosa and posterior pharynx with adherent or draining secretions. Inspection of the ears may reveal serious otitis. A computed tomographic (CT) study of the nasal passages and sinuses in the common cold has shown that widespread rhinosinusitis, which clears on resolution of the infection, is most typical.23
The findings on high resolution computed tomography (HRCT) scanning of the lung have been reported in a group of 76 young adults with a common cold.24 No important pulmonary changes were reported which is consistent with the normal findings usually reported on examination of the lower respiratory tract.
Acute cough is common in any patient presenting with pneumonia. Physical findings on examination of the chest are often very helpful and include dullness on percussion, bronchial breathing, and crackles on auscultation.
3.3.4 Treatment
Recommendations
Acute viral cough is almost invariably benign and prescribed treatment can be regarded as unnecessary.
Acute viral cough can be distressing and cause significant morbidity.
Patients report benefit from various over‐the‐counter preparations but there is little evidence of a specific pharmacological effect.
The simplest and cheapest advice may be to provide a “home remedy” such as honey and lemon.
Central modulation of the cough reflex is common; simple voluntary suppression of cough may be sufficient to reduce cough frequency.25
This may be the mechanism for the effect of simple drinks and linctuses.
Opiate antitussives have a significant adverse side effect profile and are not recommended.
Because of the variable and episodic nature of acute cough, little firm evidence has been obtained in clinical studies. Cough challenge methodologies have, however, shown suppression of the cough reflex and active agents include:
dextromethorphan;
menthol;
sedative antihistamines;
codeine or pholcodine.
Dextromethorphan
This non‐sedating opiate is a component of many over‐the‐counter cough remedies and has been shown to suppress acute cough in a single meta‐analysis.26 The generally recommended dosage is probably subtherapeutic. There is a dose response, and maximum cough reflex suppression occurs at 60 mg and can be prolonged.27 Care must be taken in recommending dextromethorphan at higher doses since some combined preparations contain other ingredients such as paracetamol.
Menthol
Menthol by inhalation suppresses the cough reflex28 and may be prescribed as menthol crystals BPC or in the form of proprietary capsules. Cough suppression is acute and short lived.
Sedative antihistamines
First generation antihistamines with sedative properties suppress cough but also cause drowsiness. They may be a suitable treatment for nocturnal cough.
Codeine or pholcodine
These opiate antitussives have no greater efficacy than dextromethorphan but have a much greater adverse side effect profile and are not recommended.
Currently available over‐the‐counter cough treatments which contain dextromethorphan and/or menthol are listed in table 4.
Table 4 Over‐the‐counter antitussive preparations contining dextromethorphan or menthol.
| Adult Meltus® Expectorant with Decongestant (guaifenesin, pseudoephedrine, menthol) |
| Benylin Chesty Coughs® Original (diphenhydramine, menthol) |
| Benylin Cough and Congestion® (dextromethorphan, diphenhydramine, menthol, pseudoephedrine) |
| Benylin Dry Cough® (dextromethorphan, diphenhydramine, menthol) |
| Benylin Non‐drowsy for Chesty Coughs® (guaifenesin, menthol) |
| Benylin Non‐drowsy for Dry Coughs® (dextromethorphan) |
| Buttercup Syrup Honey and Lemon Flavour® (ipecacuanha, menthol) |
| Cabdrivers® (dextromethorphan, menthol) |
| Covonia Bronchial Balsam® (dextromethorphan, menthol) |
| Covonia Mentholated Cough Mixture® (liquorice, menthol, squill) |
| Covonia Night Time Formula® (dextromethorphan, diphenhydramine) |
| Expulin® (chlorphenamine, menthol, pholcodine, pseudoephedrine) |
| Histalix® (ammonium chloride, diphenhydramine, menthol) |
| Junior Meltus Dry Cough® (dextromethorphan, pseudoephedrine) |
| Meltus Dry Cough® (dextromethorphan, pseudoephedrine) |
| Multi‐action Actifed Dry Coughs® (dextromethorphan, pseudoephedrine, triprolidine) |
| Night Nurse® (dextromethorphan, paracetamol, promethazine) |
| Nirolex for Dry Coughs with Decongestant® (dextromethorphan, pseudoephedrine) |
| Non‐Drowsy Sudafed Linctus® (dextromethorphan, pseudoephedrine) |
| Robitussin Dry Cough® (dextromethorphan) |
| Robitussin Soft Pastilles For Dry Cough® (dextromethorphan) |
| Vicks Medinite® (dextromethorphan, doxylamine, ephedrine, paracetamol) |
| Vicks Vaposyrup for Tickly Coughs® (menthol) |
| Vicks Vaposyrup Dry Cough® (dextromethorphan) |
4. Chronic cough
4.1 Epidemiology
In a European survey of young patients, which presumably included both acute and chronic cough, about 20% reported a non‐productive or productive cough during the winter months.29 In epidemiological surveys of the general population, persistent cough is reported in 18% of the US population, in up to 16% of a population in south‐east England, and in 11% of the Swedish population.30,31,32 The only study to grade cough severity found 7% of a general population had cough sufficient to interfere with activities of daily living on at least a weekly basis.33 A higher prevalence of nocturnal and non‐productive cough was reported in women than in men.34,35 Most studies show a preponderance of females. This may be related to the increased sensitivity of cough reflex in women.36,37 Cough is associated with a diagnosis of asthma, tobacco smoking in a dose related fashion, symptoms of reflux, irritable bowel syndrome, and obesity.33,38 In the survey in south‐east England, up to 16% of 9077 responders had cough every day on half the days of the year, and up to 13.2% had sputum every day or on half the days of the year; 54% of this cohort were current cigarette smokers.31
Exposure to pollutants or environmental irritants is an important aggravating factor. In adults and school children, productive cough or chronic nocturnal dry cough has been associated with levels of the particulates, PM10.39,40 Increases in levels of PM10 are related to increased reporting of cough, sputum production, and sore throat in children with or without asthma.41 Living close to heavy traffic may be associated with asthma symptoms and longstanding cough compared with those not living close to heavy traffic.42 In the Italian Po Valley district, the increase in air pollution has been associated with an increase in cough incidence among females but not males.43 Nocturnal cough in relation to indoor exposure to cat allergens was observed not only in sensitised but also in non‐sensitised subjects.44 There are no epidemiological data on the frequency of gastro‐oesophageal reflux and rhinosinusitis with postnasal drip associated with chronic cough.
4.2 Impact of cough on health status
Recommendations
Chronic cough has wide ranging and potentially profound effects of cough on health status.
The evaluation of a patient with chronic cough should include an assessment of health status in clinical practice and research.
The Leicester Cough Questionnaire is a well validated cough specific quality of life questionnaire that can be used to assess longitudinal changes in patients with chronic cough.
Cough visual analogue scores are an alternative to cough specific quality of life questionnaires but are less well validated.
4.2.1 Background
In acute cough, adverse effects on health status result from physical symptoms and are transient. In contrast, chronic cough is often perceived as a trivial problem but can be a disabling symptom associated with significantly impaired quality of life.3,4 The impact on health status is varied, being minimal in some patients who do not seek medical attention to disabling in others, associated with impairment of quality of life comparable to other chronic respiratory disorders such as chronic obstructive pulmonary disease.45 Physical, psychological, and social domains of health are commonly affected.3 Patients with chronic cough frequently report musculoskeletal chest pains, sleep disturbance, and hoarse voice. More marked symptoms such as blackouts, stress incontinence, and vomiting can occur. The psychological impact of cough includes a high prevalence of depressive symptoms and worry about serious underlying diseases such as cancer and tuberculosis.46 The impact of cough on social well being depends on individual circumstances and may result in difficulty in relationships, avoidance of public places, and disruption of employment.
Two recently developed self‐completed cough specific quality of life questionnaires for acute and chronic cough can be used to facilitate communication with patients and establish information on the range of problems affecting them.3,4 Both are well validated, repeatable, and have good responsiveness. The Leicester Cough Questionnaire (LCQ) is brief, easy to administer, and comprises 19 items divided into three domains: physical, psychological and social.3 The standard deviation of the 2 week repeatability within‐subject difference for the LCQ is 0.9 and a change of twice this is considered significant for an individual3(available at http://thorax.bmjjournals.com/cgi/content/full/58/4/339 – please seek permission from authors for use). The Cough Specific Quality of Life Questionnaire (CQLQ) is a 28‐item questionnaire that has been developed and tested in North America.4 The items are divided into six domains: physical complaints, extreme physical complaints, psychosocial issues, emotional well being, personal safety fears, and functional abilities. Studies to determine the minimal important clinical difference for both questionnaires are underway. Preliminary data suggest a good relationship between cough health status scores and cough visual analogue severity scores, but the relationship with cough diary scores has not been studied.3
Published evidence
Preliminary data from studies using cough specific quality of life questionnaires afford an insight into the effects of cough on health status. Quality of life is significantly impaired in acute cough; this impairment affects men and women equally.47 In patients with chronic cough, quality of life is impaired and is worse in women than in men.48 The psychological aspects of health status are particularly affected in patients with chronic cough.48,49 There is good evidence that health status improves significantly after specific treatment for the cough.3,4
4.3 Evaluation and management of chronic cough
4.3.1 Taking a history
There is little evidence in the existing literature to determine the best questions to ask when taking a history from a patient with chronic cough. The same is true for clinical examination. Much of what is currently done derives from consensus as a result of individual physicians' experience. The aim is to exclude structural disease as a cause for cough. Non‐specific associations also occur as a result of an abnormal cough reflex, itself associated with a number of factors. A detailed history will often suggest a likely association or trigger for chronic cough and should include a number of key components (table 5).
Table 5 Causes of chronic cough in patients with a normal chest radiograph.
| • Reflux disease |
| Gastro‐oesophageal reflux |
| Laryngopharyngeal reflux |
| Oesophageal dysmotility |
| • Asthma syndromes |
| Cough variant asthma |
| Eosinophilic bronchitis |
| • Rhinitis |
(a) Age and sex
Chronic cough is more likely to occur in middle aged women.
Published evidence
Observational studies have shown a significant female preponderance.50 The cough reflex is more sensitive in women with cough.37
(b) Smoking
One of the commonest causes of persistent cough is smoking, which appears to be dose related. Patients often state that their cough changes in character with smoking cessation.
Published evidence
The prevalence of chronic cough is increased in smokers.29 In a case‐control study of almost 2000 subjects, Jansen et al reported an increased prevalence of chronic cough among smokers.51 Smoking cessation leads to a short term increase in cough reflex sensitivity.52
(c) Characteristics of the cough
Interpretation of the diagnostic characteristics of the cough should be done with caution.
Published evidence
One study has suggested that the character and timing of a cough are not diagnostically helpful.53 However, others have suggested a characteristic pattern in reflux cough.54
Important areas of inquiry may establish that the cough is associated with frequent throat clearing or the sensation of post‐nasal drip, occurs mainly at night or after meals, or is made worse with exercise or cold air. However, the symptoms of post‐nasal drip in a patient may reflect only coexistent rhinitis and the absence of dyspepsia does not rule out reflux as the cause of cough. In one study the predictive values for cough characteristics and associated symptoms were calculated.55
A cough with a “honking” or “barking” quality and which disappears with sleep has been suggested as typical of a psychogenic or habit cough. Such characteristics have been frequently reported in the paediatric literature and may represent a tic cough.56
Consensus would suggest the following areas should be covered in the history in relation to the cough. It may be easier to ask the patients to complete a history questionnaire around which to structure the consultation (see Appendix 3 for suggested questionnaire framework).
(i) Onset
Did coughing begin gradually or suddenly?
Published evidence
Cough of sudden onset may be clinically associated with foreign body aspiration.
(ii) Duration
When did the cough start? How long have you had your cough?
Published evidence
There is no evidence linking the duration of cough to a particular association.
(iii) Relation to infection
Did coughing begin after an initial upper respiratory tract infection—for example, a cold or sore throat?
Published evidence
Although patients commonly describe their persistent cough as starting after an infection, there are no data specifically linking ongoing viral infection to persistent cough. Viral infection enhances the cough reflex sensitivity57 and may make subclinical bronchial hyperresponsiveness or reflux clinically apparent.
(iv) Sputum
Is the cough dry/productive?
Significant sputum production suggests primary pulmonary pathology
Published evidence
Numerous studies link chronic cough and sputum production. In a cross‐sectional study in 18 000 Italian adults there was a 11.9% prevalence of cough and phlegm for a minimum of 3 months per year.58 Primary pulmonary disease is more likely in patients attending a specialist cough clinic with a productive cough.
(v) Diurnal variation in cough
Patients cough less at night.
A cough which abates overnight may be due to reflux (closure of the lower oesophageal sphincter).
Asthma, infection, or heart failure can cause coughing which wakes patients.
Published evidence
Sleep is known to suppress the cough reflex. In a series of patients with lung disease and nocturnal cough, spontaneous cough was almost abolished during sleep stages 3 and 4.59
In an observational study in coughers, asthmatics and non‐coughers, ambulatory recordings have shown a marked reduction in cough overnight.60
(vi) Severe coughing spasms/paroxysms
Severe coughing paroxysms may be associated with syncope.
The Driver and Vehicle Licensing Agency (DVLA) stipulates that those at risk of syncope should not be driving.
Published evidence
(vii) Incontinence
Women with cough are often troubled by stress incontinence and this may be one of their major concerns.
Published evidence
In a questionnaire survey 55% of women reported urinary incontinence in association with chronic cough.54
(viii) Origin of sensation
It is unlikely to be of diagnostic value to enquire where the sensation that leads to cough arises.
Whatever the aetiology, the irritation leading to cough is usually localised to the throat or upper chest.
Published evidence
The site where cough sensations arise in the thorax tends to be poorly localised. One case study reported cough and tickly throat occurring during ventricular pacing.61,62 These C‐fibre sensations can be reproduced by systemic injections in both the throat and chest.
(ix) Cough triggers and aggravants
Persistent cough may be perpetuated because the cough reflex is abnormally sensitive.
A sensitised cough reflex is suggested if there is triggering of cough with change in air temperature, scent, sprays, aerosols, exercise.
Published evidence
Cough reflex sensitivity may vary from time to time, particularly when intercurrent respiratory infection occurs.57 Other known cough aggravants have been shown to alter the sensitivity of the cough reflex—for example, angiotensin converting enzyme (ACE) inhibitors63—and diseases including asthma and gastro‐oesophageal reflux disease (GORD).55 The effect of air temperature, scent, sprays, aerosols, and exercise has not been studied.
(x) Posture
Reflux is known to be related to posture, e.g. bending or lying down. However, there is no evidence demonstrating a connection between posture and reflux‐related cough.
Published evidence
See Bonnet et al.64
(xi) Food
Cough on eating and postprandially may indicate reflux cough.
Published evidence
Maximum stimulation for transient opening of the lower oesophageal sphincter is gastric distention which typically occurs 10 minutes postprandially.65 In a retrospective review of patients with proven reflux cough, three quarters had cough with food or postprandial cough.66
(xii) Cough on phonation
Cough on phonation such as talking on the telephone, laughing, or singing may indicate reflux because of lack of diaphragmatic closure of the lower oesophageal sphincter (LOS).
Published evidence
A retrospective review again showed that 90% of patients with reflux cough associated the symptom with phonation.66
(d) Medications
Note all medications, particularly ACE inhibitors, and consider which might be causing or potentiating the cough. The cough may take some months to settle following withdrawal of ACE inhibitors.
Published evidence
Cough associated with ACE inhibitors was first reported with captopril in 1985.67 It is a class effect, but the reported incidence variable may be as high as 16%. It is not dose related and time to onset is variable, occurring within hours or more than a year after start of treatment.68 Studies identifying predisposing factors for the development of cough associated with ACE inhibitors have been largely inconclusive. A recent large retrospective cohort study has identified smoking, East Asian ethnicity, and previous ACE inhibitor associated cough as risk factors.69 ACE inhibitors are associated with an increased sensitivity of the cough reflex, so they may aggravate cough due to other causes.63 The cough invariably resolves on cessation of the drug. The median time to resolution is 26 days although it may be longer (up to 40 weeks) in some patients.68,70 Most patients with ACE inhibitor associated cough can tolerate angiotensin II receptor blockers.71
There are only occasional reports of cough as a troublesome side effect of other drug treatments. One case report has attributed chronic cough to antiretroviral therapy in an HIV positive woman.72 Dry cough has also been reported as a rare complication of interferon alpha treatment in chronic viral gastroenteritis.73
(e) Occupation/hobbies/pets
A thorough occupational history should be sought as work place sensitisers can lead to chronic cough. The same is true of dust/chemical exposure at home.
Published evidence
Numerous studies and case reports provide accounts of persistent cough as a presenting feature of occupational sensitisation of the airways.74 Significant excess cough was reported in workers exposed to hot acidic conditions in a bottle factory75 and in workers exposed to hot chilli peppers.76
(f) Past medical history and the association of cough with underlying disease
(i) Respiratory disease
Chronic cough is a common association of respiratory diseases and a thorough respiratory history should be sought.
Persistent cough is commonly associated with asthma including eosinophilic bronchitis and upper airway disease. These will be detailed in subsequent sections of this document. Common respiratory diseases which are readily appreciated as being associated with chronic cough will be addressed below.
(ii) COPD
Although patients with COPD commonly report cough, it is usually in association with production of phlegm and breathlessness.
Published evidence
Smokers with persistent cough may be at risk of developing COPD (GOLD).77 A productive cough in patients with established airflow obstruction is predictive of lung function decline.
(iii) Bronchiectasis
Although usually associated with sputum production, “dry” bronchiectasis can cause persistent cough and a history of past respiratory insult as a potential trigger should be sought.
Published evidence
Patients with productive cough may have the same range of aetiologies as those with dry cough. The prevalence of bronchiectasis among patients attending specialist cough clinics is low, estimated at 4%.78
(iv) Lung cancer
Cough may arise as a consequence of the cancer itself, the treatment, or other co‐existent disease.
Published evidence
Cough is the fourth commonest presenting feature of lung cancer.79 Persistent cough contributes significantly to impaired quality of life. In one study, 39% of patients with inoperable non‐small cell lung cancer scored cough prominently.80
(v) Pertussis infection
Persistent pertussis infection can lead to chronic cough.
Published evidence
An increasing body of circumstantial evidence implicates pertussis as a cause of persistent cough. In a series of 180 prospective cases of chronic cough, 10% had nasal swabs positive for Bordetella.81 In a case‐control study of 201 patients with cough lasting up to 3 months, a significant increase in positive serology was reported for Bordetella in the patient group.82
(vi) Atopic disease
There is an increase in respiratory symptoms in atopic individuals.
Published evidence
In a longitudinal comparative study in 620 adults, atopy was associated with bronchial hyperresponsiveness in symptomatic patients.83 In Japan a separate syndrome of atopic cough is described,84 although whether this is indeed a separate syndrome has been called into doubt.85
(vii) Cardiovascular disease
Patients with heart disease can develop chronic cough and are at risk of myocardial infarction.
Published evidence
Analysis of the Framingham Heart Study data identified both chronic non‐productive cough and productive cough as independent risk factors for myocardial infarction.86
(viii) Organ‐specific autoimmune disease
An association between chronic cough and organ specific autoimmunity has been reported.
Published evidence
An association between organ‐specific autoimmune disease—in particular thyroid disease—has been reported.87 In a case‐control study, there was more autoimmune disease and a higher proportion of organ specific autoantibodies in patients with cough than in age and sex matched controls.87
(f) Family history
Chronic cough may be familial, suggesting either an inherited anatomical abnormality or neurological condition.
Published evidence
A kinship of autonomic sensory neuropathy prevalence with reflux cough (possibly vagal) followed by peripheral sensory neuropathy was recently described.88
4.3.2 Physical examination
Recommendation
Physical examination should concentrate on the afferent sites of the vagus nerve most commonly associated with the irritation leading to chronic cough.
The physical examination of the patient with chronic cough may demonstrate clinical signs of obstructive lung disease, lung cancer, bronchiectasis, pulmonary fibrosis, or cardiac failure. However, more often the examination reveals less specific findings.
Physical examination should concentrate on the afferent sites identified as most commonly associated with chronic cough.
An ear, nose and throat (ENT) examination may reveal evidence of nasal obstruction due to inflamed turbinates or the presence of polyps. The appearance of secretions draining in the posterior pharynx may be apparent. A “cobblestone” appearance of the oropharyngeal mucosa has been suggested but is an uncommon finding in the routine examination of patients with chronic cough.89 Tonsillar enlargement is seen in some patients with chronic cough. Tonsillectomy can improve cough reflex sensitivity.90
Evidence of irritation of the larynx and pharynx on indirect laryngoscopy could suggest proximal gastro‐oesophageal reflux.91
Examination of the chest is not useful in differentiating reversible airflow obstruction from fixed or partially reversible airflow limitation. Likewise, there are no features which easily distinguish cough variant asthma. Asking the patient to inhale may trigger paroxysms of coughing. Chest auscultation may reveal wheezes and a prolonged expiratory phase on auscultation. Coarse crackles may be a prominent finding on examination of a patient with bronchiectasis, while widespread fine late inspiratory crackles are typical of diffuse parenchymal lung disease.
The presence of finger clubbing in a smoker together with evidence of a pleural effusion or lobar collapse on examination almost certainly points to a diagnosis of bronchogenic carcinoma.
In patients with a family history of chronic cough, neurological examination of the legs should be performed to look for signs of familial neuropathy.88
4.3.3 Baseline investigations: primary care
Cough is one of the most common symptoms of patients presenting to primary care, yet there are few studies investigating its management. One descriptive study found that 46% of patients presenting with a cough of more than 2 weeks' duration (28% of whom had a cough of more than 3 months' duration) had a diagnosis of asthma or chronic obstructive pulmonary disease.92 This contrasts markedly with studies in secondary care and specialist cough clinics in which gastro‐oesophageal reflux, cough predominant asthma, and rhinitis are the main causes. A number of reviews outlining the diagnosis and management of chronic cough in primary care have been published.93,94,95 However, the evidence for these is predominately based on descriptive cohort studies and case studies/clinical experience from specialist centres.
(a) Chest radiography
Recommendations
A chest radiograph should be undertaken in all patients with chronic cough and those with acute cough demonstrating atypical symptoms (see table 2).
Published evidence
There are numerous causes of chronic cough, many of which can be diagnosed from abnormalities on the chest radiograph. A study from a general respiratory clinic found that 31% of chest radiographs requested for the diagnosis of persistent cough were abnormal or yielded a diagnosis.2 Studies using algorithms for the diagnosis of chronic cough have been validated in patients with normal chest radiographs.55,78,96,97,98,99,100 If the patient has an abnormality on the chest radiograph that would account for his/her symptoms, this should be investigated appropriately and use of a diagnostic algorithm for chronic cough is not appropriate.
(b) Assessment of pulmonary function
Recommendation
Spirometry should be performed in all patients with chronic cough.
Patients with normal spirometry and bronchodilator response in whom the diagnoses of cough predominant asthma or eosinophilic bronchitis are being considered should be offered a therapeutic trial of prednisolone.
Published evidence
Spirometry is helpful in identifying cough caused by chronic airways obstruction.101 If an obstructive pattern is identified on spirometry, forced expiratory volume in 1 second (FEV1) should be measured before and after inhalation of a short acting β2 agonist (for example, salbutamol 400 μg by metered dose inhaler and spacer or 2.5 mg by nebuliser).102 Normal spirometry does not exclude asthma as a cause of chronic cough. In addition, many patients with asthma may not have spirometric reversibility sufficient to be defined as having asthma for the purpose of clinical studies.103
Single peak expiratory flow (PEF) measurements and, in particular, PEF to assess bronchodilator response are not as accurate as FEV1 in diagnosing airflow obstruction as a cause of cough in primary care and should be avoided.104 The role of serial PEF has not been studied in patients with persistent cough. Patients with “cough variant” or “cough predominant” asthma may not exhibit airways obstruction.99,105 In addition, patients with cough due to eosinophilic bronchitis exhibit neither bronchial obstruction nor bronchial hyperresponsiveness.106 Patients in whom the diagnosis is in doubt should be referred to a specialist centre.107
4.3.4 Baseline investigations: secondary care
Studies from general respiratory clinics have reported poor diagnostic and treatment outcomes compared with specialist cough clinics that use comprehensive management algorithms.2,108,109,110 Extrapulmonary causes, particularly gastro‐oesophageal reflux, are frequently overlooked. The investigation of these conditions is dealt with under the specialist clinic section.
(a) Bronchoscopy
Recommendation
Bronchoscopy should be undertaken in all patients in whom inhalation of a foreign body is suspected.
Bronchoscopy may be useful in patients in whom other more targeted investigations are normal.
Published evidence
Bronchoscopy should be undertaken as an initial investigation in all patients suspected of having cough as a result of inhalation of a foreign body or aspiration. A retrospective study of 15 420 patients undergoing bronchoscopy without a history of inhalation of a foreign body, 91% of whom had a persistent cough and 63% of whom had normal chest radiographs, found that a foreign body was identified in only 0.3%.111
Descriptive studies have shown its diagnostic yield as part of a diagnostic algorithm for chronic cough is low (1–6%),78,98,100,112 although in carefully selected cases the yield may be higher.113,114 In addition, bronchoscopy allows inspection of the larynx for signs of chronic inflammation that may be a result of gastro‐oesophageal reflux.91,115
(b) High resolution computed tomographic (HRCT) scanning of the thorax
Recommendation
High resolution computed tomographic (HRCT) scanning may be of use in patients with persistent atypical cough in whom other more targeted investigations are normal.
Published evidence
The role of HRCT scanning of the thorax in the diagnosis of cough has not been properly evaluated. In a prospective study of patients with chronic cough and normal radiographs who had undergone a complex diagnostic protocol, HRCT scanning was claimed to be diagnostic in 24% of patients; however, multiple diagnoses were frequently reported in this study.100 In carefully selected patients the diagnostic rate may be higher.116,117 HRCT scanning is more sensitive and specific than plain chest radiography in diagnosing bronchiectasis and diffuse pulmonary diseases that may present with chronic cough. Studies have shown abnormalities on HRCT scans in up to 42% of patients thought to have had a normal chest radiograph.118,119,120
4.4 Diganosis and management of specific cough syndromes
4.4.1 Cough variant asthma and eosinophilic bronchitis
(a) Definition
An isolated cough in a patient without objective evidence of asthma—that is, variable airflow obstruction and evidence of eosinophilic inflammation. In cough variant asthma bronchial hyperresponsiveness is present, whereas in eosinophilic bronchitis it is absent.
These syndromes are a common cause of isolated cough, accounting for around 30% of cough referrals to cough clinics.50,121 Clinical indicators of cough variant asthma include cough occurring nocturnally, after exercise, or after allergen exposure, although how reliable these features are is unclear. Some studies have highlighted overdiagnosis of cough variant asthma in children.122
(b) Diagnosis of cough variant asthma
Recommendations
Current methodology for measurement of airway hyperresponsiveness is well standardised and widely accepted. A negative test excludes asthma but does not rule out a steroid responsive cough.
Published evidence
This requires the demonstration of variable airflow obstruction and/or airway hyperresponsiveness. In patients with normal or near normal spirometric values (that is, FEV1 >70% predicted), tests of airway responsiveness are more sensitive and specific than bronchodilator reversibility studies and PEF records.107,123
(c) Asthma syndromes and cough
Key points
The presence of non‐asthmatic corticosteroid responsive cough syndromes emphasises the importance of assessment of airway inflammation or, if this is not available, a trial of corticosteroids in all patients with chronic cough, irrespective of the results of tests of variable airflow obstruction and airway hyperresponsiveness.
The test box provides clear guidance on interpretation of the results of treatment trials.
The type of corticosteroid used in a trial and the duration of treatment is unclear; expert opinion is that cough is unlikely to be due to eosinophilic airway inflammation if there is no response to treatment with prednisolone 30 mg/day for 2 weeks.
In patients with apparently corticosteroid resistant cough variant asthma, an alternative diagnosis should be considered.
Published evidence
Eosinophilic bronchitis is a common cause of cough.124 It presents as an isolated chronic cough and is characterised by eosinophilic airway inflammation associated with increased Th2 cytokine expression125 in the absence of airway hyperresponsiveness or variable airflow obstruction. It is unclear whether eosinophilic bronchitis represents a distinct clinical entity. Some patients with cough and asthma have non‐eosinophilic airway inflammation.126 This pattern of airway inflammation has been associated with corticosteroid resistance;126,127 theoretically it might be associated with a bronchodilator responsive but corticosteroid resistant cough. The prevalence of non‐eosinophilic asthma in patients presenting with cough variant asthma is unclear.
(d) Management
Recommendation
Management of cough variant asthma should follow national guidelines, except at step 3 where there is no evidence for use of a long acting β agonist.
At step 3, evidence exists for the use of leukotriene receptor antagonists. Eosinophilic bronchitis and atopic cough respond to inhaled corticosteroids.
There is insufficient evidence to give guidance on dose, preparation, and duration of inhaled corticosteroid therapy but use of the BTS asthma guidelines is recommended.
There is some evidence to support the role of antihistamines and anti‐leukotrienes in cough due to asthma and its variants, but larger scale studies are required.
Published evidence
Cough variant asthma responds to treatment with corticosteroids.128 Leukotriene receptor antagonists have also been reported to be effective in reducing cough in this condition.129 High dose antihistamines have been shown to dramatically reduce cough in seasonal asthma130 but have not been specifically investigated in cough variant asthma. Eosinophilic bronchitis is characteristically resistant to treatment with inhaled bronchodilators but responds to inhaled steroids.131
Longitudinal studies have shown that up to one third of patients who present with cough variant asthma later develop the typical wheezing of classical asthma.84,132,133 In contrast, the development of wheezing or airway hyperresponsiveness is extremely uncommon in eosinophilic bronchitis.134
4.4.2 Gastro‐oesophageal reflux disease (GORD)
(a) Background
Chronic cough due to gastro‐oesophageal disorders has been reported in prospective studies in 5–41% of cases.97,100,110 Confusion between different diagnostic criteria, symptoms of dyspepsia, extra‐oesophageal reflux, and pH monitoring make quantification of cough due to GORD difficult.
Patients with GORD have an increased cough reflex sensitivity which improves with antireflux therapy.135,136,137 GORD related cough may be induced by microaspiration of gastric content into the larynx and tracheobronchial tree.138,139,140 A second proposed mechanism is through a vagally mediated oesophageal reflex stimulated by acid or non‐acid volume reflux.141,142,143 Oesophageal motor dysfunction144,145,146,147,148 and reduced oesophageal clearance149—but not delayed gastric emptying150—can contribute to both of these mechanisms. The presence of a self‐perpetuating cough‐reflux cycle has also been suggested.143,151
(b) Does antireflux therapy improve cough associated with GORD?
Recommendations
Proton pump inhibitors (PPIs) such as omeprazole 20–40 mg twice daily or equivalent taken before meals for at least 8 weeks.
Prokinetic agents such as metoclopramide 10 mg three times daily may be required in a proportion of patients.
Elimination of medications that potentially can worsen GORD should be considered.
Published evidence
A number of uncontrolled studies have reported that antireflux therapy produces an improvement in chronic reflux cough in 75–100% of cases.98,99,135 In contrast, randomised controlled trials suggest that proton pump inhibitors (PPIs) twice daily improve cough in 36–57% of patients with gastro‐oesophageal reflux related cough when given for 8 weeks.152,153 Ranitidine 300 mg daily for 2 weeks improved cough in 54% of patients.154 However, PPIs may be superior to H2 antagonists.99,155 Twice daily dosing and dosing before meals may be more appropriate.156,157 Full acid suppression may only be achieved by a combination of twice daily PPIs and nocturnal H2 antagonists.158 A trial of treatment should be at least 8 weeks.96,98,152,153,159 Prokinetic agents may be helpful in a proportion of patients.78,96,98,160 Elimination of medications potentially worsening reflux (bisphosphonates, nitrates, calcium channel blockers, theophylline, progesterones) may also help.160
The GABA agonist baclofen increases lower oesophageal tone and decreases lower oesophageal opening and, in an open study, decreased the incidence of GORD related cough resistant to other treatment.161 A non‐specific effect on the cough reflex has also been demonstrated.162
(c) What is the role of antireflux surgery?
Recommendation
Antireflux surgery may be effective in treating cough in carefully selected cases.
Published evidence
Some patients with GORD related cough may benefit from surgical intervention such as fundoplication.160,163,164 However, the timing and indications for surgery remain undefined. Prior to surgery a careful evaluation should be undertaken including exclusion of other causes of cough, oesophageal ambulatory 24 hour pH measurement (measuring acid and alkaline reflux and the temporal association between cough and reflux events), oesophageal manometry, barium meal, gastric emptying studies, Bernstein acid infusion tests, trial of PPI treatment, and elimination of medications potentially worsening reflux.160,164,165 Abnormal oesophageal motility may be associated with a less satisfactory outcome following surgery.145
4.4.3 Upper airway disease and cough
(a) Definition
Upper airway disease causes a cough commonly accompanied by nasal stuffiness, sinusitis, and the sensation of secretions draining into the posterior pharynx from the nose or sinuses, sometimes termed post‐nasal drip.
Key points
There is an association between upper airway disease and cough but a poor association between the various symptoms and cough.
There is disparity in the reported efficacy of antihistamines.
In the presence of prominent upper airway symptoms, a 1 month trial of topical corticosteroid is recommended.
Published evidence
Post‐nasal drip syndrome (PNDS) has been reported in the American literature as the most common cause of chronic cough,97,98,99 although this is not a universal finding.110 A broad range of diseases of the upper airway are associated with post‐nasal drip and cough. In contrast, many patients with observable post‐nasal secretion do not cough. Whether PNDS is a distinct syndrome or merely a symptom has been debated.166
Symptoms89 and clinical findings99 are not reliable discriminators in establishing post‐nasal drip upper airways disease as a cause of cough. A successful response to treatment directed at the upper airway is one recommended diagnostic approach.5
In the USA, recommended treatment involves a first line approach with a sedating antihistamine/decongestant combination.5 The first generation antihistamines recommended in this document are not available in the UK and there is conflicting evidence as to the efficacy of second generation (less sedating) antihistamines in the treatment of cough.167,168 There have been no randomised controlled studies evaluating the role of topical steroids in chronic cough, although one randomised placebo controlled trial has suggested that intranasal steroids given for 2 weeks are effective in the treatment of cough due to allergic rhinitis.169 Intranasal steroids appear to be ineffective in the treatment of common cold symptoms including cough.170 A number of prospective studies suggest that topical nasal steroids given for 2–8 weeks to patients with cough and post‐nasal drip are effective.55,171
4.4.4 Undiagnosed or idiopathic chronic cough
Key points
Chronic cough should only be considered idiopathic following thorough assessment at a specialist cough clinic.
The clinical history of reflux cough is often present in patients with idiopathic cough.
A typical lymphocytic airways inflammation is seen in idiopathic cough.
Published evidence
In up to 20% of referrals to cough clinics55,87,172,173 the cause of cough remains unclear after extensive investigations and treatment trials. It has been suggested that these patients represent a separate subgroup that should be labelled as idiopathic chronic cough. However, the clinical history usually suggests non‐acid reflux and opinion is divided as to whether, in the absence of a definitive diagnostic or therapeutic intervention, this represents the underlying aetiology. If reflux is the underlying cause, then the airway changes seen in these patients represent the response to the refluxate. The alternative view that there is a separate syndrome is discussed below.
Patients with idiopathic cough are predominantly middle aged women who typically present with a long standing chronic dry cough which starts around the time of the menopause87,172,174 and often appears to follow a viral respiratory tract infection.173 Organ‐specific autoimmune disease is present in up to 30%; autoimmune hypothyroidism is particularly common.87,172 Patients have objective evidence of abnormal airways with a heightened cough reflex,175 evidence of lymphocytic airway inflammation,87,174,176,177 increased numbers of mast cells in bronchoalveolar lavage fluid,178,179 and increased concentrations of tussive mediators such as histamine, prostaglandin (PG)D2 and PGE2 in induced sputum.180 A plausible explanation for the development of cough is amplification of previously subclinical airway inflammation at the time of the menopause.174,181,182 In some cases this airway inflammation may be as a result of aberrant homing of inflammatory cells to the lungs from a primary site of autoimmune inflammation.87,183,184,185
When evaluating a patient with idiopathic cough, it is important to recognise common pitfalls in managing chronic cough. Treatment for idiopathic chronic cough is disappointing and is largely limited to non‐specific antitussive therapy such as dextromethorphan and drugs with weak evidence of benefit such as baclofen and nebulised local anaesthetics (lidocaine, mepivicaine).186 Low dose morphine has recently been shown to be helpful.187
4.4.5 Treatment of cough due to other common respiratory diseases
Cough may be a prominent and debilitating symptom in a number of common respiratory diseases including lower respiratory tract infections (acute tracheobronchitis and pneumonia) COPD, lung cancer, diffuse parenchymal lung disease, and bronchiectasis.
Key points
Cough can be a debilitating symptom in many common acute and chronic respiratory diseases.
Suppression may be relatively contraindicated, especially when cough clearance is important.
Published evidence
In some conditions, in particular pneumonia and bronchiectasis, cough clearance is important and its suppression would be undesirable. The treatment of COPD is mainly directed at the control of symptoms and reduction of exacerbations, but no studies have evaluated the effectiveness of a particular treatment on the cough itself.188 The majority of lung cancer patients experience cough.189 Radiotherapy and both opioid and non‐opioid antitussives have been recommended (www.rcseng.ac.uk). Breathlessness is usually the most distressing symptom for patients with diffuse parenchymal lung disease. However, cough is frequently reported and can be debilitating;190 only limited information is available on its treatment.191 There are no randomised trials evaluating the benefit of treatment directed solely at cough. The treatment of diffuse parenchymal lung disease is outside the scope of this document and the reader is referred to the appropriate BTS guidelines on this topic (www.brit‐thoracic.org.uk).
5. Guidelines for specialist cough clinics
General recommendation
A systematic approach to diagnosis and treatment remains the most effective way to manage chronic cough. Important questions remain as to the complexity and cost effectiveness of existing diagnostic algorithms.
5.1 Introduction
The evaluation and management of cough in specialist clinics has been widely reported in the literature. Patients attending specialist cough clinics generally comprise non‐smokers with a female preponderance of approximately 2:1.50 They have often had a combination of baseline investigations and trials of empirical treatment before referral.
Studies in the primary literature from specialist cough clinics consist mainly of descriptive cohort studies and reports of clinical experience from centres with recognised expertise in cough evaluation and management. There have been no comparative studies of diagnostic methodology within or between specialty clinics. The recommendations for specialist clinics in this document will therefore comprise a review of the published evidence and the clinical experience of the Guideline Development Group.
5.2 Do specialist cough clinics offer superior diagnostic/management outcomes?
Recommendations
All clinics managing patients with chronic cough should ensure management protocols consider pulmonary and extrapulmonary causes of cough.
Published evidence
Three studies have reported poor diagnostic and treatment outcomes in hospital based clinics where no established management algorithm for cough existed.2,108,109 The experience in such clinics markedly contrasts with the generally high treatment success attributed to the specialist approach.55,98,99,171,192 In non‐specialist clinics extrapulmonary causes, particularly GORD, appears to be overlooked.
5.2.1 Comparison of specialist cough clinic protocols and outcomes
Recommendation
Specialist protocols should continue to evaluate pulmonary and extrapulmonary causes for cough. Comparative studies of cough algorithms are required. No single existing diagnostic protocol can be recommended. A combination of therapeutic trials and targeted investigation is recommended when diagnostic doubt exists.
Published evidence
No direct comparisons of management protocols between specialty clinics have been published. However, treatment success reported from specialist clinics ranges from 68% to 100%.55,97,98,99,100,171,192 Thus, despite the specialist evaluation of cough, a significant number of patients remain undiagnosed. It is not clear whether this variance reflects differences in referral population.
5.2.2 Cost effectiveness of diagnostic cough algorithms employed by specialist cough clinics
Recommendation
A combination approach of selected diagnostic testing and empirical trials of treatment is likely to be most cost effective.
Published evidence
Algorithms for cough evaluation typically used in specialist clinics range from sequential trials of empirical treatment99 to exhaustive diagnostic testing in all cases before any trial of treatment.100 Only one study has explored the cost efficacy of such diagnostic cough algorithms.193 The “investigate all then treat” approach was the most expensive, but with the shortest time to success compared with sequential trials of empirical treatment.
5.2.3 Is there a specific role for specialist cough clinics and when to refer?
Recommendation
Referral to a specialist cough clinic should be encouraged when there has been a failure of empirical treatment.
A directory of specialist centres should be made available.
The specialist cough clinics from Europe,55,124,148,194 Asia‐Pacific,84,192,195 and the Americas98,100 broadly report successful outcomes when comprehensive diagnostic protocols are implemented. It would be desirable if all physicians were able to refer to a specialist cough clinic. Advice on how to set up a specialist cough clinic is given in Appendix 4. Appropriate referral criteria are:
lack of availability of relevant diagnostic testing in primary or secondary care;
failed trials of empirical treatment directed at asthma, GORD, and rhinosinusitis;
a history suggestive of serious cough complication such as syncope or chest wall trauma;
patient preference; and
recruitment and participation in clinical trials of antitussive therapy.
5.3 Specialist investigations
5.3.1 Background
Mandatory investigations in patients with chronic cough are chest radiography and spirometry. This section will deal with more complex diagnostic tests where the interpretation remains open to debate, tests with largely research implications, and new innovations.
5.3.2 Bronchial provocation testing
Key points
Current methodology for measurement of airway hyperresponsiveness is standardised and widely accepted. A negative test excludes asthma but does not rule out a steroid responsive cough.
Recommendations
Bronchial provocation testing should be performed in patients without a clinically obvious aetiology referred to a respiratory physician with chronic cough and normal spirometric values.
Published evidence
Most of the published accounts from specialist cough clinics have described their experience with bronchial provocation testing. The methods of measurement of airway hyperresponsiveness have been well standardised. In cough clinics, direct methods using methacholine or histamine are most commonly employed,55,98,171 although indirect methods have been described.196 There is broad agreement between cough centres that a positive test is suggestive of asthma and should prompt treatment with inhaled steroids.55,98,192 The positive predictive value of this test ranges from 78% to 88%.55,98 While a negative test in a patient with cough rules out asthma, it does not eliminate a cough which may respond to steroids. A number of independent centres have reported steroid responsive cough in patients with no evidence of airway hyperresponsiveness.124,195,196
Extrathoracic airway responsiveness can be assessed by recording the maximal inspiratory flow/volume curve during conventional bronchial challenge testing. Three groups have used this method in the assessment of cough.192,197,198 There is no wide agreement as to the interpretation of this test.
5.3.3 Oesophageal testing
Key point
Failure to consider GORD as a cause of cough is a common reason for treatment failure.
Recommendation
Empirical treatment should be offered to patients with cough and typical reflux symptoms before oesophageal testing.
24 hour pH monitoring poorly predicts the therapeutic response but may be indicated in cases of diagnostic doubt and in patients thought to require fundoplication.
Published evidence
Objective investigation for GORD—including barium studies,96,100 upper gastrointestinal endoscopy,141 and ambulatory oesophageal pH testing55,84,98,100,141,149—have been described. Ambulatory oesophageal pH monitoring is often regarded as the most sensitive and specific investigation for the diagnosis of GORD. A long term follow up study (median 30 months) has recently reported that less than 30% of patients with a “positive” oesophageal pH study respond to antireflux therapy, and no features on pH monitoring accurately predict the response.199 One study has described a high prevalence of motility disorders in cough patients using oesophageal manometry testing.148 As cough may arise as a consequence of non‐acid reflux, impedance testing may offer new insights into GORD related cough.200 No published reports of its application in cough currently exist. One study has advocated the use of empirical therapy in place of oesophageal testing.152
5.3.4 Sinus imaging
Key point
Rhinosinusitis is commonly associated with chronic cough.
Recommendation
Examination of ear, nose and throat should be performed in preference to sinus imaging in patients suspected of having rhinosinusitis but with persisting cough, despite an adequate trial of treatment directed at the upper airway.
Published evidence
Existing cough guidelines make few recommendations on the role of sinus imaging, preferring to observe the response to a course of specific treatment for nasal disease.10 In selected patients (chronic cough and excess sputum production) a sinus radiograph has a reported positive predictive value of 81% and negative predictive value of 95%.78 However, sinus radiographs are less sensitive than CT imaging of the sinuses.201 In a prospective study, routine CT sinus scanning was no better than an ENT examination in accurately identifying upper airway disease as a cause of the cough.55
5.3.5 Fibreoptic laryngoscopy
Recommendation
Specialist cough clinics should have access to fibreoptic laryngoscopy, preferably within the clinic setting.
Published evidence
Pernasal fibreoptic laryngoscopy provides a quick and simple method of viewing the laryngeal apparatus without sedation. The presence of laryngopharyngeal reflux may be determined by the characteristic changes associated with laryngeal inflammation and oedema.202 These include pseudosulcus (subglottic oedema), obliteration of the laryngeal ventricle, erythema of the arytenoids, oedema of the posterior laryngeal wall, and laryngeal mucus.
5.3.6 Cough provocation testing
Recommendations
There is no current evidence to support the routine use of cough challenge testing in the management of chronic cough.
For research purposes, standardisation of methodology is required and accurate data on the distribution of cough responsiveness within the population are needed.
Published evidence
A variety of methods to measure cough reflex sensitivity have been described in the specialist cough clinic setting. These include tidal breathing challenge with low chloride solutions, and single breath challenges with capsaicin55,171,203 and citric acid.37 Although safe and relatively simple to perform, a review of cough provocation testing has highlighted the need for consensus on methodology.204
Unlike bronchial hyperresponsiveness, cough challenge reveals a wide range of normal cough reflex sensitivity. Cough provocation testing therefore has no clear diagnostic applications and is likely to be confined to the clinical research of cough.
5.4 Measurement and monitoring of cough
Key points
Accurate measurement of cough helps determine cough severity, assess treatment efficacy, and may provide diagnostic information.
Ambulatory cough recording currently offers most promise in the objective assessment of cough, although further technical refinement is required if it is to be broadly accessible to physicians.
Published evidence
A number of methods to measure cough frequency, intensity and severity have been described. Visual analogue scales and self‐report cough diary cards have been used but do not consistently correlate with objective methods such as ambulatory cough monitoring.205 The use of a series of different ambulatory cough recording monitors has been reported in both adult60,206 and paediatric207,208,209 literature. Although some technical limitations currently exist, they offer the best objective means of recording cough. Differences in the characteristics of the cough sound and flow pattern between asthma, bronchitis, and interstitial fibrosis have been reported.210 Recently, analysis of overnight cough recording determined differences in character and intensity of cough sounds between patients with cystic fibrosis and those with cryptogenic fibrosing alveolitis.211 These observations open the diagnostic possibilities for cough monitoring.
5.5 Assessing airway inflammation
5.5.1 Induced sputum
Recommendations
The demonstration of sputum eosinophilia has important treatment implications and should be available in cough clinics.
Induced sputum should be requested after exclusion of other common causes.
Published evidence
A number of independent groups have adapted conventional diagnostic strategies for chronic cough to include induced sputum.84,124,195 The demonstration of airway eosinophilia (>3% sputum eosinophil count) in patients without the functional abnormalities (particularly bronchial hyperreactivity) associated with asthma has helped define eosinophilic bronchitis as a distinct cause for chronic cough. Eosinophilic bronchitis may account for up to 15% of cases of cough referred for specialist attention,124 although debate remains as to whether eosinophilic bronchitis exists as a separate diagnostic entity.212
5.5.2 Exhaled breath
Recommendations
There is insufficient evidence to recommend the routine use of exhaled breath measurements in the clinical evaluation of chronic cough.
Published evidence
Exhaled nitric oxide (NO) levels appear to be lower in non‐asthmatic coughers, allowing some differentiation from asthmatic patients with cough.213 Exhaled NO may represent a simpler alternative to induced sputum tests but currently it has no clear diagnostic role in the management of chronic cough. An increase in nitrite levels has been reported in exhaled breath condensate from asthmatic children with cough but not from non‐asthmatic children with cough.214 Measurement of many different inflammatory molecules in breath condensate, although currently a research procedure, may have a place in the future diagnosis of chronic cough.
5.6 Recommended diagnostic protocol (see Appendix 2, Parts 1 and 2)
The evaluation and management of cough in an adult should comprise two phases. The approach suggested in phase 1 is applicable to all physicians (primary and secondary care) encountering the patient for the first time. Treatment failure should prompt phase 2 of the evaluation algorithm. The algorithm is available online only at http://www.thoraxjnl.com/supplemental.
6. Potential new treatments for cough
Recommendations
There is an urgent need for multicentre phase II trials on new drugs carried out across specialist centres using objective methods of cough counting as well as subjective quality of life and symptom indices.
6.1 Background
Chronic cough is associated with many inflammatory airways diseases such as asthma, COPD, post‐viral infections, pulmonary fibrosis, and bronchiectasis.10 In some cases certain drugs can be used to inhibit the underlying inflammatory process that, under certain conditions, cause cough—for example, corticosteroids for the treatment of asthma or COPD, or PPIs as treatment for gastro‐oesophageal reflux. However, there are patients who cough who do not respond to treatments directed at the cause of the cough, and there are patients in whom there is no identifiable cause to treat. Therefore, there is also a requirement to develop compounds that are targeted to inhibit sensory nerve activity directly (by inhibition of peripheral or central mechanisms), which should in theory inhibit cough of any aetiology.
6.2 New treatments under investigation
6.2.1 Opioids
Attempts have been made to improve the therapeutic index by topical administration of a peripherally acting polar enkephalin analogue, BW443C81, which was shown to inhibit citric acid induced cough in guinea pigs.215 However, in humans there was no effect on capsaicin induced cough in normal volunteers.215 A novel opioid peptide, nociceptin, which binds to the opioid receptor‐like 1 receptor (NOP) has been shown to suppress capsaicin induced cough in guinea pigs and mechanically induced cough in the cat, but so far no data exist in humans.216,217
6.2.2 Neurokinin receptor (NK) antagonists
The NK2 receptor antagonist SR 48968 has been shown to inhibit citric acid induced cough in conscious guinea pigs,218,219 and an antitussive effect of NK1 receptor antagonists is still under debate. Although there is a report suggesting an antitussive effect of a dual NK1/NK2 receptor antagonist (FK224) on bradykinin induced cough in asthmatics,220 other studies have failed to demonstrate any antitussive action of compounds of this type.221 Recent data have implicated a role for NK3 receptor activation in evoking a tussive response possibly via a peripheral mechanism of action,222,223 even though there have been no reports of the presence of functional NK3 receptor antagonists in the human lung.
6.2.3 Gamma‐aminobutyric acid (GABAB) receptor agonists
GABAB agonists (such as baclofen) have been shown to inhibit capsaicin induced cough in the conscious guinea pig224,225 and in normal volunteers,226 and provided some benefit in patients with chronic cough.227
6.2.4 Cannabinoid CB2 receptor agonists
CB2 receptor agonists inhibit guinea pig and human sensory nerve activation in vitro and the cough reflex in guinea pigs, which suggests that the development of CB2 agonists, devoid of CB1 mediated central effects, will provide a new and safe antitussive treatment for chronic cough.228 No clinical data exist in humans.
6.2.5 Local anaesthetics
Local anaesthetics such as lignocaine are delivered locally to the airways and have been shown to attenuate capsaicin induced cough in man.229 However, the effect is transient and the antitussive effect is accompanied by oropharyngeal anaesthesia leading to an increased risk of aspiration of airway secretions and food.
6.2.6 Transient receptor potential (TRP) channels
The cold and menthol sensitive receptor (CMR1) has recently been characterised and cloned.230 Interestingly, menthol has been proposed as an antitussive therapy and has been shown to inhibit citric acid induced cough in normal volunteers.28 The heat sensitive channel TRPV1 is activated by capsaicin, the main pungent ingredient in hot chilli peppers,231,232 and capsazepine, a blocker of this channel, inhibits capsaicin and citric acid induced cough in the guinea pig.233 An increase in epithelial nerve profiles expressing TRPV1 has been reported in patients with non‐asthmatic chronic cough.234 Compounds of this type are currently in clinical development.
6.2.7 Potassium channel openers
NS1619, an opener of large conductance calcium activated potassium (BKCa) channels, has been shown to inhibit sensory nerve function and cough induced by citric acid in the guinea pig.235 ATP sensitive potassium channels may also be a good target.
6.3 Conclusions
Treatment of the causes of cough can often be an effective treatment strategy. However, at the moment there are no effective treatments controlling the cough response per se with an acceptable therapeutic ratio. The future looks promising with several novel mechanisms identified; however, most of these studies have been carried out in animal models and these may not be predictive of effects in man as evidenced by the compound attrition rate from preclinical to clinical studies of antitussives tested in the past. Furthermore, there have been no large scale clinical trials of antitussive drugs as most of the studies illustrated have investigated drug efficacy in simple capsaicin challenge protocols in normal volunteers. There is therefore an urgent need for multicentre phase II trials of new drugs carried out across specialist centres using objective methods of cough counting as well as subjective quality of life and symptom indices in these patients with chronic cough.
7. Research directions
Determining the best methodology for investigation of antitussive therapy.
Simple diagnostic test, particularly for gastro‐oesophageal reflux.
Causes of familial cough (genetic basis?).
Relationship between cough/reflux/asthma.
Fundoplication versus medical treatment.
The algorithm for the evaluation of chronic cough in adults is shown in Appendix 2 (Parts 1 and 2) available online at http://www.thoraxjnl.com/supplemental.
Copyright © 2006 BMJ Publishing Group and British Thoracic Society
Supplementary Material
Appendix 1 Patient Information Sheet
Appendix 2 Protocol for the evaluation of chronic cough in an adult
Appendix 2 Parts 1 and 2 is available online only at http://www.thoraxjnl.com/supplemental
Appendix 3 Cough assessment questionnaire
Appendix 4 Setting up a specialist cough clinic service
Why set up a cough clinic service?
A specialist cough clinic service offers a number of distinct advantages
Improved patient outcomes: treatment success is considerably higher for patients managed in a specialist cough clinic than in general respiratory clinics.
Avoidance of inappropriate prescribing: diagnostic uncertainty often leads to inappropriate use of antibiotics and inhaled corticosteroids.
Training: specialist cough clinics provide an environment for training and skill development for physicians (often specialist registrars in respiratory training programmes), pulmonary function technicians, and respiratory nurse specialists.
Clinical research: an improved understanding of the pathophysiology of cough and need to develop and evaluate new cough treatments requires the collaboration of clinicians, scientists, and the pharmaceutical industry. Specialist cough clinics ensure the accurate characterisation of patients with cough and provide opportunities for trusts with an interest in clinical research and pharmaceutical trial participation.
Where to set up a cough clinic service?
A specialist cough clinic should provide a combination of diagnostic testing and treatment trials. Although specialist cough clinics have generally been set up in secondary care, they could be developed within a Primary Care Trust. There are no comparisons of treatment outcome or cost to recommend one or other.
Core requirements
A named consultant or GP should have responsibility for the service.
All staff should be provided with training appropriate to their role in providing care.
To adequately supervise trials of treatment including assessment of cough severity (visual analogue scales and quality of life questionnaires).
Pulmonary function testing with spirometry as a minimum requirement.
Access to chest radiography and bronchial provocation challenge testing (methacholine inhalation challenge testing).
Facility to refer for oesophageal testing in appropriate circumstances.
Ear, nose and throat (ENT) assessment either on site (facility for direct laryngoscopy) or direct access to ENT clinic.
Access to bronchoscopy and chest CT scanning in appropriate circumstances.
The outcomes of the service should be subject to regular review.
Desirable requirements
Facility to obtain and analyse induced sputum samples.
Cough provocation testing.
Cost implications
Capital costs
Essential items: spirometer (£200–2500)
Non‐essential: flexible laryngoscope (approximately £7000); cough provocation testing, dosimeter and nebuliser (approximately £4000).
Recurring costs
Staff costs should include physician, pulmonary function technician, nurse specialist, and clerical time.
Consumables—for example, methacholine challenge testing (approximately £50 per test).
Footnotes
Contributors: Professor M Belvisi, National Heart & Lung Institute, London, UK; Dr S S Birring, King's College Hospital, London, UK; Professor R Eccles, Cardiff University, Cardiff, UK; Professor K F Chung, National Heart & Lung Institute, London, UK; Professor D Geddes, The Royal Brompton Hospital, London, UK; Dr J Haughney, Aberdeen University, Alison Lea Medical Centre, Aberdeen, UK; Dr J A Kastelik, University of Hull, Castle Hill Hospital, Cottingham, UK; Dr J A McGlashan, University of Nottingham, Queen's Medical Centre, Nottingham, UK; Dr S Packham, Singleton Hospital, Swansea, UK; Dr R Stone, Taunton & Somerset Hospital, Somerset, UK
Conflicts of interest: Professor A H Morice has received research monies from Profile Respiratory Systems Ltd, Altana Pharma, AstraZeneca, GlaxoSmithKline (GSK), Schering Plough Research, Novartis; speaker honoraria from AstraZeneca, Altana Pharma, IVAX Pharmaceuticals, GSK, ReckittBenckiser Healthcare, and Novartis; advisory committee honoraria from Proctor and Gamble Healthcare and GSK; and sponsorship to attend international meetings from IVAX Pharmaceuticals and Boehringer Ingelheim. Dr L McGarvey has received speaker honoraria from GSK, AstraZeneca and Boehringer Ingelheim and consultancy honoraria from GSK. Professor I Pavord has received speakers' fees, research grants and funding to attend international meetings from GSK and AstraZeneca. Professor M Belvisi has received honoraria for consultancy work from GSK; grants from GSK and Novartis; and advisory committee honoraria for Biolipox and Euroscreen. Dr S S Birring has no conflict of interest in relation to this publication. Professor E Eccles has received consultancy fees from Proctor & Gamble and GSK. Professor K F Chung has received speakers' fees and educational grants from GSK, Novartis, Altana and Boehringer Ingelheim; consultancy honoraria from Scios, GSK, AstraZeneca, Novartis and Pfizer; and research grants from Novartis and GSK. Professor D Geddes has no conflicts of interest. Dr J Haughney has received speaker honoraria from AstraZeneca, Boehringer Ingelheim, Merck Sharp and Dohme, and consultancy honoraria from GSK, Merck Sharp & Dohme, Novartis and Schering Plough. Dr J A Kastelik has received speaker honoraria from AstraZeneca, GSK, Pfizer, Boehringer Ingelheim, Schering Plough and an educational grant from Altana Pharma. Mr J McGlashan discloses research funding, consultancy work and sponsorship to attend international meetings from Reckitt Benckiser, and an educational grant from Laryngograph Ltd. Dr S Packham has received speaker honoraria from AstraZeneca, GSK, and Boehringer Ingelheim and sponsorship to attend scientific meetings from AstraZeneca and GSK. Dr R Stone has received funds for lectures and travel from GSK, Boehringer Ingelheim, and AstraZeneca.
The algorithm for the evaluation of chronic cough in adults is shown in Appendix 2 (Parts 1 and 2) available online at http://www.thoraxjnl.com/supplemental.
References
- 1.Schappert S M. National ambulatory medical care survey: 1991 summary. Adv Data 19932301–16. [PubMed] [Google Scholar]
- 2.McGarvey L P A, Heaney L G, MacMahon J. A retrospective survey of diagnosis and management of patients presenting with chronic cough to a general chest clinic. Int J Clin Pract 199852158–161. [PubMed] [Google Scholar]
- 3.Birring S S, Prudon B, Carr A J.et al Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 200358339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.French C T, Irwin R S, Fletcher K E.et al Evaluation of cough‐specific quality of life questionnaire. Chest 20021211123–1131. [DOI] [PubMed] [Google Scholar]
- 5.Irwin R S.et al Diagnosis and management of cough: ACCP evidence‐based clinical practice guidelines. Chest 20061291–292s. [DOI] [PubMed] [Google Scholar]
- 6.Morice A H, Fontana G A, Sovijarvi A R A.et al The diagnosis and management of chronic cough. Eur Respir J 200424481–492. [DOI] [PubMed] [Google Scholar]
- 7.Anon Cough guidelines choke on evidence. Lancet 2006367276. [DOI] [PubMed] [Google Scholar]
- 8.Widdicombe J, Fontana G. Cough: what's in a name? Eur Respir J 20062810–15. [DOI] [PubMed] [Google Scholar]
- 9.Curley F J, Irwin R S, Pratter M R.et al Cough and the common cold. Am Rev Respir Dis 1988138305–311. [DOI] [PubMed] [Google Scholar]
- 10.Irwin R S, Boulet L P, Cloutier M M.et al Managing cough as a defense mechanism and as a symptom. A consensus panel report of the American College of Chest Physicians. Chest 1998114133–81S. [DOI] [PubMed] [Google Scholar]
- 11.Johnston S L, Holgate S T. Epidemiology of respiratory tract infections. In: Myint S, Taylor‐Robinson D, eds. Viral other infections of the human respiratory tract. London: Chapman & Hall, 1996
- 12.Reid D D, Williams R E, Hirch A. Colds among office workers, an epidemiological study. Lancet 19532651303–1306. [DOI] [PubMed] [Google Scholar]
- 13.Eccles R, Loose I, Jawad M.et al Effects of acetylsalicylic acid on sore throat pain and other pain symptoms associated with acute upper respiratory tract infection. Pain Med 20034118–124. [DOI] [PubMed] [Google Scholar]
- 14.Proprietary Association of Great Britain (PAGB) Annual review and report 2002. London: Proprietary Association of Great Britain, 20021–30.
- 15.Office of Population Censuses and Surveys Morbidity statistics from general practice: 4th national study 1991–1992. Series MB5,3. London: HMSO, 1995
- 16.Morice A H. Epidemiology of cough. Pulm Pharmacol Ther 200215253–259. [DOI] [PubMed] [Google Scholar]
- 17.Schaberg T, Gialdroni‐Grassi G, Huchon G.et al An analysis of decisions by European general practitioners to admit to hospital patients with lower respiratory tract infections. The European Study Group of Community Acquired Pneumonia (ESOCAP) of the European Respiratory Society. Thorax 1996511017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loudon R G. Weather and cough. Am Rev Respir Dis 196489352–359. [DOI] [PubMed] [Google Scholar]
- 19.Monto A S, Gravenstein S, Elliott M.et al Clinical signs and symptoms predicting influenza infection. Arch Intern Med 20001603243–3247. [DOI] [PubMed] [Google Scholar]
- 20.Fleming D, Harcourt S, Smith G. Influenza and adult hospital admissions for respiratory conditions in England 1989–2001. Commun Dis Public Health 20036231–237. [PubMed] [Google Scholar]
- 21.Fendrick A M, Monto A S, Nightengale B.et al The economic burden of non‐influenza‐related viral respiratory tract infection in the United States. Arch Intern Med 2003163487–494. [DOI] [PubMed] [Google Scholar]
- 22.Bramley T J, Lerner D, Sames M. Productivity losses related to the common cold. J Occup Environ Med 200244822–829. [DOI] [PubMed] [Google Scholar]
- 23.Gwaltney J M, Jr, Phillips C D, Miller R D.et al Computed tomographic study of the common cold. N Engl J Med 199433025–30. [DOI] [PubMed] [Google Scholar]
- 24.Puhakka T, Lavonius M, Varpula M.et al Pulmonary imaging and function in the common cold. Scand J Infect Dis 200133211–214. [DOI] [PubMed] [Google Scholar]
- 25.Hutchings H A, Eccles R, Smith A P.et al Voluntary cough suppression as an indication of symptom severity in upper respiratory tract infections. Eur Respir J 199361449–1454. [PubMed] [Google Scholar]
- 26.Parvez L, Vaidya M, Sakhardande A.et al Evaluation of antitussive agents in man. Pulm Pharmacol 19969299–308. [DOI] [PubMed] [Google Scholar]
- 27.Manap R A, Wright C E, Gregory A.et al The antitussive effect of dextromethorphan in relation to CYP2D6 activity. Br J Clin Pharmacol 199948382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morice A H, Marshall A E, Higgins K S.et al Effect of inhaled menthol on citric acid induced cough in normal subjects. Thorax 1994491024–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janson C, Chinn S, Jarvis D.et al Determinants of cough in young adults participating in the European Community Respiratory Health Survey. Eur Respir J 200118647–654. [DOI] [PubMed] [Google Scholar]
- 30.Barbee R A, Halonen M, Kaltenborn W T.et al A longitudinal study of respiratory symptoms in a community population sample. Correlations with smoking, allergen skin‐test reactivity, and serum IgE. Chest 19919920–26. [DOI] [PubMed] [Google Scholar]
- 31.Cullinan P. Persistent cough and sputum: prevalence and clinical characteristics in south east England. Respir Med 199286143–149. [DOI] [PubMed] [Google Scholar]
- 32.Lundback B, Nystrom L, Rosenhall L.et al Obstructive lung disease in northern Sweden: respiratory symptoms assessed in a postal survey. Eur Respir J 19914257–266. [PubMed] [Google Scholar]
- 33.Ford A C, Forman D, Moayyedi P.et al Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax. 2006 (epub ahead of print) [DOI] [PMC free article] [PubMed]
- 34.Bjornsson E, Plaschke P, Norrman E.et al Symptoms related to asthma and chronic bronchitis in three areas of Sweden. Eur Respir J 199472146–2153. [DOI] [PubMed] [Google Scholar]
- 35.Ludviksdottir D, Bjornsson E, Janson C.et al Habitual coughing and its associations with asthma, anxiety, and gastroesophageal reflux. Chest 19961091262–1268. [DOI] [PubMed] [Google Scholar]
- 36.Fujimura M, Kasahara K, Kamio Y.et al Female gender as a determinant of cough threshold to inhaled capsaicin. Eur Respir J 199691624–1626. [DOI] [PubMed] [Google Scholar]
- 37.Kastelik J A, Thompson R H, Aziz I.et al Sex‐related differences in cough reflex sensitivity in patients with chronic cough. Am J Respir Crit Care Med 2002166961–964. [DOI] [PubMed] [Google Scholar]
- 38.Turner D, Wailoo A, Nicholson K.et al Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technol Assess 20037iii–xiii. [DOI] [PubMed] [Google Scholar]
- 39.Zemp E, Elsasser S, Schindler C.et al Long‐term ambient air pollution and respiratory symptoms in adults (SAPALDIA study). The SAPALDIA Team. Am J Respir Crit Care Med 19991591257–1266. [DOI] [PubMed] [Google Scholar]
- 40.Braun‐Fahrlander C, Wuthrich B, Gassner M.et al Validation of a rhinitis symptom questionnaire (ISAAC core questions) in a population of Swiss school children visiting the school health services. SCARPOL team. Swiss Study on Childhood Allergy and Respiratory Symptom with respect to Air Pollution and Climate. International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol 1997875–82. [DOI] [PubMed] [Google Scholar]
- 41.Vedal S, Petkau J, White R.et al Acute effects of ambient inhalable particles in asthmatic and nonasthmatic children. Am J Respir Crit Care Med 19981571034–1043. [DOI] [PubMed] [Google Scholar]
- 42.Montnemery P, Bengtsson P, Elliot A.et al Prevalence of obstructive lung diseases and respiratory symptoms in relation to living environment and socio‐economic group. Respir Med 200195744–752. [DOI] [PubMed] [Google Scholar]
- 43.Viegi G, Pedreschi M, Baldacci S.et al Prevalence rates of respiratory symptoms and diseases in general population samples of North and Central Italy. Int J Tuberc Lung Dis 199931034–1042. [PubMed] [Google Scholar]
- 44.Gehring U, Heinrich J, Jacob B.et al Respiratory symptoms in relation to indoor exposure to mite and cat allergens and endotoxins. Indoor Factors and Genetics in Asthma (INGA) Study Group. Eur Respir J 200118555–563. [DOI] [PubMed] [Google Scholar]
- 45.French C L, Irwin R S, Curley F J.et al Impact of chronic cough on quality of life. Arch Intern Med 19981581657–1661. [DOI] [PubMed] [Google Scholar]
- 46.Dicpinigaitis P V, Tso R. Prevalence of depressive symptoms in patients with chronic cough (abstract). Proceedings of the American Thoracic Society 20052A520 [Google Scholar]
- 47.French C T, Fletcher K E, Irwin R S. A comparison of gender differences in health‐related quality of life in acute and chronic coughers. Chest 20051271991–1998. [DOI] [PubMed] [Google Scholar]
- 48.French C T, Fletcher K E, Irwin R S. Gender differences in health‐related quality of life in patients complaining of chronic cough. Chest 2004125482–488. [DOI] [PubMed] [Google Scholar]
- 49.Birring S S, Patel R B, Prudon B.et al Quality of life in chronic cough (abstract). Am J Respir Crit Care Med 2003167A135 [Google Scholar]
- 50.Morice A H, Kastelik J A. Cough – 1: Chronic cough in adults. Thorax 200358901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jansen D F, Schouten J P, Vonk J M.et al Smoking and airway hyperresponsiveness especially in the presence of blood eosinophilia increase the risk to develop respiratory symptoms: a 25‐year follow‐up study in the general adult population. Am J Respir Crit Care Med 1999160259–264. [DOI] [PubMed] [Google Scholar]
- 52.Dicpinigaitis P V. Cough reflex sensitivity in cigarette smokers. Chest 2003123685–688. [DOI] [PubMed] [Google Scholar]
- 53.Mello C J, Irwin R S, Curley F J. Predictive values of the character, timing, and complications of chronic cough in diagnosing its cause. Arch Intern Med 1996156997–1003. [PubMed] [Google Scholar]
- 54.Everett C F, Ojoo J C, Thompson R H.et al A questionnaire survey of individuals complaining of chronic cough (abstract). Am J Respir Crit Care Med 2003167(Suppl)A316 [Google Scholar]
- 55.McGarvey L P, Heaney L G, Lawson J T.et al Evaluation and outcome of patients with chronic non‐productive cough using a comprehensive diagnostic protocol. Thorax 199853738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ojoo J C, Kastelik J A, Morice A H. A boy with a disabling cough. Lancet 2003361674. [DOI] [PubMed] [Google Scholar]
- 57.O'Connell F, Thomas V E, Studham J M.et al Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med 199690279–286. [DOI] [PubMed] [Google Scholar]
- 58.Cerveri I, Accordini S, Corsico A.et al Chronic cough and phlegm in young adults. Eur Respir J 200322413–417. [DOI] [PubMed] [Google Scholar]
- 59.Power J T, Stewart I C, Connaughton J J.et al Nocturnal cough in patients with chronic bronchitis and emphysema. Am Rev Respir Dis 1984130999–1001. [DOI] [PubMed] [Google Scholar]
- 60.Hsu J Y, Stone R A, Logan Sinclair R B.et al Coughing frequency in patients with persistent cough: assessment using a 24 hour ambulatory recorder. Eur Respir J 199471246–1253. [DOI] [PubMed] [Google Scholar]
- 61.Hargreaves M, Channon K. Mechanism of pacemaker induced cough. Br Heart J 199471484–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raj H, Singh V K, Anand A.et al Sensory origin of lobeline‐induced sensations: a correlative study in man and cat. J Physiol 1995482235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morice A H, Lowry R, Brown M J.et al Angiotensin converting enzyme and the cough reflex. Lancet 198721116–1118. [DOI] [PubMed] [Google Scholar]
- 64.Bonnet R, Jorres R, Downey R.et al Intractable cough associated with the supine body position. Effective therapy with nasal CPAP. Chest 1995108581–585. [DOI] [PubMed] [Google Scholar]
- 65.Mittal R K, Balaban D H. The esophagogastric junction. N Engl J Med 1997336924–932. [DOI] [PubMed] [Google Scholar]
- 66.Everett C F, Morice A H. Clinical history in gastroesophageal cough. Respir Med. 2006 (epub ahead of print) [DOI] [PubMed]
- 67.Sesoko S, Kaneko Y. Cough associated with the use of captopril. Arch Intern Med 19851451524. [PubMed] [Google Scholar]
- 68.Yeo W W, Foster G, Ramsay L E. Prevalence of persistent cough during long‐term enalapril treatment: controlled study versus nifedipine. Q J Med 199180763–770. [PubMed] [Google Scholar]
- 69.Morimoto T, Gandhi T K, Fiskio J M.et al An evaluation of risk factors for adverse drug events associated with angiotensin‐converting enzyme inhibitors. J Eval Clin Pract 200410499–509. [DOI] [PubMed] [Google Scholar]
- 70.Ojoo J C, Kastelik J A, Morice A H. Duration of angiotensin converting enzyme inhibitor (ACEI) induced cough (abstract). Thorax 200156(Suppl III)iii72 [Google Scholar]
- 71.Pitt B, Segal R, Martinez F A.et al Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet 1997349747–752. [DOI] [PubMed] [Google Scholar]
- 72.Peyriere H, Mauboussin J M, Arnaud A.et al Chronic cough induced by abacavir apart from a context of hypersensitivity. Allerg Immunol (Paris) 200234359–360. [PubMed] [Google Scholar]
- 73.Isler M, Akhan G, Bardak Y.et al Dry cough and optic neuritis: two rare complications of interferon alpha treatment in chronic viral hepatitis. Am J Gastroenterol 2001961303–1304. [DOI] [PubMed] [Google Scholar]
- 74.Kern J, Mustajbegovic J, Schachter E N.et al Respiratory findings in farmworkers. J Occup Environ Med 200143905–913. [DOI] [PubMed] [Google Scholar]
- 75.Gordon S B, Curran A D, Wong C H.et al Chronic respiratory symptom excess in bottle factory workers. Eur Respir J 1996923s [Google Scholar]
- 76.Blanc P, Liu D, Juarez C.et al Cough in hot pepper workers. Chest 19919927–32. [DOI] [PubMed] [Google Scholar]
- 77.Pauwels R A, Buist A S, Ma P.et al Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care 200146798–825. [PubMed] [Google Scholar]
- 78.Smyrnios N A, Irwin R S, Curley F J. Chronic cough with a history of excessive sputum production. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Chest 1995108991–997. [DOI] [PubMed] [Google Scholar]
- 79.Hopwood P, Stephens R J. Symptoms at presentation for treatment in patients with lung cancer: implications for the evaluation of palliative treatment. The Medical Research Council (MRC) Lung Cancer Working Party. Br J Cancer 199571633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bergman B, Aaronson N K, Ahmedzai S.et al The EORTC QLQ‐LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ‐C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer 199430A635–642. [DOI] [PubMed] [Google Scholar]
- 81.Galdi E, Moscato G. Pertussis in the aetiology of chronic cough in adults. Monaldi Arch Chest Dis 200257229–230. [PubMed] [Google Scholar]
- 82.Birkebaek N H, Kristiansen M, Seefeldt T.et al Bordetella pertussis and chronic cough in adults. Clin Infect Dis 1999291239–1242. [DOI] [PubMed] [Google Scholar]
- 83.Jansen D F, Rijcken B, Schouten J P.et al The relationship of skin test positivity, high serum total IgE levels, and peripheral blood eosinophilia to symptomatic and asymptomatic airway hyperresponsiveness. Am J Respir Crit Care Med 1999159924–931. [DOI] [PubMed] [Google Scholar]
- 84.Fujimura M, Ogawa H, Nishizawa Y.et al Comparison of atopic cough with cough variant asthma: is atopic cough a precursor of asthma? Thorax 20035814–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McGarvey L, Morice A H. Atopic cough: little evidence to support a new clinical entity. Thorax 200358736–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haider A W, Larson M G, O'Donnell C J.et al The association of chronic cough with the risk of myocardial infarction: the Framingham Heart Study. Am J Med 1999106279–284. [DOI] [PubMed] [Google Scholar]
- 87.Birring S S, Brightling C E, Symon F A.et al Idiopathic chronic cough: association with organ specific autoimmune disease and bronchoalveolar lymphocytosis. Thorax 2003581066–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kok C, Kennerson M L, Spring P J.et al A locus for hereditary sensory neuropathy with cough and gastroesophageal reflux on chromosome 3p22–p24. Am J Hum Genet 200373632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McGarvey L P, Heaney L G, Lawson J T.et al Evaluation and outcome of patients with chronic non‐productive cough using a comprehensive diagnostic protocol. Thorax 199853738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Birring S S, Passant C, Patel R B.et al Chronic tonsillar enlargement and cough: preliminary evidence of a novel and treatable cause of chronic cough. Eur Respir J 200423199–201. [DOI] [PubMed] [Google Scholar]
- 91.Koufman J A. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24‐hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope 19911011–78. [DOI] [PubMed] [Google Scholar]
- 92.Thiadens H A, De Bock G H, Dekker F W.et al Identifying asthma and chronic obstructive pulmonary disease in patients with persistent cough presenting to general practitioners: descriptive study. BMJ 19983161286–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu M L, Ryu J H. Assessment of the patient with chronic cough (see comment). Mayo Clin Proc 199772957–959. [DOI] [PubMed] [Google Scholar]
- 94.Lawler W R. An office approach to the diagnosis of chronic cough. Am Fam Physician 1998582015–2022. [PubMed] [Google Scholar]
- 95.D'Urzo A, Jugovic P. Chronic cough. Three most common causes. Can Fam Physician 2002481311–1316. [PMC free article] [PubMed] [Google Scholar]
- 96.Irwin R S, Corrao W M, Pratter M R. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis 1981123413–417. [DOI] [PubMed] [Google Scholar]
- 97.Poe R H, Harder R V, Israel R H.et al Chronic persistent cough. Experience in diagnosis and outcome using an anatomic diagnostic protocol. Chest 198995723–728. [DOI] [PubMed] [Google Scholar]
- 98.Irwin R S, Curley F J, French C L. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis 1990141640–647. [DOI] [PubMed] [Google Scholar]
- 99.Pratter M R, Bartter T, Akers S.et al An algorithmic approach to chronic cough. Ann Intern Med 1993119977–983. [DOI] [PubMed] [Google Scholar]
- 100.Palombini B C, Villanova C A, Araujo E.et al A pathogenic triad in chronic cough: asthma, postnasal drip syndrome, and gastroesophageal reflux disease. Chest 1999116279–284. [DOI] [PubMed] [Google Scholar]
- 101.Celli B R, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 200423932–946. [DOI] [PubMed] [Google Scholar]
- 102.British Thoracic Society/Scottish Intercollegiate Guidelines Network British guideline on the management of asthma. Thorax 200358(Suppl I)i1–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cockcroft D W, Jokic R, Marciniuk D D.et al The current dilemma with spirometric inclusion criteria for asthma drug trials. Ann Allergy Asthma Immunol 199779226–228. [DOI] [PubMed] [Google Scholar]
- 104.Thiadens H A, De Bock G H, Van Houwelingen J C.et al Can peak expiratory flow measurements reliably identify the presence of airway obstruction and bronchodilator response as assessed by FEV1 in primary care patients presenting with a persistent cough? Thorax 1999541055–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corrao W M, Braman S S, Irwin R S. Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med 1979300633–637. [DOI] [PubMed] [Google Scholar]
- 106.Gibson P G, Dolovich J, Denburg J.et al Chronic cough: eosinophilic bronchitis without asthma. Lancet 198911346–1348. [DOI] [PubMed] [Google Scholar]
- 107.Hunter C J, Brightling C E, Woltmann G.et al A comparison of the validity of different diagnostic tests in adults with asthma. Chest 20021211051–1057. [DOI] [PubMed] [Google Scholar]
- 108.Puolijoki H, Lahdensuo A. Causes of prolonged cough in patients referred to a chest clinic. Ann Med 198921425–427. [DOI] [PubMed] [Google Scholar]
- 109.Al‐Mobeireek A F, Al‐Sarhani A, Al‐Amri S.et al Chronic cough at a non‐teaching hospital: are extrapulmonary causes overlooked? Respirology 20027141–146. [DOI] [PubMed] [Google Scholar]
- 110.Kastelik J A, Aziz I, Ojoo J C.et al Investigation and management of chronic cough using a probability‐based algorithm. Eur Respir J 200525235–243. [DOI] [PubMed] [Google Scholar]
- 111.Mise K, Sviliicic A, Bradaric A. Foreign bodies in the bronchial system of adults (abstract). Eur Respir J Suppl 20042448s [Google Scholar]
- 112.Poe R H, Israel R H, Utell M J.et al Chronic cough: bronchoscopy or pulmonary function testing? Am Rev Respir Dis 1982126160–162. [DOI] [PubMed] [Google Scholar]
- 113.Packham S. The role of bronchoscopy in the mangement of chronic cough (abstract). Eur Respir J 200118(Suppl 33)378s [Google Scholar]
- 114.Sen R P, Walsh T E. Fiberoptic bronchoscopy for refractory cough. Chest 19919933–35. [DOI] [PubMed] [Google Scholar]
- 115.El Hennawi D D, Iskander N M, Ibrahim I H.et al Persistent cough: prevalence of gastroesophageal reflux and study of relevant laryngeal signs. Otolaryngol Head Neck Surg 2004131767–772. [DOI] [PubMed] [Google Scholar]
- 116.Packham S. The sensitivity of high resolution computed tomography and flexible bronchoscopy in the diagnosis of chronic cough (abstract). Eur Respir J Suppl 200220450s [Google Scholar]
- 117.Ojoo J C, Kastelik J A, Mulrennan S A. Selective use of thoracic computed tomographs in patients with chronic cough (abstract). Eur Respir J Suppl 200220449s [Google Scholar]
- 118.Padley S P, Hansell D M, Flower C D.et al Comparative accuracy of high resolution computed tomography and chest radiography in the diagnosis of chronic diffuse infiltrative lung disease. Clin Radiol 199144222–226. [DOI] [PubMed] [Google Scholar]
- 119.van der Bruggen‐Bogaarts B A, van der Bruggen H M, van Waes P F.et al Screening for bronchiectasis. A comparative study between chest radiography and high‐resolution CT. Chest 1996109608–611. [DOI] [PubMed] [Google Scholar]
- 120.Volpe J, Storto M L, Lee K.et al High‐resolution CT of the lung: determination of the usefulness of CT scans obtained with the patient prone based on plain radiographic findings. AJR Am J Roentgenol 1997169369–374. [DOI] [PubMed] [Google Scholar]
- 121.Irwin R S, Madison J M. The diagnosis and treatment of cough. N Engl J Med 20003431715–1721. [DOI] [PubMed] [Google Scholar]
- 122.Fitch P S, Brown V, Schock B C.et al Chronic cough in children: bronchoalveolar lavage findings. Eur Respir J 2000161109–1114. [DOI] [PubMed] [Google Scholar]
- 123.Higgins B G, Britton J R, Chinn S.et al Comparison of bronchial reactivity and peak expiratory flow variability measurements for epidemiologic studies. Am Rev Respir Dis 1992145588–593. [DOI] [PubMed] [Google Scholar]
- 124.Brightling C E, Ward R, Goh K L.et al Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med 1999160406–410. [DOI] [PubMed] [Google Scholar]
- 125.Brightling C E, Bradding P, Symon F A.et al Mast cell infiltration of airway smooth muscle in asthma. N Engl J Med 20023461699–1705. [DOI] [PubMed] [Google Scholar]
- 126.Pavord I D, Brightling C E, Woltmann G.et al Non‐eosinophilic corticosteroid unresponsive asthma (letter]. Lancet 19993532213–2214. [DOI] [PubMed] [Google Scholar]
- 127.Green R H, Brightling C E, Woltmann G.et al Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 200257875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheriyan S, Greenberger P A, Patterson R. Outcome of cough variant asthma treated with inhaled steroids. Ann Allergy 199473478–480. [PubMed] [Google Scholar]
- 129.Dicpinigaitis P V, Dobkin J B, Reichel J. Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough‐variant asthma. J Asthma 200239291–297. [DOI] [PubMed] [Google Scholar]
- 130.Rafferty P, Jackson L, Smith R.et al Terfenadine, a potent histamine H1‐receptor antagonist in the treatment of grass pollen sensitive asthma. Br J Clin Pharmacol 199030229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brightling C E, Ward R, Wardlaw A J.et al Airway inflammation, airway responsiveness and cough before and after inhaled budesonide in patients with eosinophilic bronchitis. Eur Respir J 200015682–686. [DOI] [PubMed] [Google Scholar]
- 132.Braman S S, Corrao W M. Chronic cough. Diagnosis and treatment. Prim Care 198512217–225. [PubMed] [Google Scholar]
- 133.Koh Y Y, Jeong J H, Park Y.et al Development of wheezing in patients with cough variant asthma during an increase in airway responsiveness. Eur Respir J 199914302–308. [DOI] [PubMed] [Google Scholar]
- 134.Hancox R J, Leigh R, Kelly M M.et al Eosinophilic bronchitis. Lancet 20013581104. [DOI] [PubMed] [Google Scholar]
- 135.O'Connell F, Thomas V E, Studham J M.et al Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med 199690279–286. [DOI] [PubMed] [Google Scholar]
- 136.Ferrari M, Olivieri M, Sembenini C.et al Tussive effect of capsaicin in patients with gastroesophageal reflux without cough. Am J Respir Crit Care Med 1995151557–561. [DOI] [PubMed] [Google Scholar]
- 137.Benini L, Ferrari M, Sembenini C.et al Cough threshold in reflux oesophagitis: influence of acid and of laryngeal and oesophageal damage. Gut 200046762–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chernow B, Johnson L F, Janowitz W R.et al Pulmonary aspiration as a consequence of gastroesophageal reflux: a diagnostic approach. Dig Dis Sci 197924839–844. [DOI] [PubMed] [Google Scholar]
- 139.Corwin R W, Irwin R S. The lipid‐laden alveolar macrophage as a marker of aspiration in parenchymal lung disease. Am Rev Respir Dis 1985132576–581. [DOI] [PubMed] [Google Scholar]
- 140.Crausaz F M, Favez G. Aspiration of solid food particles into lungs of patients with gastroesophageal reflux and chronic bronchial disease. Chest 198893376–378. [DOI] [PubMed] [Google Scholar]
- 141.Irwin R S, French C L, Curley F J.et al Chronic cough due to gastroesophageal reflux. Clinical, diagnostic, and pathogenetic aspects. Chest 19931041511–1517. [DOI] [PubMed] [Google Scholar]
- 142.Giudicelli R, Dupin B, Surpas P.et al Gastroesophageal reflux and respiratory manifestations: diagnostic approach, therapeutic indications and results (in French). Ann Chir 199044552–554. [PubMed] [Google Scholar]
- 143.Ing A J, Ngu M C, Breslin A B. Pathogenesis of chronic persistent cough associated with gastroesophageal reflux. Am J Respir Crit Care Med 1994149160–167. [DOI] [PubMed] [Google Scholar]
- 144.Patti M G, Debas H T, Pellegrini C A. Esophageal manometry and 24‐hour pH monitoring in the diagnosis of pulmonary aspiration secondary to gastroesophageal reflux. Am J Surg 1992163401–406. [DOI] [PubMed] [Google Scholar]
- 145.DeMeester T R, Bonavina L, Iascone C.et al Chronic respiratory symptoms and occult gastroesophageal reflux. A prospective clinical study and results of surgical therapy. Ann Surg 1990211337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fouad Y M, Katz P O, Hatlebakk J G.et al Ineffective esophageal motility: the most common motility abnormality in patients with GERD‐associated respiratory symptoms. Am J Gastroenterol 1999941464–1467. [DOI] [PubMed] [Google Scholar]
- 147.Knight R E, Wells J R, Parrish R S. Esophageal dysmotility as an important co‐factor in extraesophageal manifestations of gastroesophageal reflux. Laryngoscope 20001101462–1466. [DOI] [PubMed] [Google Scholar]
- 148.Kastelik J A, Redington A E, Aziz I.et al Abnormal oesophageal motility in patients with chronic cough. Thorax 200358699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ing A J, Ngu M C, Breslin A B. Chronic persistent cough and clearance of esophageal acid. Chest 19921021668–1671. [DOI] [PubMed] [Google Scholar]
- 150.Kastelik J A, Jackson W, Davies T W.et al Measurement of gastric emptying in gastroesophageal reflux‐related chronic cough. Chest 20021222038–2041. [DOI] [PubMed] [Google Scholar]
- 151.Ing A J, Ngu M C, Breslin A B. Chronic persistent cough and gastro‐oesophageal reflux. Thorax 199146479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ours T M, Kavuru M S, Schilz R J.et al A prospective evaluation of esophageal testing and a double‐blind, randomized study of omeprazole in a diagnostic and therapeutic algorithm for chronic cough. Am J Gastroenterol 1999943131–3138. [DOI] [PubMed] [Google Scholar]
- 153.Kiljander T O, Salomaa E R, Hietanen E K.et al Chronic cough and gastro‐oesophageal reflux: a double‐blind placebo‐controlled study with omeprazole. Eur Respir J 200016633–638. [DOI] [PubMed] [Google Scholar]
- 154.Ing A J, Ngu M C, Breslin A B. A randomised double‐blind placebo controlled crossover study of ranitidine in patients with chronic persistent cough associated with gastroesophageal reflux. Am Rev Respir Dis 1992145A11 [Google Scholar]
- 155.Vaezi M F, Richter J E. Twenty‐four‐hour ambulatory esophageal pH monitoring in the diagnosis of acid reflux‐related chronic cough. South Med J 199790305–311. [DOI] [PubMed] [Google Scholar]
- 156.Kamel P L, Hanson D, Kahrilas P J. Omeprazole for the treatment of posterior laryngitis. Am J Med 199496321–326. [DOI] [PubMed] [Google Scholar]
- 157.Kuo B, Castell D O. Optimal dosing of omeprazole 40 mg daily: effects on gastric and esophageal pH and serum gastrin in healthy controls. Am J Gastroenterol 1996911532–1538. [PubMed] [Google Scholar]
- 158.Xue S, Katz P O, Banerjee P.et al Bedtime H2 blockers improve nocturnal gastric acid control in GERD patients on proton pump inhibitors. Aliment Pharmacol Ther 2001151351–1356. [DOI] [PubMed] [Google Scholar]
- 159.Nilsson M, Johnsen R, Ye W.et al Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA 200329066–72. [DOI] [PubMed] [Google Scholar]
- 160.Novitsky Y W, Zawacki J K, Irwin R S.et al Chronic cough due to gastroesophageal reflux disease: efficacy of antireflux surgery. Surg Endosc 200216567–571. [DOI] [PubMed] [Google Scholar]
- 161.Menon M S, Mulrennan S A, Everett C F.et al Experience with baclofen in cough secondary to gastro‐oesophageal reflux disease (abstract). Proceedings of the American Thoracic Society 20052A323 [Google Scholar]
- 162.Dicpinigaitis P V. Effect of the GABA‐agonist baclofen on bronchial responsiveness in asthmatics. Pulm Pharmacol Ther 199912257–260. [DOI] [PubMed] [Google Scholar]
- 163.Allen C J, Anvari M. Gastro‐oesophageal reflux related cough and its response to laparoscopic fundoplication. Thorax 199853963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Allen C J, Anvari M. Preoperative symptom evaluation and esophageal acid infusion predict response to laparoscopic Nissen fundoplication in gastroesophageal reflux patients who present with cough. Surg Endosc 2002161037–1041. [DOI] [PubMed] [Google Scholar]
- 165.Irwin R S, Zawacki J K, Wilson M M.et al Chronic cough due to gastroesophageal reflux disease: failure to resolve despite total/near‐total elimination of esophageal acid. Chest 20021211132–1140. [DOI] [PubMed] [Google Scholar]
- 166.Morice A H. Post‐nasal drip syndrome‐a symptom to be sniffed at? Pulm Pharmacol Ther 200417343–345. [DOI] [PubMed] [Google Scholar]
- 167.Tanaka S, Hirata K, Kurihara N.et al Effect of loratadine, an H1 antihistamine, on induced cough in non‐ asthmatic patients with chronic cough. Thorax 199651810–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dicpinigaitis P V, Gayle Y E. Effect of the second‐generation antihistamine fexofenadine on cough reflex sensitivity and pulmonary function. Br J Clin Pharmacol 200356501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gawchik S, Goldstein S, Prenner B.et al Relief of cough and nasal symptoms associated with allergic rhinitis by mometasone furoate nasal spray. Ann Allergy Asthma Immunol 200390416–421. [DOI] [PubMed] [Google Scholar]
- 170.Puhakka T, Makela M J, Malmstrom K.et al The common cold: effects of intranasal fluticasone propionate treatment. J Allergy Clin Immunol 1998101726–731. [DOI] [PubMed] [Google Scholar]
- 171.O'Connell F, Thomas V E, Pride N B.et al Capsaicin cough sensitivity decreases with successful treatment of chronic cough. Am J Respir Crit Care Med 1994150374–380. [DOI] [PubMed] [Google Scholar]
- 172.Birring S S, Murphy A C, Scullion J E.et al Idiopathic chronic cough and organ‐specific autoimmune diseases: a case‐control study. Respir Med 200498242–246. [DOI] [PubMed] [Google Scholar]
- 173.Haque R A, Usmani O S, Barnes P J. Chronic idiopathic cough: a discrete clinical entity? Chest 20051271710–1713. [DOI] [PubMed] [Google Scholar]
- 174.Mund E, Christensson B, Gronneberg R.et al Noneosinophilic CD4 lymphocytic airway inflammation in menopausal women with chronic dry cough. Chest 20051271714–1721. [DOI] [PubMed] [Google Scholar]
- 175.Prudon B, Birring S S, Vara D D.et al Cough and glottic‐stop reflex sensitivity in health and disease. Chest 2005127550–557. [DOI] [PubMed] [Google Scholar]
- 176.Lee S Y, Cho J Y, Shim J J.et al Airway inflammation as an assessment of chronic nonproductive cough. Chest 20011201114–1120. [DOI] [PubMed] [Google Scholar]
- 177.Boulet L P, Milot J, Boutet M.et al Airway inflammation in nonasthmatic subjects with chronic cough. Am J Respir Crit Care Med 1994149482–489. [DOI] [PubMed] [Google Scholar]
- 178.McGarvey L P, Forsythe P, Heaney L G.et al Bronchoalveolar lavage findings in patients with chronic nonproductive cough. Eur Respir J 19991359–65. [DOI] [PubMed] [Google Scholar]
- 179.Niimi A, Torrego A, Nicholson A G.et al Nature of airway inflammation and remodeling in chronic cough. J Allergy Clin Immunol 2005116565–570. [DOI] [PubMed] [Google Scholar]
- 180.Birring S S, Parker D, Brightling C E.et al Induced sputum inflammatory mediator concentrations in chronic cough. Am J Respir Crit Care Med 200416915–19. [DOI] [PubMed] [Google Scholar]
- 181.Mund E, Christensson B, Larsson K.et al Sex dependent differences in physiological ageing in the immune system of lower airways in healthy non‐smoking volunteers: study of lymphocyte subsets in bronchoalveolar lavage fluid and blood. Thorax 200156450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Birring S S, Pavord I D. Idiopathic chronic cough and organ‐specific autoimmune disease. Chest 2006129213–214. [DOI] [PubMed] [Google Scholar]
- 183.Birring S S, Patel R B, Parker D.et al Airway function and markers of airway inflammation in patients with treated hypothyroidism. Thorax 200560249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Brightling C E, Symon F A, Birring S S.et al A case of cough, lymphocytic bronchoalveolitis and coeliac disease with improvement following a gluten free diet. Thorax 20025791–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Birring S S, Morgan C N, Prudon B.et al Respiratory symptoms in patients with treated hypothyroidism and inflammatory bowel disease. Thorax 200358533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Raphael J H, Stanley G D, Langton J A. Effects of topical benzocaine and lignocaine on upper airway reflex sensitivity. Anaesthesia 199651114–118. [DOI] [PubMed] [Google Scholar]
- 187.Jackson J, Wright C E, Menon M.et al Opiate therapy in chronic intractable cough (abstract). Proceedings of the American Thoracic Society 20052A321 [Google Scholar]
- 188.National Collaborating Centre for Chronic Conditions Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 200459(Suppl I)1–232. [PMC free article] [PubMed] [Google Scholar]
- 189.Muers M F, Round C E. Palliation of symptoms in non‐small cell lung cancer: a study by the Yorkshire Regional Cancer Organisation Thoracic Group. Thorax 199348339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Crystal R G, Fulmer J D, Roberts W C.et al Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med 197685769–788. [DOI] [PubMed] [Google Scholar]
- 191.Hope‐Gill B D, Hilldrup S, Davies C.et al A study of the cough reflex in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003168995–1002. [DOI] [PubMed] [Google Scholar]
- 192.Carney I K, Gibson P G, Murree‐Allen K.et al A systematic evaluation of mechanisms in chronic cough. Am J Respir Crit Care Med 1997156211–216. [DOI] [PubMed] [Google Scholar]
- 193.Lin L, Poh K L, Lim T K. Empirical treatment of chronic cough: a cost‐effectiveness analysis. Proc AMIA Symp 2001383–387. [PMC free article] [PubMed]
- 194.Chung K F, Lalloo U G. Diagnosis and management of chronic persistent dry cough. Postgrad Med J 199672594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kim C K, Kim J T, Kang H.et al Sputum eosinophilia in cough‐variant asthma as a predictor of the subsequent development of classic asthma. Clin Exp Allergy 2003331409–1414. [DOI] [PubMed] [Google Scholar]
- 196.Gibson P G, Hargreave F E, Girgis‐Gabardo A.et al Chronic cough with eosinophilic bronchitis: examination for variable airflow obstruction and response to corticosteroid. Clin Exp Allergy 199525127–132. [DOI] [PubMed] [Google Scholar]
- 197.Irwin R S, Pratter M R, Holland P S.et al Postnasal drip causes cough and is associated with reversible upper airway obstruction. Chest 198485346–352. [DOI] [PubMed] [Google Scholar]
- 198.Bucca C, Rolla G, Brussino L.et al Are asthma‐like symptoms due to bronchial or extrathoracic airway dysfunction? Lancet 1995346791–795. [DOI] [PubMed] [Google Scholar]
- 199.Patterson R N, Johnston B T, MacMahon J.et al Oesophageal pH monitoring is of limited value in the diagnosis of “reflux‐cough”. Eur Respir J 200424724–727. [DOI] [PubMed] [Google Scholar]
- 200.Sifrim D, Holloway R, Silny J.et al Acid, nonacid, and gas reflux in patients with gastroesophageal reflux disease during ambulatory 24‐hour pH‐impedance recording. Gastroenterology 20011201588–1598. [DOI] [PubMed] [Google Scholar]
- 201.Davidson T M, Brahme F J, Gallagher M E. Radiographic evaluation for nasal dysfunction: computed tomography versus plain films. Head Neck 198911405–409. [DOI] [PubMed] [Google Scholar]
- 202.Belafsky P C, Postma G N, Koufman J A. The validity and reliability of the reflux finding score (RFS). Laryngoscope 20011111313–1317. [DOI] [PubMed] [Google Scholar]
- 203.Dicpinigaitis P V. Short‐ and long‐term reproducibility of capsaicin cough challenge testing. Pulm Pharmacol Ther 20031661–65. [DOI] [PubMed] [Google Scholar]
- 204.Morice A H, Kastelik J A, Thompson R. Cough challenge in the assessment of cough reflex. Br J Clin Pharmacol 200152365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chang A B, Newman R G, Carlin J B.et al Subjective scoring of cough in children: parent‐completed vs child‐completed diary cards vs an objective method. Eur Respir J 199811462–466. [DOI] [PubMed] [Google Scholar]
- 206.Coyle M A, Keenan B D, Mayleben D W.et al Objective assessment of cough over a 24‐hr period in patients with COPD (abstract). Am J Respir Crit Care Med 2004A606
- 207.Chang A B, Newman R G, Phelan P D.et al A new use for an old Holter monitor: an ambulatory cough meter. Eur Respir J 1997101637–1639. [DOI] [PubMed] [Google Scholar]
- 208.Corrigan D L, Paton J Y. Pilot study of objective cough monitoring in infants. Pediatr Pulmonol 200335350–357. [DOI] [PubMed] [Google Scholar]
- 209.Munyard P, Bush A. How much coughing is normal? Arch Dis Child 199674531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Piirila P, Sovijarvi A R. Differences in acoustic and dynamic characteristics of spontaneous cough in pulmonary diseases. Chest 19899646–53. [DOI] [PubMed] [Google Scholar]
- 211.Hall L E, Smith A, Earis J E.et al Patterns of cough in cystic fibrosis and cryptogenic fibrosing alveolitis (abstract). Thorax 200156(Suppl III)iii71 [Google Scholar]
- 212.McGarvey L, Heaney L, MacMahon J.et al Eosinophilic bronchitis is an important cause of chronic cough (letter). Am J Respir Crit Care Med 20001611763–1764. [PubMed] [Google Scholar]
- 213.Chatkin J M, Ansarin K, Silkoff P E.et al Exhaled nitric oxide as a noninvasive assessment of chronic cough. Am J Respir Crit Care Med 19991591810–1813. [DOI] [PubMed] [Google Scholar]
- 214.Formanek W, Inci D, Lauener R P.et al Elevated nitrite in breath condensates of children with respiratory disease. Eur Respir J 200219487–491. [DOI] [PubMed] [Google Scholar]
- 215.Adcock J J, Schneider C, Smith T W. Effects of codeine, morphine and a novel opioid pentapeptide BW443C, on cough, nociception and ventilation in the unanaesthetized guinea‐pig. Br J Pharmacol 19889393–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mcleod R L, Parra L E, Mutter J C.et al Nociceptin inhibits cough in the guinea‐pig by activation of ORL(1) receptors. Br J Pharmacol 20011321175–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bolser D C, Mcleod R L, Tulshian D B.et al Antitussive action of nociceptin in the cat. Eur J Pharmacol 2001430107–111. [DOI] [PubMed] [Google Scholar]
- 218.Advenier C, Girard V, Naline E.et al Antitussive effect of SR 48968, a nonpeptide tachykinin NK(2) receptor antagonist. Eur J Pharmacol 1993250169–171. [DOI] [PubMed] [Google Scholar]
- 219.Girard V, Naline E, Vilain P.et al Effect of the two tachykinin antagonists, SR 48968 and SR 140333, on cough induced by citric acid in the unanaesthetized guinea‐pig. Eur Respir J 199581110–1114. [DOI] [PubMed] [Google Scholar]
- 220.Ichinose M, Nakajima N, Takahashi T.et al Protection against bradykinin‐induced bronchoconstriction in asthmatic patients by neurokinin receptor antagonist. Lancet 19923401248–1251. [DOI] [PubMed] [Google Scholar]
- 221.Fahy J V, Wong H H, Geppetti P.et al Effect of an NK1 receptor antagonist (CP‐99,994) on hypertonic saline‐induced bronchoconstriction and cough in male asthmatic subjects. Am J Respir Crit Care Med 1995152879–884. [DOI] [PubMed] [Google Scholar]
- 222.Daoui S, Cognon C, Naline E.et al Involvement of tachykinin NK3 receptors in citric acid‐induced cough and bronchial responses in guinea pigs. Am J Respir Crit Care Med 199815842–48. [DOI] [PubMed] [Google Scholar]
- 223.Hay D W, Giardina G A, Griswold D E.et al Nonpeptide tachykinin receptor antagonists. III. SB 235375, a low central nervous system‐penetrant, potent and selective neurokinin‐3 receptor antagonist, inhibits citric acid‐induced cough and airways hyper‐reactivity in guinea pigs. J Pharm Exp Ther 2002300314–323. [DOI] [PubMed] [Google Scholar]
- 224.Bolser D C, Aziz S M, DeGennaro F C.et al Antitussive effects of GABAB agonists in the cat and guinea‐pig. Br J Pharmacol 1993110491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bolser D C, DeGennaro F C, O'Reilly S.et al Peripheral and central sites of action of GABA‐B agonists to inhibit the cough reflex in the cat and guinea pig. Br J Pharmacol 19941131344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dicpinigaitis P V, Dobkin J B, Rauf K.et al Inhibition of capsaicin‐induced cough by the gamma‐aminobutyric acid agonist baclofen. J Clin Pharmacol 199838364–367. [DOI] [PubMed] [Google Scholar]
- 227.Dicpinigaitis P V, Dobkin J B. Antitussive effect of the GABA‐agonist baclofen. Chest 1997111996–999. [DOI] [PubMed] [Google Scholar]
- 228.Patel H J, Birrell M A, Crispino N.et al Inhibition of guinea‐pig and human sensory nerve activity and the cough reflex in guinea‐pigs by cannabinoid (CB2) receptor activation. Br J Pharmacol 2003140261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Choudry N B, Fuller R W, Anderson N.et al Separation of cough and reflex bronchoconstriction by inhaled local anaesthetics. Eur Respir J 19903579–583. [PubMed] [Google Scholar]
- 230.McKemy D D, Neuhausser W M, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 200241652–58. [DOI] [PubMed] [Google Scholar]
- 231.Caterina M J, Schumacher M A, Tominaga M.et al The capsaicin receptor: a heat‐activated ion channel in the pain pathway. Nature 1997389816–824. [DOI] [PubMed] [Google Scholar]
- 232.Caterina M J, Leffler A, Malmberg A B.et al Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000288306–313. [DOI] [PubMed] [Google Scholar]
- 233.Lalloo U G, Fox A J, Belvisi M G.et al Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol 1995791082–1087. [DOI] [PubMed] [Google Scholar]
- 234.Groneberg D A, Niimi A, Dinh Q T.et al Increased expression of transient receptor potential vanilloid‐1 in airway nerves of chronic cough. Am J Respir Crit Care Med 20041701276–1280. [DOI] [PubMed] [Google Scholar]
- 235.Fox A J, Barnes P J, Venkatesan P.et al Activation of large conductance potassium channels inhibits the afferent and efferent function of airway sensory nerves in the guinea pig. J Clin Invest 199799513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.