Abstract
This study examined the communicative behavior of 49 captive chimpanzees (Pan troglodytes), particularly their use of vocalizations, manual gestures, and other auditory- or tactile-based behaviors as a means of gaining an inattentive audience’s attention. A human (Homo sapiens) experimenter held a banana while oriented either toward or away from the chimpanzee. The chimpanzees’ behavior was recorded for 60 s. Chimpanzees emitted vocalizations faster and were more likely to produce vocalizations as their 1st communicative behavior when a human was oriented away from them. Chimpanzees used manual gestures more frequently and faster when the human was oriented toward them. These results replicate the findings of earlier studies on chimpanzee gestural communication and provide new information about the intentional and functional use of their vocalizations.
An affective communication system enables a signaler to intentionally transfer a message to an audience (Smith, 1977). This message can be either affective in nature by providing information about the signaler’s emotional state or symbolic by expressing a more complex, semantic idea (Gouzoules, Gouzoules, & Ashley, 1995; Marler, 1980; Seyfarth & Cheney, 1997). In either case, the audience must receive the message intact with an understanding that is close to the original intent of the signaler or the communication is not effective. Therefore, although some simple forms of affective communication may be produced without the intention of the signaler, in order for a communication system to be valuable to a species in terms of obtaining a goal, both the message and the means of communication must be intentional on the part of the signaler. Otherwise, the meaning of communicative repertoire depends more on the subjective interpretation of the audience than on the actual desired message of the signaler. Therefore, for behavior to be truly communicative, it is necessary for communicative actions, whether they are vocal or nonvocal, to be intentional and controlled by the signaler.
Unfortunately, establishing whether or not a behavior is intentionally communicative is not a simple task. Developmental researchers typically use the following three criteria in making such a distinction: (a) the behavior changes in accordance with the attentional states of the audience; (b) the behavior is accompanied by gaze alternation between an observer and a distal object or event of interest; and (c) the behavior is goal directed and continues until the desired outcome is reached (Bard, 1992; Bates, Camaioni, & Volterra, 1975; Leavens & Hopkins, 1999). A number of studies have attempted to assess whether manual gestures produced by chimpanzees (largely housed in captivity) meet the above criteria for intentional communication (see Leavens & Hopkins, 1999, for review). Many studies have reported that apes gesture much less frequently in the absence of a human observer than in their presence (the so-called audience effect; see Call & Tomasello, 1994; Krause & Fouts, 1997; Leavens & Hopkins, 1998; Leavens, Hopkins, & Bard, 1996). In other words, apes typically do not gesture toward an object if there is no social agent present who may retrieve the object for them. Similarly, Povinelli and Eddy (1996) found that young chimpanzees seem to recognize eye contact and head orientation as attentional cues; chimpanzees were more likely to gesture to an experimenter who was making direct eye contact than to an experimenter who was averting his or her eye gaze. This evidence suggests that chimpanzees (a) understand the function of their gestures as communicative; (b) distinguish, at least to some extent, attentional states in others; and (c) recognize this attention as an important prerequisite for successful gestural communication.
Although chimpanzees may recognize that an audience must be present and visually attentive for their gestural communication to be effective, how well chimpanzees understand the possible function of their communicative behavior in other sensory modalities is unclear. Specifically, gaining insight into chimpanzees’ understanding of their vocalizations or other acoustic signals could have important implications in tracing the evolution of human language and speech. A primary step in the evolution of speech was presumably the ability to control vocal activity and to intentionally use these vocalizations as a means of communication. Despite early theories to the contrary (Erwin, 1975; Myers, 1976), there is some empirical evidence suggesting that nonhuman primates can voluntarily control their vocalizations (Steklis & Raleigh, 1979a). For example, Sutton, Larson, Taylor, and Linderman (1973) found that rhesus monkeys can learn to produce a specific type of vocalization as a response to an arbitrary visual stimulus. Similarly, Randolph and Brooks (1967) were able to use social reinforcement to modify the vocal behavior of a chimpanzee. Although this evidence suggests that vocalizations can be conditioned or brought under stimulus control, the ability to control vocalizations and facial expressions should not solely define this behavior as intentionally communicative behavior. Showing that primates can produce vocalizations voluntarily does not necessarily mean that they typically do so in a natural setting or that these behaviors are intentionally communicative.
However, evidence suggesting that nonhuman primates alter their vocal production and other communicative behaviors in concordance with changes in their audience’s attentional state could provide new insights into the origin of speech and language evolution. In one of the first studies of this type, Tomasello, Call, Nagell, Olguin, and Carpenter (1994) found that chimpanzees used significantly more auditory- and tactile-based gestures in communicating with a conspecific recipient that was not looking at them. Tomasello et al. concluded that chimpanzees seem to typically understand that a gesture alone is an insufficient means of communication when their audience is not attending to them. However, the observed auditory-based gestures in the Tomasello et al. study only included actions such as ground slaps, foot stomps, and hand claps. Vocalizations were not systematically recorded because they “did not seem to be an important part of the group’s communicative repertoire” (Tomasello et al., 1994, p. 140). Theall and Povinelli (1999) specifically examined whether chimpanzees use their vocalizations to fit the attentional states of others. Theall and Povinelli recorded the use of vocalizations by 7 juvenile chimpanzees in response to human observers who were holding food and had their eyes either open or closed. Theall and Povinelli found no evidence for a higher incidence of vocal communicative behavior in the closed-eyed condition contrasted with the open-eyed condition, which would have been predicted if the chimpanzees were monitoring the attentional status of the human subject.
The present study was designed to examine whether or not chimpanzees use their vocalizations differently in response to variation in the attentional status of a human respondent. This study differs from the Tomasello et al. (1994) study in that (a) we put specific emphasis on the use of vocal behavior and other acoustic means of communication and (b) we required the chimpanzees to communicate with humans rather than with other chimpanzees. Testing the chimpanzees’ behavior in relation to communication with humans allowed for better experimental control of situational factors that might or might not have influenced the socially housed chimpanzees studied by Tomasello et al. This study also differs from the Theall and Povinelli (1999) study in that we used a much larger sample of chimpanzees, and most of the chimpanzees were adults. Leavens and Hopkins (1998) reported that chimpanzees under the age of 7 (or juveniles) never intentionally vocalize toward food that is positioned outside their home cage. Thus, one reason Theall and Povinelli might not have found any significant results was because their sample size was too small and their chimpanzees were too young.
The paradigm we used was similar to those that have previously been used to assess intentionality in gestural communication; however, the range of communicative behaviors we measured was expanded to include vocalizations and other forms of behavior that could serve as a communicative function. We predicted that if chimpanzees could discriminate the attentional states of a human respondent and could understand their own ability to alter these states (two crucial prerequisites for intentional communication), then the initial types of communication a chimpanzee would use when a human was oriented away from it should include more vocalizations and attention-getting behaviors than the initial types of communication a chimpanzee would use when the human was oriented toward it. Similarly, if chimpanzees could use vocalizations as a means of obtaining attention from a nonattentive human and not merely as an affective expression of emotion (whether it be frustration or excitement), then the vocalizations a chimpanzee would use when a human was oriented away should be produced more quickly than when the human was oriented toward the chimpanzee because it would use vocalizations as an intentional means of gaining the audience’s attention. The converse could also be predicted. That is, if chimpanzees could understand that vocalizations were not needed when a human was oriented toward them, then they should exhibit fewer attention getting behaviors, vocalize less, and instead use significantly more visual means of communication, notably gestures.
Method
Subjects
Subjects were 49 chimpanzees (Pan troglodytes) housed at the Yerkes Regional Primate Research Center in Atlanta, Georgia. Of the 49 chimpanzees (23 females and 26 males), 30 were nursery reared and 19 were mother reared. All of the chimpanzees were adults or subadults with a mean age of 21 years, 5 months (SD = 10 years, 6 months).
Materials and Apparatus
A Hitachi VM-2500A VHS video camera recorder (Hitachi, Ltd., Peoria, IL) and a Velbon PH-656Q tripod (Velbon, Los Alamitos, CA) were used to video record the chimpanzees’ behavior during testing. The behavior was then scored using a Sony SLV-393 video cassette recorder (Sony Electronics, New York, NY) and a ProScan color television (Thomson Multimedia, San Diego, CA)
Procedure
To investigate the chimpanzees’ ability to differentiate between the functions of different types of communication, we designed an experiment with three experimental conditions and compared the types of behavior exhibited by the chimpanzees across these conditions. The three conditions included were oriented away, oriented toward, and baseline. In the oriented-away condition, a human (Homo sapiens), the experimenter, knelt down approximately 1 m in front of the chimpanzee’s cage with his or her back facing the chimpanzee while holding half of a banana behind his or her back; thus, the chimpanzee could see the banana but not the human’s face. In the oriented-toward condition, the human knelt down approximately 1 m in front of the chimpanzee’s cage while holding half of a banana in front of him or her and looking directly at the chimpanzee; thus, the chimpanzee could see both the banana and the human’s face. In the third condition, the baseline condition, the human placed half of a banana on the ground about 1 m in front of the chimpanzee’s cage, where the banana could easily be seen but not reached by the chimpanzee. In this condition, the human left the testing area once the banana had been placed on the ground.
To facilitate behavioral coding of the data, we set up a video camera approximately 2 m from the front of the chimpanzee’s cage. In each condition, the human approached the cage and called the chimpanzee’s name if necessary to get the chimpanzee positioned on camera. Then the human announced the chimpanzee’s name and the condition of the trial, positioned him- or herself and the banana in the appropriate orientation for the experimental condition, and used a stopwatch to begin timing a trial duration of 60 s. Once the announcement had been made to the camera, the trial began and the chimpanzee’s behavior was recorded on a videotape for the 60-s duration. At the end of each 60-s trial, the human gave the chimpanzee the banana regardless of its behavior during the trial. Each chimpanzee received one 60-s trial in each of the three experimental conditions. The order of presentation of the three conditions was randomized across subjects using a Latin square procedure, and each chimpanzee received the three conditions consecutively with a 60-s time lapse between trials in which the chimpanzee was allowed to eat the banana from the previous trial. Chimpanzees were tested in their home cages on an individual basis, unless 2 chimpanzees sharing a cage were both within the video camera’s range during the same trial. In these cases, a single set of trials was completed for the cage, and the data were then analyzed twice using a different focal chimpanzee each time.
Behavioral Ethogram
From the videotape, we transcribed the behavior of each chimpanzee during each experimental condition into an ethogram containing 11 behavioral categories. The behavioral categories included vocalization, manual gesture, vocalization and gesture, cage bang, spit, throw, clap, display, lip pout, depart, and other. A vocalization was defined as any noise produced by the focal chimpanzee’s mouth or throat during the trial. No attempt was made to classify the vocalizations into different types. Manual gestures were recorded when the chimpanzees produced a food beg, whole hand point, or indexical point (see Leavens, Hopkins, & Bard, 1996, for descriptions). Although a distinction in the type of gesture produced was noted, this distinction was not retained in the subsequent analyses. When a vocalization and gesture occurred simultaneously, we recorded the behavior as a vocalization and gesture, and the individual components were not recorded separately although the specific type of gesture was noted. Cage bang and clap were recorded as the total number of times one hand made contact with either the cage (for cage bang) or the other hand (for clap) during the trial. An expression was classified as a lip pout when the chimpanzee manipulated its face so that the inside of the bottom lip was exposed, a distinction not unlike that described by Goodall (1986). A throw was considered to occur any time the chimpanzee picked up an object from its cage and forcefully threw it through the cage bars (this did not include gently dropping a piece of food through the bars, which were attempts by some chimpanzees to share food). A spit was recorded any time the chimpanzee expelled saliva or water from its mouth in the direction of the human. A display was recorded when the chimpanzees engaged in the typical display behavior described in chimpanzees by Goodall. A chimpanzee was considered to depart when no part of the chimpanzee could be seen by the video camera. This included departures to the outside portion of the chimpanzee’s cage as well as shifts in its position within the cage where the chimpanzee could no longer be seen by the range of the camera lens. Any other significant communicative action exhibited by the chimpanzee was classified as other (e.g., stomping on the ground, presentation of sexual swelling by females, and sharing food).
In addition to recording the frequencies of each behavior, an experimenter recorded on the videotape the starting time for the onset of each trial and for the first occurrence of each behavior within each 60-s trial. The difference between the onset time for the trial and for the first occurrence of each behavior was then calculated to produce a measure of latency (0 s < x < 60 s) for each exhibited behavior within the trial. Thus for each category, the experimenter recorded two sets of data: the frequency that the behavior occurred and the latency in the production of each behavior.
Interrater Reliability
The data for all 49 chimpanzees were scored by one primary coder. A second coder recorded the frequencies of each behavioral category in 20 chimpanzees for the purposes of assessing interrater reliability. The second coder was naive to the hypothesis of the study but was aware of the conditions that each chimpanzee was exposed to during testing because the conditions were indicated by the experimenter on the videotape. Cohen’s kappa was used to assess reliability, and the coefficients exceeded chance (p < .001) for all categories of behavior except spit (κs = .90, .75, .96, .79, .90, .78, .93, 1.00, and .10 for vocalization, gesture, vocalization and gesture, cage bang, lip pout, depart, other, clap, and spit, respectively). Throw and display were excluded from the analysis because they were not recorded by either coder in the 20 chimpanzees used to determine reliability.
Results
Descriptive Statistics
There was a substantial amount of variation in the frequencies of different categories of behavior as well as interindividual variation in the types of communicative behaviors produced by chimpanzees. Depicted in Table 1 are the total frequencies of each behavior produced in each testing condition as well as the number of chimpanzees contributing to each of the frequencies. Within the oriented-away condition, the most frequently observed behaviors were cage bangs, vocalizations, and gestures. Within the oriented-toward condition, the most frequently observed behaviors were gestures, cage bangs, and claps. Very little communicative behavior was seen in the baseline condition, and the most frequently observed behavior was depart.
Table 1.
Pooled Frequencies and Interindividual Variation in Communicative Behaviors
| Testing condition |
|||
|---|---|---|---|
| Behavioral category | Away | Toward | Baseline |
| Vocalization | |||
| No. of occurrences | 67 | 31 | 10 |
| No. of chimpanzees | 19 | 14 | 9 |
| Gestures | |||
| No. of occurrences | 46 | 128 | 5 |
| No. of chimpanzees | 21 | 38 | 5 |
| Gesture and Vocalization | |||
| No. of occurrences | 21 | 34 | 1 |
| No. of chimpanzees | 11 | 15 | 1 |
| Cage Bang | |||
| No. of occurrences | 100 | 79 | 2 |
| No. of chimpanzees | 16 | 19 | 1 |
| Throw | |||
| No. of occurrences | 4 | 0 | 0 |
| No. of chimpanzees | 1 | 0 | 0 |
| Spit | |||
| No. of occurrences | 27 | 8 | 0 |
| No. of chimpanzees | 8 | 3 | 0 |
| Display | |||
| No. of occurences | 1 | 2 | 1 |
| No. of chimpanzees | 1 | 2 | 1 |
| Clap | |||
| No. of occurrences | 18 | 39 | 1 |
| No. of chimpanzees | 4 | 5 | 1 |
| Lip Pout | |||
| No. of occurrences | 1 | 19 | 0 |
| No. of chimpanzees | 1 | 10 | 0 |
| Depart | |||
| No. of occurrences | 16 | 10 | 35 |
| No. of chimpanzees | 13 | 8 | 24 |
| Other | |||
| No. of occurrences | 16 | 37 | 7 |
| No. of chimpanzees | 9 | 11 | 3 |
Frequency Distribution of First Communicative Behavior
In addition to analyzing the frequency and latency of communicative behavior, we also examined the association between the test condition and the type of communicative behavior that was produced first by the chimpanzees. For this analysis, we calculated the number of chimpanzees that exhibited a vocalization, gesture, vocalization and gesture, spit, clap, cage bang, throw, lip pout, depart, or no behavioral response as their first communicative behavior (throw and display were omitted because they were never observed as the first response in either condition). On the basis of this analysis, the chimpanzee could produce any of the 10 behaviors first for each condition. Thus, there was a .10 probability that a chimpanzee could be categorized into each of these groupings for each behavior. To determine significance, we analyzed the frequencies using a z score to determine whether the number of chimpanzees exhibiting each behavior in each condition was greater than would be predicted by chance. The number of chimpanzees in each category for each behavioral response can be seen in Table 2. Two significant findings were found. In the oriented-away condition, chimpanzees vocalized significantly more than would be predicted by chance (z = 2.90, p < .01). In the oriented-toward condition, chimpanzees gestured significantly more than would be predicted by chance (z = 11.00, p < .001).
Table 2.
Distribution of First Responses Across Different Behavior Categories
| Behavior | Away only | Toward only |
|---|---|---|
| Vocalization | 11 | 5 |
| Gesture | 9 | 28 |
| Gesture and vocalization | 5 | 6 |
| Cage bang | 3 | 2 |
| Clap | 2 | 2 |
| Spit | 3 | 0 |
| Lip pout | 0 | 1 |
| Other | 4 | 2 |
| Depart | 5 | 1 |
| Nothing | 7 | 2 |
Quantitative Analyses of Overall Communicative Behaviors
In the initial analysis, we examined whether there were differences in communicative behavior in the presence or absence of a human. For this analysis, we summed the frequencies of communicative behaviors across all behaviors in the oriented-away, oriented-toward, and baseline conditions. The total frequency of communicative behaviors was compared using a 2 (sex) × 2 (rearing history) × 3 (condition) mixed model analysis of variance. Sex and rearing history were the between-groups variables, and condition was the repeated measure. A significant main effect for condition was found, F(1, 42) = 16.18, p < .05. The mean number of communicative behaviors produced in the oriented-away, oriented-toward, and baseline conditions were 5.71, 6.89, and 0.34, respectively. Post hoc analysis was performed using a paired t test with alpha adjusted using Bonferroni’s correction procedure. Significantly more communicative behaviors were produced in the oriented-away, t(43) = 5.58, p < .001, and oriented-toward, t(43) = 5.62, p < .001, conditions contrasted with the baseline condition. In contrast, no significant difference was found in the total number of communicative behaviors produced between the oriented-away and the oriented-toward conditions, t(48) = 0.88, p > .05.
These findings indicate that the chimpanzees made very few attempts to communicate in the baseline condition, and therefore this condition was not considered in subsequent analyses. Instead, focus was given to determining whether the attentive state of the human affected the specific types of communication used by the chimpanzees. To test this hypothesis, we used a series of paired t tests to compare the mean frequency of each type of communicative behavior in the oriented-toward condition contrasted with the oriented-away condition. For this analysis, if a chimpanzee did not produce the behavior, then it was given a frequency score of zero.
Orientation Effects on Specific Frequencies of Behaviors
A significant effect was found for manual gesture, t(48) = 5.56, p < .001. The chimpanzees produced significantly more manual gestures when the human was oriented toward them (M = 2.61) than when the human was oriented away from them (M = 0.94). Of the 38 chimpanzees that produced this behavior, 31 produced more manual gestures in the oriented-toward condition. A significant effect was also found for lip pout, t(48) = 2.39, p < .05, with significantly more lip pouts produced in the oriented-toward, (M = 0.39) condition than in the oriented-away (M = 0.02) condition. Of the 10 chimpanzees that produced this behavior, only 1 produced it when the human was oriented away. Finally, there was a borderline significant difference for vocalizations, t(48) = 1.68, p < .09, with more vocalizations produced in the oriented-away condition (M = 2.80) than in the oriented-toward condition (M = 1.28). None of the other behaviors differed significantly beyond p < .10.
Orientation Effects on Latency to Produce Specific Behaviors
In the next set of analyses, we analyzed the chimpanzees’ latency to produce a communicative behavior as a function of the attentional status of the human. The number of chimpanzees that produced at least one of the communicative behaviors in either condition varied across the different behaviors. If a chimpanzee produced the behavior in one condition but not in the other, then we gave the chimpanzee a score of 60 s for the condition in which they did not produce the behavior. For example, if a chimpanzee vocalized 15 s into their oriented-away trial and did not vocalize at all during their oriented-toward trial, then we gave the chimpanzee a score of 15 s for the oriented-away condition and a score of 60 s for the oriented-toward condition. If a chimpanzee did not produce the behavior in at least one of the two conditions, then we did not include these scores in the analysis. Thus, the degrees of freedom varied for each behavioral measure according to the number of chimpanzees that produced the behavior.
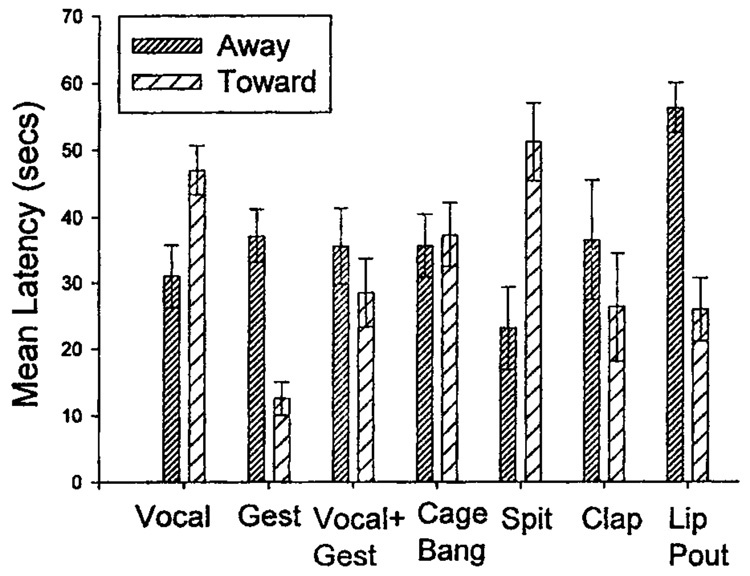
A second set of paired t tests was done to compare the latency of each behavior in the oriented-away and the oriented-toward conditions. The mean latency for each behavior and condition can be seen in Figure 1. The chimpanzees produced their first vocalization faster when the human was oriented away from them than when the human was oriented toward them, t(27) = 2.48, p < .05. As predicted, the opposite was found to be true for gestures. The first occurrence of a manual gesture occurred faster when the human was oriented toward the chimpanzee than when the human was oriented away from the chimpanzee, t(36) = 4.98, p < .001. There was also a significant effect for spit, t(8) = 3.57, p < .01. Chimpanzees were quicker to spit when the human was oriented away from them than when the human was oriented toward them. Conversely, chimpanzees were slower to display a lip pout when the human was oriented away from them than when the human was oriented toward them, t(9) = 5.79, p < .001.
Figure 1.
The mean latency (in seconds) for the first production of each type of communicative behavior in both the oriented-away and the oriented-toward conditions. Vocal = vocalization; Gest = gesture; Vocal + Gest = vocalization and gesture.
Discussion
The results of this study indicate that chimpanzees modify their communicative behavior in response to the attentional state of a human. When the human was oriented away from the chimpanzee, it was more likely to produce a vocalization as its first communicative response. In addition, in the oriented-away condition, chimpanzees produced either a vocalization or a spit faster compared with the oriented-toward condition. In contrast, when the human was oriented toward the chimpanzee, it was more likely to gesture as its first communicative behavior. Chimpanzees were also faster to gesture or to lip pout in the oriented-toward condition compared with the oriented-away condition. Finally, in terms of frequency, the chimpanzees produced significantly more gestures and lip pouts in the oriented-toward condition compared with the oriented-away condition. In contrast, the chimpanzees produced more vocalizations in the oriented-away condition compared with the oriented-toward condition, although this effect was significant only at the p < .10 level.
In the initial analysis, we found that the overwhelming majority of chimpanzees did not produce any communicative behaviors when there was no human present. In short, communicative behaviors were only produced in the presence of a human. These findings are in agreement with previous research on the influence of a social agent on the production of communicative behavior in chimpanzees and in other great apes (the so-called audience effect; see Call & Tomasello, 1994; Hyatt & Hopkins, 1998; Krause & Fouts, 1997; Leavens & Hopkins, 1998; Leavens et al., 1996; Tomasello et al., 1994).
The basic premise of our hypothesis was that chimpanzees would modify the type of communicative behavior they produced in response to the attentional state of a human. Evidence from earlier studies (Povinelli & Eddy, 1996) suggesting that chimpanzees understand the attentional state of their audience on the basis of eye gaze and body orientation was replicated in our study, as the chimpanzees used significantly more visual means of communication (manual gestures and facial expressions) when the human was oriented toward them than when the human was oriented away from them. Similarly, chimpanzees produced manual gestures and facial expressions significantly faster when the human was oriented toward them than when the human was oriented away from them. Because the production of visual communicative behavior changed as a function of the human’s attentional state, it seems highly likely that chimpanzees can distinguish a human’s attentional state on the basis of the human’s orientation and can understand this orientation (and thus attention) as a prerequisite for successful communication in the visual domain.
The unique finding from this study was the use of communication in other modalities, specifically in the chimpanzees’ use of auditory or tactile means as a way of gaining a human’s attention. Analyses of the latency and, to a lesser extent, frequency of the first communicative behavior all indicated that chimpanzees used vocalizations to capture the attention of an otherwise inattentive human. This conclusion is principally supported by the fact that the chimpanzees were more likely to vocalize as their first communicative behavior when the human was oriented away from them compared with oriented toward them. Moreover, the chimpanzees vocalized and spit faster when the human was oriented away from them compared with oriented toward them. Although Tomasello et al. (1994) dismissed the possibility that chimpanzees might use vocalizations to capture the attention of an otherwise inattentive audience, our results clearly suggest that chimpanzees can. Our results are also at odds with those reported by Theall and Povinelli (1999), and this may be explained, in part, by differences in sample size (7 vs. 49) and the age of the chimpanzees (juveniles contrasted with adults). The issue of sample size seems particularly relevant considering the amount of interindividual variation in the production and use of vocalizations seen in our sample (see Table 1).
In light of the fact that the chimpanzees modified their use of vocalizations in response to the different attentional states of the human, these results suggest that the chimpanzees have some voluntary control of their vocalizations. Historically, primate vocalizations have typically been recognized as by-products of affective states primarily controlled by the limbic system (Locke, 1995; Steklis & Raleigh, 1979b; Sutton, Trachy, & Linderman, 1981) and not under voluntary control. Previous studies with monkeys and apes have demonstrated that they can be taught to produce species-specific vocalizations in response to specific visual cues in classic operant-conditioning paradigms (e.g., Randolph & Brooks, 1967). The results from this study differ from these previous reports in two ways. First, the stimulus controlling the vocal behavior of the chimpanzees was social (visual orientation of a human) rather than an arbitrary nonsocial visual stimulus. Second, the vocalizations produced by the chimpanzees were not sounds that are typically produced in the presence of food. Although no spectrographic analyses were performed, few if any of the vocalizations produced by the chimpanzees were food calls or grunts that are typically produced in the presence of food. In fact, few if any of the vocalizations produced by the chimpanzees fell neatly into any of the vocal categories described for wild or captive chimpanzees (see Goodall, 1986, for summary). Rather, they were idiosyncratic sounds and grunts that have probably been learned as a result of their captive rearing, and they have learned to use them in instrumental ways to capture the attention of an otherwise inattentive audience. Whether specific vocalizations are associated with specific gestures or other communicative behaviors is unclear from this study, but this issue warrants further investigation.
One limitation of this study was the fact that not all chimpanzees were tested alone. It could be argued that the observed effects for increased use in vocal communication in the oriented-away condition were due, in part, to the cage mate of one chimpanzee vocalizing in response to their cage mate’s vocalizing rather than to the orientation of the human holding the food. Although this explanation cannot be completely ruled out, examination of the data does not support this interpretation. Specifically, of the chimpanzees that were housed together and observed to vocalize in either the oriented-toward or the oriented-away condition, in fewer than 20% of the observations were the chimpanzees from the same group. In other words, there were many more dyads or groups of chimpanzees in which only one chimpanzee was observed to vocalize compared with dyads or groups in which more than one chimpanzee vocalized.
In summary, the results of this study indicate that chimpanzees modify their communicative behavior in response to the attentional status of a human. Although the chimpanzees may have been producing vocalizations as a result of their affective state, the present study provides evidence to suggest that they are capable of manipulating this behavior for seemingly intentional communicative purposes (getting an inattentive audience’s attention). Moreover, it suggests that chimpanzees have at least partial volitional control over their vocalizations and use them in a functionally meaningful way. How the communicative behaviors expressed by the chimpanzees in this study were acquired remains unclear. Whether similar differential use of vocal, tactile, and gestural communication would be found in wild chimpanzees under comparable circumstances is unknown. However, continued laboratory and field studies with chimpanzees should provide important information on both of these dimensions of communicative behavior and on its relationship to the evolution of human language and speech (Jürgens, 1995; Lieberman, 1995; Steklis & Raleigh, 1979b).
Acknowledgments
This research was supported by National Institutes of Health Grants NS-29574, NS-36605, HD-38051, and RR-00165 to the Yerkes Regional Primate Research Center and to the Living Links Center for the Advanced Study of Ape and Human Evolution. The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. American Psychological Association guidelines for the ethical treatment of animals were adhered to during all aspects of this study.
Contributor Information
Autumn B. Hostetter, Department of Psychology, Berry College, and Division of Psychobiology, Yerkes Regional Primate Research Center, Emory University
Monica Cantero, Department of Foreign Languages, Berry College..
William D. Hopkins, Department of Psychology, Berry College, and Division of Psychobiology, Yerkes Regional Primate Research Center, Emory University
References
- Bard KA. Intentional behavior and intentional communication in young free-ranging orangutans. Child Development. 1992;63:1186–1197. [PubMed] [Google Scholar]
- Bates E, Camaioni L, Volterra V. Performatives prior to speech. Merrill-Palmer Quarterly. 1975;21:205–226. [Google Scholar]
- Call J, Tomasello M. Production and comprehension of referential pointing by orangutans (Pongo pygmaeus) Journal of Comparative Psychology. 1994;108:307–317. doi: 10.1037/0735-7036.108.4.307. [DOI] [PubMed] [Google Scholar]
- Erwin J. Rhesus monkey vocal sounds. In: Bourne GH, editor. The rhesus monkey. Vol. 1. New York: Academic Press; 1975. pp. 365–380. [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns in adaptation. Cambridge, MA: Harvard University Press; 1986. [Google Scholar]
- Gouzoules H, Gouzoules S, Ashley J. Representational signaling in non-human primate vocal communication. In: Zimmermann E, Newman JD, Jürgens U, editors. Current topics in primate vocal communication. New York: Plenum Press; 1995. pp. 235–252. [Google Scholar]
- Hyatt CW, Hopkins WD. Interspecies object exchange: Bartering in apes? Behavioural Processes. 1998;42:177–187. doi: 10.1016/s0376-6357(97)00075-2. [DOI] [PubMed] [Google Scholar]
- Jürgens U. Neuronal control of vocal production in non-human and human primates. In: Zimmerman E, Newmann JD, Jürgens U, editors. Current topics in primate vocal communication. New York: Plenum Press; 1995. pp. 199–206. [Google Scholar]
- Krause MA, Fouts RS. Chimpanzee (Pan troglodytes) pointing: Hand shapes, accuracy, and the role of eye gaze. Journal of Comparative Psychology. 1997;111:330–336. doi: 10.1037/0735-7036.111.4.330. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD. Intentional communication by chimpanzees: A cross-sectional study of the use of referential gestures. Developmental Psychology. 1998;34:813–822. doi: 10.1037//0012-1649.34.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD. The whole-hand point: The structure and function of pointing from a comparative perspective. Journal of Comparative Psychology. 1999;113:417–425. doi: 10.1037/0735-7036.113.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD, Bard KA. Indexical and referential pointing in chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1996;110:346–353. doi: 10.1037/0735-7036.110.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. What primate calls can tell us about human evolution. In: Zimmerman E, Newmann JD, Jürgens U, editors. Current topics in primate vocal communication. New York: Plenum Press; 1995. pp. 273–282. [Google Scholar]
- Locke JL. Linguistic capacity: An ontogenetic theory with evolutionary implications. In: Zimmerman E, Newmann JD, Jürgens U, editors. Current topics in primate vocal communication. New York: Plenum Press; 1995. pp. 253–272. [Google Scholar]
- Marler P. Primate vocalization: Affective or symbolic? In: Sebeok TA, Umiker-Seboek J, editors. Speaking of apes. New York: Plenum Press; 1980. pp. 221–229. [Google Scholar]
- Myers RE. Comparative neurology of vocalization and speech: Proof of a dichotomy. Annals of the New York Academy of Sciences. 1976;280:745–757. doi: 10.1111/j.1749-6632.1976.tb25537.x. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ, Eddy TJ. Factors influencing young chimpanzees’ (Pan troglodytes) recognition of attention. Journal of Comparative Psychology. 1996;110:336–345. doi: 10.1037/0735-7036.110.4.336. [DOI] [PubMed] [Google Scholar]
- Randolph MC, Brooks BA. Conditioning of a vocal response in a chimpanzee through social reinforcement. Folia Primatologica. 1967;5:70–79. doi: 10.1159/000161938. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL. Behavioral mechanisms underlying vocal communication in nonhuman primates. Animal Learning & Behavior. 1997;25:249–267. [Google Scholar]
- Smith WJ. The behavior of communicating. Cambridge, MA: Harvard University Press; 1977. [Google Scholar]
- Steklis HD, Raleigh MJ. Behavioral and neurobiological aspects of primate vocalization and facial expression. In: Steklis HD, Raleigh MJ, editors. Neurobiology of social communication in primates. New York: Academic Press; 1979a. pp. 257–282. [Google Scholar]
- Steklis HD, Raleigh MJ. Requisites for language: Inter-specific and evolutionary aspects. In: Steklis HD, Raleigh MJ, editors. Neurobiology of social communication in primates. New York: Academic Press; 1979b. pp. 283–314. [Google Scholar]
- Sutton D, Larson C, Taylor EM, Linderman RC. Vocalizations in rhesus monkeys: Conditionability. Brain Research. 1973;53:225–231. doi: 10.1016/0006-8993(73)90660-4. [DOI] [PubMed] [Google Scholar]
- Sutton D, Trachy RE, Linderman RC. Primate phonation: Unilateral and bilateral cingulate lesion effects. Behavioural Brain Research. 1981;3:99–114. doi: 10.1016/0166-4328(81)90031-0. [DOI] [PubMed] [Google Scholar]
- Theall LA, Povinelli D. Do chimpanzees tailor their gestural signals to fit the attentional states of others? Animal Cognition. 1999;2:207–214. [Google Scholar]
- Tomasello M, Call J, Nagell K, Olguin R, Carpenter M. The learning and use of gestural signals by young chimpanzees: A trans-generational study. Primates. 1994;35:137–154. [Google Scholar]