Abstract
Research on the regulation and function of ascending noradrenergic, dopaminergic, serotonergic, and cholinergic systems has focused on the organization and function of individual systems. In contrast, evidence describing co-activation and interactions between multiple neuromodulatory systems has remained scarce. However, commonalities in the anatomical organization of these systems and overlapping evidence concerning the post-synaptic effects of neuromodulators strongly suggest that these systems are recruited in concert; they influence each other and simultaneously modulate their target circuits. Therefore, evidence on the regulatory and functional interactions between these systems is considered essential for revealing the role of neuromodulators. This postulate extends to contemporary neurobiological hypotheses of major neuropsychiatric disorders. These hypotheses have focused largely on aberrations in the integrity or regulation of individual ascending modulatory systems, with little regard for the likely possibility that dysregulation in multiple ascending neuromodulatory systems and their interactions contribute essentially to the symptoms of these disorders. This review will paradigmatically focus on neuromodulator interactions in the PFC and be further constrained by an additional focus on their role in cognitive functions. Recent evidence indicates that individual neuromodulators, in addition to their general state-setting or gating functions, encode specific cognitive operations, further substantiating the importance of research concerning the parallel recruitment of neuromodulator systems and interactions between these systems.
Keywords: acetylcholine, dopamine, serotonin, norepinephrine, cognition, co-modulation
1. Ascending modulatory systems and cognition: focus on the modulation of prefrontal functions
Ascending neuromodulatory systems include the noradrenergic, serotonergic, dopaminergic, and cholinergic projections from brainstem and basal forebrain regions to the cortex. Traditionally, neuromodulators have been defined as such based on: 1) the reticular organization of the soma of these neurons and the limited topographic organization of their projections; 2) extensive collateralization of projections to the forebrain, which also reflects a relatively low ratio between the number of projection neurons of the neuromodulator system and estimates of the number of neurons targeted by individual systems; 3) evidence for spatially distributed and relatively uniform effects on target neurons, based in part on axonal collateralization and volume transmission; 4) evidence for slowly changing, or tonic effects of target neurons based, again, in part on volume transmission (e.g., Agnati et al., 2006; Katz, 1999).
The usefulness of these criteria and, more fundamentally, the uniform categorization of these neuronal systems as neuromodulator systems has remained a subject of debate. For example, the degree of collateralization of cholinergic projections to the cortex appears to be limited (Price et al., 1990; Price and Stern, 1983; Walker et al., 1985). In addition, the degree to which ACh acts extra-synaptically is unclear considering the highly concentrated presence and extremely potent metabolizing enzyme AChE in terminal regions. Furthermore, results from ultrastructural analyses of the density and location of pre- and postsynaptic sites question the suggestion that ACh acts extra-synaptically (Mechawar et al., 2000; Turrini et al., 2001).
It is not just the characterization of ACh that incites debate, however; anatomical and functional analyses of ascending modulatory systems have suggested that individual systems are composed of multiple subsystems, or modules, that could differentially influence the processing of information in different telencephalic regions (Aston-Jones and Cohen, 2005a; 2005b; Golmayo et al., 2003; Zaborszky, 2002). Furthermore, as will be discussed below, recent experiments indicate that in addition to the relatively slow, or tonic, changes in the activity of neuromodulator systems (over minutes), faster, transient, or phasic, components of activity are evoked by defined cognitive and behavioral activities. Therefore, in addition to the more global regulation of “arousal” or the readiness for input processing (Pribram and McGuinness, 1975), neuromodulators appear to influence and perhaps even initiate the processing of highly specific cognitive operations.
Examples of these specific actions include evidence suggesting that decision outcomes are encoded by noradrenergic neurons in the LC of animals performing cued signal detection tasks (Aston-Jones and Cohen, 2005b), and the incorporation of conditioned stimuli into the cognitive process by cholinergic inputs to the PFC (Parikh et al., 2006). Likewise, studies on the effects of neuromodulator-specific lesions on cognitive performance have yielded highly specific hypotheses about the necessity of neuromodulatory projections for cognitive functions (e.g., McGaughy et al., 1996; Mobini et al., 2000; Stefani and Moghaddam, 2006; Turchi and Sarter, 1997; 2000; see below for further references).
Such evidence indicates that traditional hypotheses describing ascending modulatory systems merely as “state-setting” or “gating” systems, to regulate “arousal” or the “readiness for cortical information processing”, are incomplete. The separate categorization of neuromodulators as such has been further challenged by evidence indicating extra-synaptic effects of amino acid neurotransmitters (e.g., Del Arco et al., 2003) or that glutamatergic projections are a component of ascending brain stem and basal forebrain systems that have traditionally been considered arousal-mediating systems (e.g., Gritti et al., 2003).
Although recent evidence indicates that modulator systems exert highly specific effects on forebrain systems (see below), promising a wide-ranging revision of traditional conceptualizations of the regulation and function of neuromodulator systems, the functional significance of the more global, tonic, influences of these systems on forebrain circuits likewise remains inadequately understood. The fact that hypotheses about dysregulated neuromodulator systems represent a central tenet in theories on the neuronal mediation of the core symptoms of neuropsychiatric disorders, including schizophrenia, anxiety disorders, and depression, is a stark reminder that abnormalities in the regulation of the tonic components of neuromodulator activity may be key to understanding these disorders.
This review is guided by the general hypothesis that understanding the regulation and function of a neuromodulator system requires evidence on the interactions between these modulators (for an early account concerning the significance of such interactions see Decker and McGaugh 1991). As will be pointed out below, there are striking commonalities in the anatomical organization of modulator systems, as well as direct interactions between these systems. Collectively, these commonalities illustrate that these systems are regulated, perhaps necessarily, in a highly orchestrated fashion. Conversely, these organizational properties, including closed-loop circuits with prefrontal regions, suggest that it is extremely unlikely that individual systems are recruited separately. Unfortunately, information about the interactions between these neuromodulatory systems, both at the neuronal level and with respect to functional implications, has remained scarce.
This review is intended to stress the significance of such research. It will not provide a comprehensive overview of the anatomical organization and functions of individual modulator systems as can be found elsewhere (e.g., Aston-Jones and Cohen, 2005b; Berridge et al., 1993; Everitt and Robbins, 1997; Geyer, 1996; Sarter et al., 2005a). In an attempt to further focus this review, we will constrain our discussion to modulator interactions in the PFC and the significance of these interactions for cognitive functions. Additionally, we will focus particularly on the regulation of cholinergic activity in this region by other neuromodulators. The BFCS represents the most rostral of all ascending modulatory systems. Its neurons and terminals are targeted by the other modulators (see below and Figure 2), and the basic cognitive functions mediated via prefrontal cholinergic activity are relatively well defined (e.g., Apparsundaram et al., 2005; Kozak et al., 2006; Sarter et al., 2006; Sarter et al., 2005a). Therefore, the goal of this review is to conceptualize the role of noradrenergic, dopaminergic, and serotonergic modulation of cholinergic function, and vice versa, in prefrontal regions. Following a brief overview of the organization and functions of the main neuromodulatory systems, the functional implications of common anatomical features will be addressed. We will then review the evidence concerning modulation of prefrontal cholinergic neurotransmission by serotonergic, noradrenergic, and dopaminergic ascending systems. Finally, hypotheses and speculations about the behavioral and cognitive impact of modulation of cholinergic activity in the PFC will be discussed. As neuromodulators act in parallel and in collaboration with one another (e.g., Yu and Dayan, 2005), research addressing co-modulation and interactions between modulator systems, as opposed to the isolated investigation of individual neuromodulator systems, is critical for the development of hypotheses that describe the fundamental roles of the ascending neuromodulatory systems.
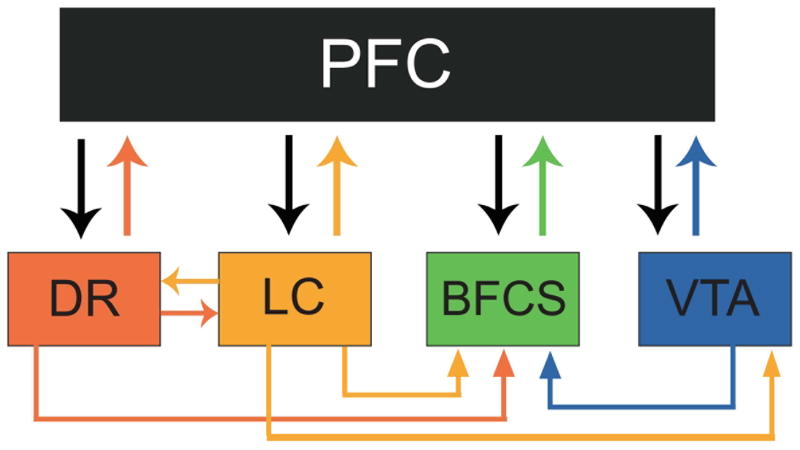
Figure 2.
The dorsal raphe (DR), locus coeruleus (LC), basal forebrain cholinergic system (BFCS), and the ventral tegmental area (VTA) all send projections to and receive projections from the PFC. These parallel feedback loops provide a mechanism through which serotonergic, noradrenergic, cholinergic, and dopaminergic modulatory systems can directly affect cortical targets and be modulated by descending glutamatergic projections. Feedback to ascending neuromodulatory systems can occur directly from the PFC or indirectly via feedback loops from other modulatory nuclei at the levels of the brainstem, midbrain, and basal forebrain. For example, as shown above, the caudal principal LC sends substantial projections to the caudal, ventromedial, and intrafasicular DR and in return, the caudal DR and ipsilateral wing of the DR sends heavy projections to the mid-ventral and caudal dorsal LC. Ascending noradrenergic and serotonergic projections, along with projections from the VTA innervate the BFCS. In addition to sharing a parallel and closed-loop system organization, 5-HT, NE (NE), DA, and ACh neuromodulator systems exhibit overlapping innervation patterns and terminal fields.
1.1. The prefrontal cortex: a brief review of structure and function
The PFC is generally considered to include those regions of the frontal cortex that extend anterior to the motor and premotor regions of the frontal lobes (Uylings and van Eden, 1990). Since Brodmann first described the anatomical boundaries of the PFC, there have been several historical revisions of the definition of PFC proper and the rules governing cross-species homology. Although considerable differences exist across species in size and cytoarchitecture, relative comparisons can be drawn based on neural connections and anatomical necessity for behavioral function (Kolb, 1984). Early revisionists such as Walker (1940) and Rose and Woolsey (1948) proposed defining PFC regions based on anatomical connectivity with thalamic and cortical regions. Using this delineation, PFC regions were defined as having “essential cortical projections” from the MD (Rose and Woolsey, 1948). Later, with the advent of better staining techniques, it also became clear that reciprocal connections existed between the MD and many frontal brain regions. Several investigators have proposed that this feature should also be applied when making cross-species comparisons of mammalian PFC homology (Nauta, 1962; Leonard, 1969; for a comprehensive review on this topic see Campbell and Hodos, 1970 and Uylings and van Eden, 1990). The application of this standard, however, produces an unresolved debate about the validity of species comparisons between the anatomical boundaries of different mammalian PFC regions, and subsequently, comparisons of apparent cognitive function (Brown and Bowman, 2002). The debate is less contentious in comparisons of non-human primates with humans; yet, in comparisons of primates with rodents it remains controversial. The argument stems primarily from the basis that the MD sends projections to dorsal lateral regions in humans and non-human primates; however, no such projections exist to dorsal lateral regions in rodents. Instead, the rodent forebrain only receives projections to medial and orbital regions from the MD. Nonetheless, recent studies purport that functions requiring dorsal lateral integrity in primates and humans can be mapped onto orbital and medial regions of the rat frontal lobe – including experimental data that provides behavioral support for shared functional comparisons in working memory tasks in monkeys (Miller, 2000) and rats (Kesner, 2000), as well as in attentional set-shifting behavior in humans (Owen et al., 1991) and rats (Birrell and Brown, 2000; for a detailed review of cross-species homology see Kolb, 1990; Dalley et al., 2004).
The PFC collectively consists of an interconnected network of sub-regions that sends and receives projections from virtually all cortical sensory and motor systems, as well as a number of subcortical structures. Findings from human, non-human primate, and rodent species suggest that specific aspects of cognitive processing are deferentially weighted across distinct sub-regions of the PFC. The lateral and mid-dorsal PFC are thought to be closely associated with sensory processing, and these regions receive auditory, visual, and somatosensory information from temporal, occipital, and parietal cortices (Goldman-Rakic and Schwartz, 1982; Barbas and Pandya, 1989). The medial PFC, along with orbital regions, shares connections with limbic structures critical for memory and the processing of internal states such as motivation and affect (Amaral and Price, 1984; Barbas and De Olmos, 1990). This region is also thought to be important for the process of behavioral inhibition (Fuster, 1980; Goldman-Rakic, 1987). The vlPFC is thought to be primarily involved in the perceptual processing of face and object visual stimuli, the integration of mnemonic information from limbic regions (Funahashi et al., 1990; Wilson et al., 1993; Miller, 2000), and the maintenance of directed attention (Goldman-Rakic, 1987; Fuster, 1980). The dlPFC is most robustly connected with motor system structures, including supplementary motor areas, pre–supplementary motor areas, and the cerebellum, as well as the cingulate and the superior colliculus (Goldman and Nauta, 1976; Bates and Goldman-Rakic, 1993; Lu et al., 1994; Schmahmann and Pandya, 1997). Additionally, the dlPFC is thought to play an integral part in the regulation of reflexive or mechanistic behaviors (Burgess, 2000; Fuster, 2000; Goel and Dolan, 2000), and has been implicated in several working memory tasks including the encoding of the locations of visual objects in space (Cavada and Goldman-Rakic, 1993; Kinberg, 1993; Chafee and Goldman-Rakic, 1998; for further anatomical sub-divisions of the PFC see, e.g., Fuster, 1980; Damasio, 1998; Goldman-Rakic, 1998).
The importance of ascending neuromodulator regulation of PFC function can be exemplified by looking at studies involving selective lesions of neuromodulatory systems or the pharmacological manipulations of receptors in the PFC. For example, pharmacological alteration of normal DA levels results in impaired performance in set-shifting tasks (Goto and Grace, 2005) as well as the initiation of goal-directed behaviors (Kiyatkin and Rebec, 2001). Changes in optimal levels of NE and ACh act to decrease performance in tasks of attentional processing (Arnsten, 1997; Kozak et al., 2006), while neurotoxic lesions of DA and NE terminals in the dlPFC produce working memory deficits in spatial working memory tasks nearly as severe as animals with direct lesions of the dlPFC itself (Brozoski et al., 1979). Similar results have been described in other mammalian species including marmosets and rats (Roberts et al., 1994; Schroder et al., 2003).
As indicated by an ever-expanding body of findings similar to those described above, PFC function is thought to underlie many of our cognitive or “executive” actions, including working memory, behavioral inhibition, attentional processing, and future planning. PFC-mediated impairments can occur as a process of aging (Bartus et al., 1979) or disease (Kalaria and Andorn, 1991; Muller et al., 2000) and normal cognitive functioning can be temporarily disrupted during periods of stress or anxiety (Hartley and Adams, 1974). Additionally, nearly all hypotheses of psychiatric disorders, including Alzheimer’s disease (Kalaria and Andorn, 1991), Parkinson’s disease (Muller et al., 2000), schizophrenia (Breier et al., 1997; Laruelle et al., 1999; Kapur, 2003), depression (Drevets et al., 1998; Arango et al., 2002), and attentional disorders (Mattes 1980; Barkley et al., 1992; Rubia et al., 2005), theorize that abnormal PFC function is a result of the dysregulation of one or more of the ascending neuromodulatory systems discussed in this review. A key tenet for many of the disorders listed above is a similar etiology and symptomatology that can be attenuated or augmented by a range of treatments that all target the activity of neuromodulator systems. The degree of overlap of the terminal fields of neuromodulators in the PFC (Figure 1) suggests the significance of modulator interactions for prefrontal functioning, and of abnormal interactions for the manifestation of cognitive symptoms.
Figure 1.
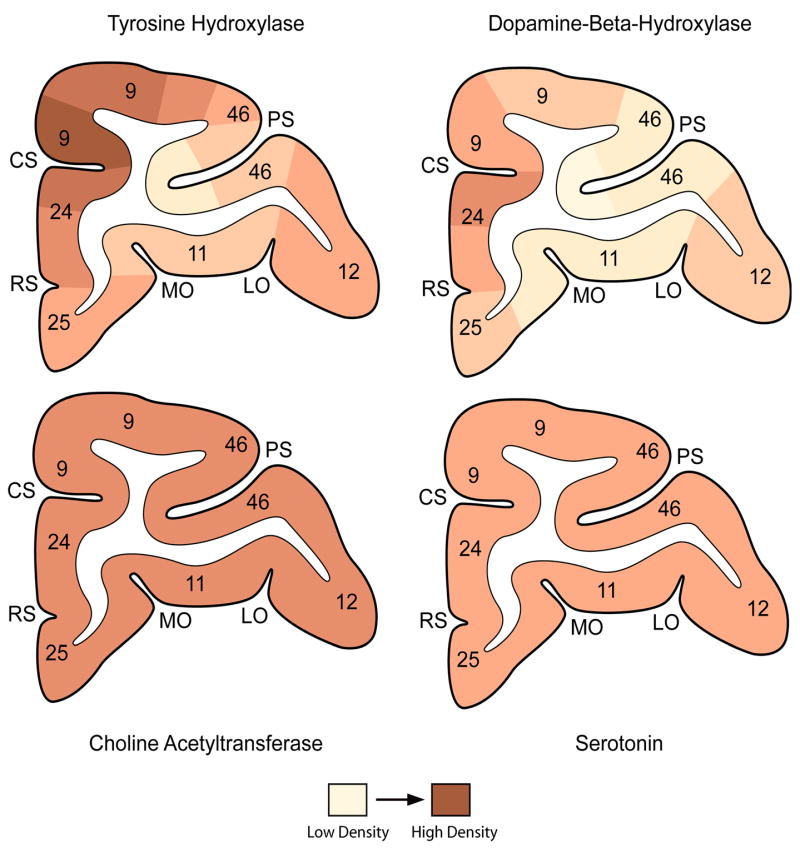
Schematic representation of coronal sections from the macaque monkey PFC illustrating the relative densities of tyrosine hydroxylase (DA), dopamine β-hydroxylase (NE), choline acetyltransferase (ChAT), and serotonin-containing axons. Numbers refer to the cortical areas described by Walker (1940). CS, cingulate sulcus; LO, lateral orbital sulcus; MO, medial orbital sulcus; PS, principal sulcus; and RS, rostral sulcus. This figure was published originally in Lewis et al. (1992) and re-drawn and colorized by Mary L. Brady (Photomicrography & Graphics Specialist, Translational Neuroscience Program, University of Pittsburgh). Reproduced with permission of the author (D.A. Lewis) and Nature Publishing Group (License Number 1676551084929).
2. Cholinergic projections to prefrontal regions: anatomical organization and function
The ascending cholinergic system originates from nuclei located within the brainstem and basal forebrain (Mesulam et al., 1983). The cholinergic neurons of the brainstem, the pedunculopontine nucleus and laterodorsal tegmental nucleus, innervate several thalamic nuclei and basal forebrain areas (Rotter and Jacobowitz, 1981; Rye et al., 1987; Woolf and Butcher, 1986) and have been hypothesized to initiate paradoxical (REM) sleep (Maloney et al., 1999). The term “basal forebrain” refers to cholinergic neurons that are present in the horizontal and vertical limbs of the diagonal band, the nbM, SI, the magnocellular preoptic nucleus, and the nucleus ansa lenticularis (Armstrong et al., 1983; Mesulam et al., 1983; Woolf et al., 1983), which collectively provide the major source of cholinergic innervation to the cortex. Given the functional significance of this terminal field, the cholinergic contribution to cognitive processes is overwhelmingly discussed in terms of these cortically projecting cholinergic neurons and, as such, will serve as the focus of the following review.
Neurons of the BFCS project along two major anatomical pathways (Saper, 1984). The medial pathway begins in the medial septal nucleus, the nucleus of the diagonal band, the medial SI, and the ventral wall of the GP. Axons traveling along this pathway traverse through the septum and run laterally along the genu of the corpus callosum. From this point, these axons diverge and either innervate the medial frontal cortex or travel back around the genu of the corpus callosum, where they bifurcate and project either to the structures of the medial cingulate bundle, such as the anterior cingulate and retrosplenial cortex, or turn ventrally to innervate the hippocampus (Saper, 1984). The lateral pathway is comprised of two subsections. The first consists of cells within the medial septal nucleus, nucleus of the diagonal band, and the magnocellular preoptic nucleus. These axons project laterally through the SI to innervate the piriform, entorhinal, and perirhinal cortices. Projections from the peripherally situated portions of the SI and GP represent the second component of this pathway and extend laterally through the ventral striatum into the external capsule and innervate the cortex (Saper, 1984).
In primates, the primary source of cholinergic innervation of the PFC arises from the magnocellular neurons of the nBM. The projections from these neurons to the PFC exhibit a rough topographic organization; cells of the anteromedial and anterolateral nBM preferentially project to the medial PFC, while those situated in the intermediate and posterior regions project to orbitofrontal and lateral PFC areas, respectively (Ghashghaei and Barbas, 2001). The primary source of neocortical cholinergic input in the rodent stems from projection neurons of both the nBM and SI (Armstrong et al., 1983; Luiten et al., 1987; Mesulam et al., 1983). In this species, more overlap is observed in the pattern of prefrontal innervation; both cells situated in the anterior and intermediate nBM/SI innervate the medial PFC, although, as observed in non-human primates, projections arising from cells in the posterior regions target more lateral areas of the frontal cortex (Luiten et al., 1987).
The axons of cortically projecting basal forebrain cholinergic neurons innervate all layers of the PFC, although most notably layers I, III, and V (Mrzljak and Goldman- Rakic, 1992; Mrzljak et al., 1993; Mrzljak et al., 1995). Cholinergic projections synapse primarily on to pyramidal neurons, the largest concentrations of which are found in cortical layers III and V (Mrzljak and Goldman-Rakic, 1992), a lamina-specific pattern of innervation that may be indicative of the regions most directly involved in cholinergic modulation of cognitive function (Lysakowski et al., 1989).
The nBM receives inputs from telencephalic, diencephalic, and brainstem regions including the amygdala, hippocampal formation, thalamus, reticular formation, LC, laterdorsal tegmental nucleus, and raphe nucleus (Carnes et al., 1990). Dopaminergic neurons from the VTA (Gaykema and Zaborszky, 1996; Smiley et al., 1999) and noradrenergic regions A1 and A2 both provide substantial input to the basal forebrain and provide direct synaptic contact with cholinergic neurons (Espana and Berridge, 2006; Smiley et al., 1999). At the cortical level, the medial prefrontal and orbitofrontal cortices, which receive projections from the nBM, share reciprocal connections with the basal forebrain. Glutamatergic projections from the PFC represent a major source of input to the basal forebrain and therefore are likely to influence cortically projecting cholinergic neurons (Zaborszky et al., 1997). Furthermore, approximately 40% of prefrontal projections to the basal forebrain terminate on parvalbumin-immunoreactive dendritic shafts and, therefore, presumably GABAergic neurons (Zaborszky et al., 1997). These feedback loops to the basal forebrain can hypothetically modulate the reactivity of the BFCS and, thus, have been hypothesized to be a component of the neuronal mechanisms that serve to enhance input processing and the allocation of attentional resources to behaviorally significant stimuli under challenging conditions (Sarter et al., 2006; Sarter et al., 2005a).
ACh released at cholinergic terminals acts on two groups of receptors, metabotropic muscarinic and ionotropic nicotinic ACh receptors. Five isoforms of the muscarinic receptor, separated into two families, M1 (m1, m3, and m5) and M2 (m2 and m4), have been sequenced from brain tissue. In the cortex, both M1 and M2 receptors are distributed throughout each layer, with the M1 family most prevalent in layers I and II, and M2 most concentrated in layers III and V (Spencer et al., 1986). Specifically, m1 receptors have their highest densities in layer I and II, while layer VI houses the largest concentration of m2 receptors. The m3 receptors primarily populate layers IV and V, while m4 receptors are concentrated in layers I and VI. Finally, m5 receptors are distributed evenly across all layers except for a notable absence within layer IV (Tayebati et al., 2006). Although both m1 and m2 receptors are located on postsynaptic neurons, m2 primarily functions as a presynaptic autoreceptor (Mrzljak et al., 1993). Among the nicotinic receptors, the most widespread configuration of the nine possible α-subunits (α2–10) and three identified β-subunits (β2-β4) is the α4β2 subtype, which is dispersed in many areas of the brain, including the frontal cortex (Perry et al., 2002).
Several techniques have been utilized in attempt to parse out the functional relevance of BFCS projections to the cortex. The effects of endogenously released ACh have been modeled via the exogenous application of the neurotransmitter in discrete brain regions. Metherate and Weinberger (1989) demonstrated in the auditory cortex that the administration of ACh, paired with a repeated, pure-tone stimulus, enhanced stimulus-specific neuron activity, or “signal”, while having no effect on spontaneous firing, or “noise”. Furthermore, ACh can enhance responses to previously “weak” signals by decreasing the threshold needed to elicit an action potential (Metherate et al., 1990). This phenomena is believed to be mediated through muscarinic, specifically M1, receptors, and reflects the ability of the cholinergic system to heighten the signal-to-noise ratio for relevant sensory stimuli. Additional evidence supporting the role of ACh in the modulation of the signal to noise ratio comes directly from electrophysiological studies performed by Metherate and Ashe. The investigators were able to demonstrate that stimulation of the BFCS during periods of thalamocortical stimulation directly gates the physiological response of auditory cortex neurons. This research has recently been interpreted to suggest an ability of ACh to both enhance ascending input to the cortex from sensory systems while suppressing spontaneous spiking (Hasselmo and McGaughy, 2004). Thus, the combined effects of ACh in cortical regions can shape the integration of stimuli into associative networks involved in attention (Hasselmo and McGaughy, 2004). Via stimulation of both nAChRs and muscarinic receptors, ACh generally has been suggested to gate information flow between the cortical layers, allowing for greater attentional selectivity for salient stimuli (Munk et al., 1996; Xiang et al., 1988).
Studies employing the immunotoxin 192 IgG-saporin, which selectively targets the p75 low-affinity nerve growth factor expressed by neurons of the BFCS (Book et al., 1992; Wiley et al., 1991), have served as the primary source of information regarding the cognitive functions subserved by cortical cholinergic inputs. Infusions of this cholinotoxin into the nbM/SI or cortical regions results in persistent and robust deficits in attentional performance, specifically impairing animals’ ability to detect cues indicative of reward (McGaughy et al., 1996; McGaughy and Sarter, 1998; Turchi and Sarter, 1997, 2000). Similarly, microdialysis studies have supported a role of the ascending cholinergic system in attentional performance, via the demonstration of increased ACh release in the PFC of animals performing an operant, sustained attention task, and suggest that this release is a function of attentional demand (Arnold et al., 2002; Himmelheber et al., 2000; Kozak et al., 2006).
Although the necessity of the BFCS for essential cognitive functions like attention is well documented, the mechanisms through which this is accomplished remain poorly defined. Both muscarinic and nicotinic receptor function contribute to cholinergic mediation of cognitive processes. nAChRs agonists enhance attentional performance (McGaughy et al., 1999), and nAChRs antagonists were frequently demonstrated to impair such performance (Grottick and Higgins, 2000; Turchi et al., 1995). Systemic administration of the muscarinic antagonist scopolamine impairs working memory, declarative memory, sustained attention, and psychomotor speed (Ellis et al., 2006). Additionally, blockade of muscarinic receptors in the PFC decrease mnemonic function (Broersen et al., 1994; Ragozzino and Kesner, 1998). Furthermore, recent evidence has suggested two modes of cholinergic activity that may uniquely contribute to cognition. The slow, tonic increases in cholinergic activity as indicated by microdialysis studies may be indicative of the state of arousal or readiness for input processing, while transient, or phasic, increases in cholinergic activity, as demonstrated by newly developed electrochemical methods of monitoring ACh release (i.e. Parikh et al., 2006), may underlie specific cognitive functions such as signal detection.
3. Modulator projections to prefrontal regions and the cholinergic basal forebrain
3.1. Noradrenergic system
The ascending noradrenergic system originates within discrete medullary and pontine nuclei of the brainstem (Dahlstrom and Fuxe, 1964). There are three major noradrenergic nuclei that make up this ascending system. At the level of the medulla, the A1 locus resides within the lateral region of the ventral column adjacent to the nucleus ambiguous (Dahlstrom and Fuxe, 1964). Its projections ascend primarily to innervate the nuclei of the hypothalamus. The A2 cells reside within the same horizontal plane but are located within the dorsal columns as part of the nucleus of the solitary tract (Dahlstrom and Fuxe, 1964). Fibers from the A2 region extend to target nuclei that additionally reside in the hypothalamus. The best studied of the ascending noradrenergic nuclei is the LC - or the A6 region – which lies just dorsal to periaqueductal gray of the pons and medial to the trigeminal nucleus (Dahlstrom and Fuxe, 1964; Russell, 1955; Swanson, 1976). The LC is the primary source of noradrenergic input to forebrain regions and is the exclusive provider of NE to regions essential for higher order executive functioning including cognition and affect (Jones and Moore, 1977; Waterhouse et al., 1983). With the notable exceptions of the basal ganglia, the olfactory tubercle, and the NAc, all regions of the telencephalon receive modulatory input via the LC (Berridge and Waterhouse, 2003). Because of the emphasis on cognitive function in this review, the remainder of this discussion will be limited to this pontine nucleus and its projections.
The LC is one of several pigmented nuclei of the brainstem and has been shown to be highly conserved across mammalian species in terms of its location and appearance (Russell, 1955). The LC contains a relatively small number of neurons, with estimates ranging from 3000 neurons bilaterally in the rat to 30,000 neurons in humans (Bondareff et al., 1981; Swanson, 1976; Vijayashankar and Brody, 1979). Despite their relatively small number, LC neurons show a dramatic level of influence across all brain regions. Axons show an immense level of branching in both ascending and descending directions with descending collaterals innervating the spinal cord as well as most other brainstem nuclei and ascending axons terminating in almost every region of the diencephalon and telencephalon (Moore and Bloom, 1979; Jones and Moore 1977).
Retrograde tract tracing studies have identified substantial inputs to the LC from the periaqueductal gray and the mesencephalic reticular nucleus, as well as various preoptic nuclei and the lateral hypothalamus (Lee et al., 2005). Descending projections also extend back from prefrontal, anterior cingulate, and orbitofrontal cortices (Arnsten and Goldman-Rakic, 1984; Jodo et al., 1998; Lee et al., 2005) and, more caudally, from the bed nucleus of the stria terminalis, diagonal band of Broca, and several brainstem nuclei including the RN, nucleus prepositus hypoglossus and the paragigantocellularis nucleus (Aston-Jones et al., 1986; Lee et al., 2005).
The LC can be divided into both dorsal and ventral segments based on anatomical location of characteristic cell types subdivided by neurons of the medial vestibular nucleus (Swanson, 1976; Swanson and Hartman, 1975). Early immunohistochemical studies determined that LC neurons produce and release NE (Dahlstrom and Fuxe, 1965). Further quantitative studies by Swanson and Hartman (1975) determined that the LC is the origin of the majority of NE producing neurons within the brain. Work over several decades using retrograde tracers has identified the primary targets of the LC. The efferent projections of the LC ascend in an organized topographical distribution; cells situated in dorsal portions of the LC innervate the most caudal regions of the brainstem, midbrain, hippocampus, and posterior cortical regions including the occipital cortex while the ventrally located cells of the LC project rostrally to the olfactory bulb, olfactory cortex, frontal cortex, and parietal cortices. Subcortical regions, including the cerebellum, receive projections from both dorsal and ventral regions of the LC (Loughlin et al., 1982; Loughlin et al., 1986; Mason and Fibiger, 1979; Waterhouse et al., 1993; Waterhouse et al., 1983). Projection fibers branch to nearly all regions of the telencephalon as they ascend, and the amygdala, hippocampus, and PFC receive noradrenergic input exclusively from the LC as quantified via immunocytochemical staining in rodents and monkeys (Freedman et al., 1975; Levitt and Moore, 1978; Lidov et al., 1978). Projections to cortical regions remain highly lateralized with as many as 80–85% of projections from the LC tending to remain ipsilateral to their origin (Waterhouse et al., 1983; Espana and Berridge 2006).
It was originally thought that noradrenergic input to the neocortex was sparse within most cortical regions and generally restricted to the molecular layer (Anden et al., 1966; Fuxe et al., 1968). Later work using DA-β-hydroxylase immunocytochemistry in rats and non-human primates identified that LC noradrenergic projections actually innervate all layers of neocortex, with the highest density present in layers III and IV (Levitt and Moore, 1978; Morrison et al., 1978; Kosofsky et al., 1984). An additional level of specialization is added by the unique level of branching within discrete axon terminal fields. Fiber collaterals that reach neocortical regions form precise networks that appear to be highly specialized for the particular field in which they terminate (Lindvall and Bjorklund, 1974). Fiber tracts have been shown to extend parallel to the pial surface in some layers or to extend radially based upon the cortical region and/or cortical layer(s) they innervate (Moore and Bloom, 1979). Axons that run radially show varying levels of collateralization such that the fibers that terminate in the third and fourth layers of neocortex show a lesser degree of collateralization than those terminating in the molecular layer (Papadopoulos et al., 1987).
At the receptor level, norepinephrine (NE) works on variety of metabotropic receptor subtypes that can be classified as belonging to one of three broad categories - each of which contains several known isoforms of that subtype. Type α-1 and βsubtypes are thought to exist primarily post-synaptically, whereas α-2 subtypes can be found both pre- and post-synaptically (Berridge and Waterhouse, 2003; MacDonald et al., 1997). βreceptor subtypes are found dispersed across all cortical layers, while α-1 and α-2 receptors are distributed in the more superficial layers (Rainbow et al., 1984; Goldman-Rakic et al., 1990). Pharmacological studies have indicated that α-2 agonists can rescue working memory deficits associated with naturally occurring or artificially induced catecholamine depletion (Arnsten et al., 1988; Arnsten and Goldman-Rakic, 1985; Cai et al., 1993). The effect of NE on α-2 receptors is starkly contrasted by its effect on α-1 receptors; activation of α-1 receptors via α-1 specific agonists impairs performance on tasks of working memory in both rats and monkeys when administered systemically or intra-cranially to the PFC (Arnsten et al., 1999; Mao et al., 1999). As mentioned earlier, both α-1 and α-2 receptors are found co-localized within the same target tissues. Current theories of attention processing posit that increases in noradrenergic release results in the initial activation of α-2 receptors based on its higher affinity for NE when compared to α-1 receptors (Arnsten, 2000). However, at very high levels of noradrenergic release (e.g. stress), the secondary activation of α-1 receptors actively impairs working memory. This would suggest that the antagonistic relationship of α-1 and α-2 receptors serve an important evolutionary function; under these conditions, stimulus-driven behavioral responses are favored over cognition-directed behaviors, and, therefore, could provide important survival-driven modulation of action via subcortical brain regions independent of executive influence (Berridge and Waterhouse, 2003). Lastly, the β-adrenergic receptor subtype shows the lowest affinity for NE of all the receptor subtypes (Arnsten, 2000). Therefore, it has been suggested that βsubtypes play a limited role in prefrontal cortical functions (Arnsten and Goldman-Rakic, 1985). Noradrenergic receptors have been isolated at both the post-synaptic density and in the extrasynaptic space, indicating that noradrenergic receptors can be activated via specific synaptic transmission or more globally, through volume transmission (Aoki, 1992).
The LC-NE system plays a specific role in the regulation of cognitive functions, including the regulation of sustained attention, working memory, impulse control, and the planning of voluntary behavior (Arnsten and Li, 2005; Dalley et al., 2004). The application of NE directly to cortical neurons decreases the spontaneous firing rate of the neuron while maintaining or enhancing the level of response to direct sensory or thalamic inputs (Berridge et al., 1993; Ciombor et al., 1999; Rogawski and Aghajanian, 1980a; 1980b). Neurons of the LC have also been shown to discharge in both tonic and phasic fashions. Tonic firing is associated with the regulation of the sleep-wake cycle while phasic discharge is associated with salient or intense stimuli that occur during the wake cycle (Aston-Jones and Bloom, 1981; Aston-Jones et al., 2000; Berridge and Waterhouse, 2003; Foote et al., 1980; Hobson et al., 1975). Further work by Aston-Jones and colleagues indicates that phasic activity is locked to responses and “import” information about outcome decisions to the LC. In turn, the LC influences the forebrain to optimize performance as a function of task utility (Clayton el al., 2004; Aston-Jones et al., 1997; Aston-Jones and Cohen, 2005a; Aston-Jones et al., 1996). Tonic activity, alternatively, particularly at high levels, is important for a scanning level of attentiveness and a search for alternate behaviors. This dynamic property allows NE to enhance the signal-to-noise ratio of the neural network, and this property can be represented as an interaction between the tonic and phasic levels of neurotransmitter release.
3.2. Serotonergic system
Serotonin, or 5-HT, is synthesized by brainstem cell bodies located near the midline (Leger et al., 2001). These serotonergic nuclei can be divided into superior (ascending) nuclei, consisting of the caudal linear nucleus, MRN, DRN, and B9 cells located along the medial lemniscus, and inferior (descending) nuclei, consisting of the nucleus raphe obscurus, pallidus, and magnus, the ventral lateral medulla, and the area postrema, groups based on their appearance during early development (Dahlstrom and Fuxe, 1965; Jacobs and Azmitia, 1992).
Serotonergic projections to the cortex arise primarily from the DRN and MRN (O’Hearn and Molliver, 1984). The DRN consists primarily of ipsilateral projections to the frontal cortex while the MRN projects bilaterally to frontal, parietal, and occipital cortices (Jacobs and Azmitia, 1992; O’Hearn and Molliver, 1984). Although both consist of beaded fibers, afferents arising from the DRN show distinctly different morphology than MRN afferents. While MRN fibers are characterized by large, spherical beads (3–5 μm diameter in rat), DRN fibers are more heterogenous and also contain fibers with fine axons and irregularly spaced small, granular and fusiform varicosities (Kosofsky and Molliver, 1987; Leger et al., 2001). These differences in morphology may have functional consequences; for example, the fine 5-HT fibers seem to be more vulnerable to amphetamine toxicity than the larger beaded fibers (Mamounas et al., 1991).
While it is clear that the dorsal raphe sends ascending projections to the PFC, it has only recently been determined that it receives reciprocal connections from the PFC as well. Aghajanian & Wang (1977) found staining in the PFC when they utilized retrograde tracing techniques to determine afferents into the DRN. As retrograde tracing studies are vulnerable to false positives due to fibers of passage, these results were not convincing until recent work by Peyron and colleagues utilizing both retrograde and anterograde tracing studies to determine the specific innervation patterns of these serotonergic inputs. Retrograde tracing indicates that these afferents primarily originate from the lateral orbital, cingulate and infralimbic cortices and anterograde tracing studies confirmed the same (Peyron et al., 1998). These tracing studies are supported by microdialysis studies confirming the release of 5-HT from the DRN following prefrontal stimulation (Celada et al., 2001). Celada et al. also utilized electrophysiological recording to demonstrate that in anesthetized conditions dorsal raphe neurons responded to stimulation of the prefrontal projection neurons with a short-latency poststimulus inhibition. Although it is not clear from this study how DRN neurons respond to PFC activation in functionally relevant situations, together with the anatomical findings, it provides support that the PFC provides feedback regulation to the DRN (see Figure 2). This feedback suggests a functional implication for a role of cognitive control in the mediation of behavioral affect and recent work by Amat and colleagues has provided support for the role of this prefrontal-raphe feedback projection in the cognitive control of stress (Amat et al., 2006).
Cortical serotonergic innervation patterns tend to exhibit laminar specificity, but the layers of the cortex that are most innervated by serotonergic fibers varies by species (Leger et al., 2001). Examination of the distribution of 5-HT-containing nerve fibers within the PFC of non-human primates indicates a fairly uniform high density innervation, whereas layer II and upper layer III are more densely innervated in the cat PFC (Berger et al., 1988; Leger et al., 2001; Wilson and Molliver, 1991).
Accompanying these species differences in laminar distribution are also structural and electrophysiological differences across species. The rat serotonergic system consists of only fine unmyelinated axonal collaterals, while primates also exhibit myelinated serotonergic axons with significantly less collateralization. Along with these two different anatomical populations, there is also support for two physiological firing patterns in the primate serotonergic system. The typical 5-HT neuron exhibits a slow and highly regular rhythmic pattern of activity (<2–3Hz) (Aghajanian et al., 1968; Bramwell, 1974; Mosko and Jacobs, 1974). However, there is a second subpopulation of cells that alter their firing patterns in response to specific actions, such as grooming, licking, or chewing (Fornal et al., 1996). It has been speculated that these electrophysiological responsive neurons may consist of myelinated fibers, while the typical rhythmic serotonergic neuron is unmeyelinated. This may represent a phylogenetic trend towards a specialized serotonergic system in more encephalized brains (Jacobs and Azmitia, 1992).
Along with traditional synaptic release, 5-HT can also be released non-synaptically onto neurons, glial cells, ependymal cells, endothelial cells, endocrine cells, or into the cerebral spinal fluid (Azmitia and Gannon, 1983; Chan-Palay, 1976; Descarries et al., 1975; Descarries et al., 1982). The proportion of synaptic and non-synaptic 5-HT release varies among species, cortical brain regions, and cortical layers. For example, in rats, almost all 5-HT released in layer I is non-synaptic, but in deeper layers of the cortex 50–90% of 5-HT is released synaptically (Jacobs and Azmitia, 1992).
Seven major 5-HT receptor subtypes (5-HT1–7) have been described, with five variants of 5-HT1 receptors (1A, 1B, 1D, 1E, 1F), three variants of 5-HT2 receptors (2A, 2B, and 2C), and two variants of 5-HT5 receptors (5A and 5B). Although many of the different receptor subtypes are located in the PFC, their specific neuronal locations (postsynaptically vs. somatodendritically; pyramidal cells vs. GABA interneurons) may allow for highly specific serotonergic effects on postsynaptic targets. The 5-HT2A receptor is the predominant 5-HT receptor found in the cortex, where it is located on all cortical pyramidal cells as well as parvalbumin- and calbindin-containing GABAergic interneurons. While the action at 5-HT2A receptors on GABAergic neurons is known to be involved in perisomatic inhibition of pyramidal cells, in pyramidal cells this receptor subtype is located postsynaptically and its activation increases the excitability of PFC neurons (Buhot, 1997; Harvey, 1996; Jakab and Goldman-Rakic, 2000). The 5-HT1A receptor is also found on the majority of pyramidal neurons and more than 25% of the GABAergic interneurons (Buhot, 1997; Gu, 2002). These receptors are located somatodendritically and are generally thought to decrease neuronal excitability (Buhot, 1997). Along with these predominant receptor subtypes, 5-HT3 receptors are found only on GABAergic interneurons in the PFC.
Research over the past decade supports the hypothesis that the DRN 5-HT system plays a specific role in prefrontal function. For example, prefrontal 5-HT depletion in marmosets acts to impair reversal learning, while leaving attentional set shifting intact Clarke et al. (2005). Similarly, 5-HT depletion has also been shown to impair performance on a serial discrimination reversal task (Clarke et al., 2004). Although a decrease in prefrontal 5-HT leads to a decrease in cognitive flexibility, it seems as though this may work to increase focused attention (Schmitt et al., 2000). As the DRN is the primary source of prefrontal serontonin, these projections and their reciprocal descending connections clearly play a role in specific cognitive functions.
3.3. Dopaminergic system
DA is produced by two groups of cell bodies in the mesencephalon: the SN and the VTA. The nigrostriatal DA pathway projects from the SN to the dorsal striatum (caudate-putamen) and the degeneration of this pathway is responsible for the pathology of Parkinson’s disease (Groenewegen, 2003). The mesolimbic system projects from the VTA to the ventral striatum (NAc and olfactory tubercle) and limbic structures and is the pathway implicated in mediating reward related behaviors (Hyman et al., 2006). The mesocortical system also originates in the VTA, terminating in the frontal cortex and innervating predominantly the infralimbic and prelimbic subregions of the medial PFC (Fluxe et al., 1974). The mesoaccumbal and mesocortical dopaminergic projections arise from two distinct cell populations in the VTA. Cells located in the nucleus paranigralis predominantly project to subcortical sites while cells located in the nucleus parabrachialis pigmentosus predominantly project to cortical targets (Goldman-Rakic et al., 1989; Smiley and Goldman-Rakic, 1993; Smiley et al., 1992). Projections from the VTA have been shown to form synapses with cortical pyramidal glutamatergic neurons and non-pyramidal GABAergic interneurons.
As with all neuromodulator systems, the VTA receives reciprocal input from the PFC (Sesack and Pickel, 1992). Afferents from the PFC innervate mesoaccumbens GABA but not DA cells, as well as mesofrontal DA but not GABA neurons (Sesack and Carr, 2002). This specificity of PFC innervation creates a 1-to-1 relationship with prefrontal efferents synapsing on VTA DA cells that form reciprocal prefrontal connections (see Figure 2). Current theories posit that this input is important for facilitating learning through the influence of prediction errors (Schultz, 1997; Schultz et al., 1997).
Dopaminergic innervation of the PFC shows a laminar distribution, with the deep layers (V, VI) receiving more input than the superficial layers (Emson and Koob, 1978). Dopaminergic transmission in the PFC is mediated by two DA receptor subtypes: D1 and D2 receptors. Although both receptor subtypes are present in the PFC, they display only a partially overlapping distribution. D2 receptor expression is considerably less dense than that of D1 receptors. D2 receptors are found almost exclusively in layer V while D1 receptors are most densely distributed in the superficial layers (I–III), although they can be found in all layers (Goldman-Rakic et al., 1990). The differential distribution of D1 and D2 receptors is important due to their different second messenger cascades; D1 receptors are coupled to stimulatory g-proteins while D2 receptors are coupled to inhibitory g-proteins (Kebabian et al., 1984). Along with these differences in signaling mechanism, D2 & D1 receptors exhibit differences in binding affinity, with D2 receptors responding to much lower levels of dopamine than D1 receptors (Grace, 2000).
Similar to LC neurons, VTA DA neurons discharge in both tonic and phasic fashions and these firing patterns result in tonic and phasic release of DA in the PFC (Grace, 1991; Stoof and Kebabian, 1981). Tonic DA release is activated by sustained increases in DA neuronal firing or presynaptic stimulation of DA terminals by glutamate. In contrast, phasic DA release results from spike-dependent mechanisms and is in response to behaviorally relevant stimuli (Finlay et al., 1995; Rebec et al., 1997). Tonic activity (∼1–6 Hz) of DA neurons occurs in the absence of salient stimuli and results in very low, tightly controlled levels of DA release (Grace and Bunney, 1984). These tonic levels of DA are not confined to the synaptic cleft and are tightly controlled by feedback systems (Parsons and Justice, 1992). It has been suggested that D2 receptors are continually stimulated by tonic DA release while phasic DA release preferentially activates D1 receptors (Grace, 2000).
Hypotheses concerning the role of DA in cognitive function have focused on its ability to modulate executive functions such as working memory, planning, and attention (Roitman et al., 2004; Sawaguchi and Goldman-Rakic, 1991; Zahrt et al., 1997). During the performance of a delayed alternation task, a measure of working memory, monkeys display an increase in prefrontal DA release (Matsuda et al., 2002). Additionally, both overstimulation and inhibition of the prefrontal DA system have been shown to decrease performance in working memory function (Abi-Dargham et al., 2002; Aultman and Moghaddam, 2001; Kellendonk et al., 2006; Muller et al., 1998; Sawaguchi and Goldman-Rakic, 1991). The ability of both insufficient and excess DA to decrease performance in tasks of cognitive functioning further emphasizes the importance of a proper balance of dopaminergic activity. The ability of dopaminergic agents to influence performance on specific cognitive tasks, while leaving performance on others intact, provides further support for a tightly regulated and specialized role for DA in prefrontal cortical function.
3.4. Modulatory systems are organized in parallel: functional implications
The classic ascending neuromodulatory systems are closed-loop circuits that are simultaneously arranged in both distributed and parallel fashions. Figure 2 outlines the parallel organization that exists among the modulatory systems. Within each loop, each neurotransmitter system sends projections directly to its prefrontal cortical targets as well as receiving modulatory input from these targets via feedback loops.
Located in the brainstem, the LC and the RN are the most caudally located sources of ascending neuromodulatory projections. The efferent projections of the LC ascend topographically, based upon their anatomical site of origin, to innervate prefrontal cortical regions (Mason and Fibiger, 1979; Loughlin, 1986; Waterhouse, 1983). In addition, nearly all regions of the LC are reciprocally connected via direct glutamatergic projections from the prefrontal, anterior cingulate, and orbitofrontal corticies (Arnsten and Goldman-Rakic, 1984; Jodo et al., 1998; Lee et al., 2005). The other brainstem nuclei, the RN, sends projections to innervate the entire neocortex. The DRN sends projections primarily to the frontal cortex with the heaviest level of innervation occurring in the medial PFC (Leger et al., 2001). The medial component of the DRN innervates the frontal, parietal, and occipital cortices equally (O’Hearn and Molliver, 1984). Reciprocal connections also project back to the DRN, which receives the highest number of inputs from the lateral orbital, cingulate, and infralimbic cortices (Peyron et al., 1998).
More rostrally, at the level of the midbrain, the VTA is the exclusive provider of dopaminergic innervation to the PFC. The mesocortical DA system projects from the VTA and terminates primarily at the infralimbic and prelimbic subregions of the medial PFC (Fluxe et al., 1974). The VTA, in turn, is modulated via reciprocal projections from the PFC (Sesack and Pickel, 1992).
The BFCS is the most rostrally located group of nuclei of the ascending neuromodulatory systems. Its projections to the PFC primarily innervate the medial prefrontal and orbitofrontal cortices via ascending fibers from the nbM/SI (Mesulam et al., 1983). The basal forebrain nuclei of the cholinergic system also receive reciprocal connections from PFC regions as well as modulatory input from subcortical and brainstem nuclei that will be discussed below (Carnes et al., 1990).
Feedback to ascending neuromodulatory systems is not only direct from the PFC but can also occur indirectly via feedback loops from other modulatory nuclei at the levels of the brainstem, midbrain, and basal forebrain. At the most caudal regions, the DRN and LC are both tonically active during states of waking and become increasingly quiescent as the animal enters deeper stages of sleep (Hobson et al., 1975). This early observation suggested that these two nuclei could be functionally connected. Recent studies involving retrograde tracers have confirmed this theory by identifying reciprocal connections between both regions. The caudal principal LC sends substantial projections to the caudal, ventromedial, and intrafasicular DRN. In return, the caudal DRN and ipsilateral wing of the DRN sends heavy projections to the mid-ventral and caudal dorsal LC (Kim et al., 2004). Ascending noradrenergic projections also innervate the BFCS extensively via projections from the LC and the A1/C1 and A2/C2 regions of the brainstem (Espana and Berridge, 2006; Hajszan and Zaborszky 2002). Serotonergic neurons of the RN have also been shown to project to and form synapses at cholinergic neurons of the basal forebrain by electron microscope studies (Dinopoulos et al., 1997). Furthermore, projections from the VTA innervate primarily the nbM and SI (Jones and Cuello, 1989; Gaykema and Zaborszky, 1996; see also Geula and Slevin, 1989). This connection between the VTA and basal forebrain is bidirectional in that cholinergic neurons send reciprocal projections to the VTA as well (Omelchenko and Sesack, 2006). Therefore, at the level of the basal forebrain, cholinergic nuclei are reciprocally connected to the VTA in addition to receiving modulatory input from the LC and the RN. Thus, cholinergic cells receive direct functional innervation from all biogenic amine systems, and this arrangement of interconnectedness has been demonstrated in both rodent and primate basal forebrain regions (Gaykema and Zaborszky, 1996; Smiley et al., 1999). Although anatomical evidence implies cross regulation via interactions at cell bodies, further electrophysiological and pharmacological studies have provided evidence that substantiates the interconnectedness of each of these systems at their terminal fields. For example, PFC brain slices treated with nicotine show an altered release for all biogenic amines in a concentration dependent manner (Rao et al., 2003).
In addition to sharing a parallel and closed-loop system organization, 5-HT, NE, DA, and ACh neurotransmitter systems exhibit overlapping terminal fields and innervation patterns (Figure 1). However, some differentiation exists based on specific cortical targets as well as the localized distribution of collateralized fibers. For example, 5-HT and NE fibers show robust axon collateralization, while cholinergic fibers show minimal collateralization, suggesting cholinergic modulation is more specifically targeted to discrete cortical regions (Jacobs and Azmitia, 1992; Malone, 2004; Price et al., 1990; Price and Stern, 1983; Walker et al., 1985). Notably, all neuromodulator systems, with the exception of 5-HT, appear to share the ability to affect target tissues in a tonic and phasic manner. Tonic discharge rates are modulated state-specifically and appear to be directly related to the behavioral sleep-wake and arousal state of the animal. Additionally, all modulators show a phasic response to specific salient stimuli or responses that act to optimize cognitive performance. Furthermore, with the possible exception of cholinergic cells, the remaining neuromodulator systems share the ability to influence cortical targets through the release of neurotransmitter via volume transmission. It has been purported that the action of volume transmission maybe be an integral component to the tonic effects of modulatory systems (Agnati et al., 2006; Katz, 1999).
A fundamental question in neuroscience concerns the development of a complex systems model of the brain complete with algorithms capable of predicting cognitive and behavioral outcomes from dynamic and nonlinear networks of neurons. The nonlinear nature of modulatory neurotransmitter interactions may explain the rich, seemingly stochastic, and context-specific actions that characterize cognitive and behavioral function. Given the level of interaction presented here, it is likely that these systems are recruited in synchrony, as opposed to autonomous and separate activation of individual neuromodulator systems. Likewise, all modulator systems are regulated by prefrontal descending, direct as well as multi-synaptic, projection systems, and it is extremely unlikely that such telencephalic feedback is segregated by target system. Still, to an overwhelming degree, ascending modulator systems have been studied in isolation. A future challenge for researchers will be to uncover how these modulatory systems work in parallel to dictate cognitive function.
4. Modulation of prefrontal cholinergic activity and function
4.1. Noradrenergic modulation of prefrontal cholinergic function
While the effects of noradrenaline release on cortical neurons have been well documented, a surprisingly limited number of studies have looked at the interaction of NE with other modulatory projections at overlapping terminal locations. Studies in guinea-pig cortical regions demonstrate that systemic administration of NE inhibits the release of cortical ACh and that this effect can be reversed via the administration of an α-2 antagonist (Beani et al., 1978). Using in vivo microdialysis in freely behaving animals, systemic administration of α-2 agonists or α-1 antagonists modify ACh release at cortical targets during periods of tactile stimulation. However, evidence concerning the regulation of basal ACh release by α-2 agonists or α-1 antagonists remains complex and at times conflicting primarily as a result of concentration differences between studies (Moroni et al., 1983; Telles et al., 1995, 1997). Finally, in a study examining noradrenergic influence on cholinergic systems, Tzavara and colleagues demonstrated that systemic administration of a NE reuptake inhibitor, atomoxetine, leads to an increase in cortical ACh release, which was dependent upon noradrenergic α-1 receptor activation. This enhancement of cortical ACh release by atomoxetine also resulted in enhanced performance on both recognition and spatial memory tasks (Tzavara et al., 2006).
Noradrenergic-cholinergic interactions were also studied at the level of the basal forebrain. As mentioned above, inputs from the LC (Espana and Berridge, 2006; Jones and Cuello, 1989) and to a lesser degree the A1/C1 and A2/C2 nuclei of medulla (Espana and Berridge, 2006; Hajszan et al. 2002) terminate on basal forebrain cholinergic cells. In agreement with anatomical studies, neurophysiological recordings have substantiated the role of noradrenergic modulation of cholinergic activity in the basal forebrain. Bath-applied NE depolarized cholinergic cells primarily through the activation of post-synaptic α-1 adrenergic receptors and secondarily via the activation of β-adrenergic receptors (Fort et al., 1995). In freely behaving animals, Berntson and colleagues demonstrated that auditory-evoked responses are enhanced by the infusion of noradrenergic α-1 agonists into the basal forebrain and attenuated by infusion of α-1 antagonists into this region (Knox et al., 2004). These studies together indicate the functional significance of the noradrenergic modulation of the BFCS.
4.2. Serotonergic modulation of prefrontal cholinergic function
Relatively few studies have been designed to determine the interactions between serotonergic and cholinergic inputs to the PFC (Levin et al., 2005; Matrenza et al., 2004). Furthermore, most of these studies examined serotonergic modulation of basal cholinergic activity, as opposed to addressing interactions with a recruited or activated cholinergic system. Nair and Gudelsky (2004) demonstrated that systemic administration of a 5-HT2A/2C, 5-HT2, or 5-HT2C agonist results in an increase in prefrontal ACh release, and these increases are blocked by the administration of serotonin receptor antagonists. Agonists at 5-HT4 or 5-HT1A receptors also increase basal ACh release (Consolo et al., 1994; Nair and Gudelsky, 2004). Research conduced in guinea pigs suggests that, in contrast, administration of 5-HT3 agonists decrease cortical ACh release (Bianchi et al., 1990). Nair and Gudelsky (2004) also found that intracortical infusions of the 5-HT2A/2C agonist DOI increased prefrontal ACh release, suggesting that the serotonergic modulation of ACh is occurring at the synaptic terminals of cholinergic projections to the PFC (see Table 1).
Table 1.
Summary of evidence on the serotonergic and dopaminergic modulation of cortical ACh release
| Pharmacological Manipulation | Injection Site | Condition | Effect on ACh release | Reference |
|---|---|---|---|---|
| 5-HT1A agonist | Systemic | Basal Release | ↑ ACh | Millan et al. 2004 |
| 5-HT2A/2C agonist | Systemic | Basal Release | ↑ ACh | Nair & Gudelsky, 2004 |
| 5-HT4 agonist | Systemic | Basal Release | ↑ ACh | Consolo et al., 1994 |
| 5-HT1A agonist | Systemic | Basal Release | ↑ ACh | Nair & Gudelsky, 2004 |
| 5-HT1B antagonist | Systemic | Basal Release | ↑ ACh | Hu et al. 2007 |
| 5-HT2A/2C agonist | Intracortical | Basal Release | ↑ ACh | Nair & Gudelsky, 2004 |
| D1 antagonist | Systemic | Basal Release | ↓ ACh | Day & Fibiger, 1992 Acquas et al., 1994; |
| D1 agonist | Systemic | Basal Release | ↑ ACh | Di Cara et al. 2006 |
| D2 agonist | Systemic | Basal Release | No change | Day and Fibiger, 1993 |
| D1 antagonist | Systemic | Tactile Stimulation | No effect on sensory evoked increase | Acquas et al., 1998 |
| D2 antagonist | Systemic | Tactile Stimulation | No effect on sensory evoked increase | Acquas et al., 1998 |
| Co-administration of D1 & D2 antagonists | Systemic | Tactile Stimulation | ↓ sensory-evoked ACh increase | Acquas et al., 1998 |
| D3 antagonist | Systemic | Basal Release | ↑ACh | Millan et al. 2007 |
As 5-HT1A receptors serve as somatodendritic autoreceptors, the activation of these receptors primarily stimulate 5-HT release in the DRN. This notion was confirmed by experiments demonstrating that 5-HT1A, but not 5-HT2A, receptor stimulation-induced augmentation of ACh release is blocked by DRN lesions (Somboonthum et al., 1997). It should be noted that although 5-HT receptor antagonists block the effects of agonists, antagonists generally do not seem to affect ACh release when administered alone (Nair and Gudelsky, 2004; 2006). The recent demonstration of increases in cortical ACh release as a result of systemic blockade of 5-HT1B receptors represents a significant exception to this rule (Hu et al. 2007).
Consistent with the general lack of effects of 5-HT receptor antagonists on cortical ACh release, selective lesions of the serotoninergic system did not affect basal prefrontal ACh release (Dekker and Thal, 1993). Although these microdialysis studies have added to our understanding of the interaction between the serotonergic and cholinergic systems, it is not clear how these systems may modulate one another in functionally relevant contexts. For example, stress has been shown to increase both 5-HT and ACh release in the PFC (Nair and Gudelsky, 2006; Storey et al., 2006). Further research needs to be done to determine whether these behaviorally induced increases in 5-HT activity are modulating ACh release.
Serotonergic fibers from the dorsal raphe form a dense network through the BFCS. These fibers were once thought to pass through only the BFCS; however, electron microscopic analysis has determined that serotonergic varicosities form synapses with the dendritic shafts of cholinergic neurons in this region (Dinopoulos et al., 1997). The regulatory and functional significance of basal forebrain serotonergic-cholinergic interactions remains unknown.
Despite their different anatomical locations and functional effects on signaling cascades, research in rats generally indicates that stimulation of 5-HT receptor subtypes produces increases in cortical ACh release (see also Millan et al. 2004). It is unknown, however, if and how these findings from studies on basal ACh release generalize to situations characterized by activation of the BFCS in the context of cognitive performance.
4.3. Dopaminergic modulation of prefrontal cholinergic function
Although there is a general paucity of studies involving the interactions of modulatory neurotransmitters on cognitive function (see also Decker and McGaugh, 1991), the dopaminergic modulation of cholinergic function has received more attention than other modulatory systems. The first studies examining ACh release utilized a cortical cup (a technique in which the dura is removed, a cup is tightly adhered to the top of the brain, and transmitter seeping into the cup is collected and quantified) to show that amphetamine-evoked increases in ACh output were eliminated following selective dopaminergic lesions of the SN (Casamenti et al., 1986). The stimulatory effects of DA receptor agonists on cortical ACh release have been confirmed by studies utilizing in vivo microdialysis. ACh release can be attenuated by the co-administration of either D1 or D2 receptor antagonists; however only D1 antagonists were able to decrease basal ACh levels when administered alone (Day and Fibiger, 1992). When specific DA receptor agonists were administered systemically, D1 agonists were shown to increase cortical ACh release while D2 agonists had no effect (Di Cara et al., 2006; Acquas et al., 1994; Day and Fibiger, 1993), suggesting that dopaminergic modulation of prefrontal cholinergic outputs is mediated primarily through activation of D1 and also D5 receptors (Fitch et al. 2006; Laplante et al., 2004; Hersi et al., 2000).
The neuronal circuits mediating the effects of systemically administered DA receptor ligands on ACh release are not clear. However, we know that dopaminergic mechanisms in the NAc are not necessary for the demonstration of a amphetamine-induced increases in cortical ACh release (Arnold et al., 2000), perhaps suggesting that dopaminergic projections from the VTA to the BFCS or PFC are involved in these effects (Emson and Koob, 1978; Geula and Slevin 1989). The modulatory role of D5 receptors has also been suggested based on evidence indicating the presence of D5 receptors on the somata, dendrites and axons of forebrain cholinergic neurons (Berlanga et al. 2005).
The overlapping of dopaminergic and cholinergic axon collaterals in the PFC (Fig. 1) suggests that dopamine-ACh interactions indeed are based on local mechanisms. Yang and Mogenson (1990) studied the effects of co-administration of ACh and DA on the firing rate of prefrontal neurons. They found that co-administration of DA increased the ACh-evoked signal-to-noise ratio by both increasing the ACh-evoked responses and simultaneously decreasing the spontaneous activity of the prefrontal neurons. This increase in signal-to-noise was blocked by D2 receptor antagonists.
Acquas et al. (1994) examined the ability of dopaminergic antagonists to modulate tactile stimulation-induced increases in cortical ACh release. This study revealed that neither D1 nor D2 antagonists alone were sufficient to affect the magnitude of the sensory-evoked increase in ACh release. However, combined blockade of D1 and D2 receptors attenuated tactile stimulation-induced increases in ACh release. Collectively, the available evidence suggests that D1 receptor stimulation increases ACh release. The effects of D2 receptor manipulations remain less well understood (see also Millan et al. 2007) and may depend on complex interactions with levels of D1 receptor activity.
Millan and colleagues recently reported that systemic blockade of D3 receptors resulted in sustained increases in frontal but not hippocampal ACh release (Millan et al., 2007). As they point out, the regional selectivity of D3-mediated effects contrast with the increases in ACh release in both regions that result from stimulation of D1 receptors. The basis for the selective effect of D3 antagonists remains unclear. However, the potent increases in ACh release seen following D3 receptor blockade indicate that frontocortical cholinergic inputs are tonically and potently inhibited by dopamine acting at D3 receptors.
4.4. Cholinergic modulation of prefrontal cholinergic function
In addition to the influence exerted by the other ascending modulator systems, the activity of the cholinergic system is modulated by the binding of ACh to presynaptic cholinergic receptors located on cholinergic terminals, as well as by way of the recruitment of other neurotransmitter systems through cholinergic heteroreceptors. This capacity for self-modulation further adds to the number of mechanisms by which the cholinergic system can be modulated.
Evidence suggests that reflexive modulation of prefrontal cholinergic inputs is primarily accomplished through the actions mediated by nicotinic receptors. Summers and Giacobini (1995) demonstrated, in freely moving animals, that systemic and local infusions of nicotine into the frontoparietal cortex increased cortical levels of ACh, an effect attributed to ligand-binding at presynaptic nicotinic receptors. Subsequent studies demonstrated that selective agonists of α4β nAChRs augment ACh release in the forebrain and that nicotine-induced increases in ACh release are attenuated by an α4β nAChR antagonist (Tani et al., 1998).
The α4β nAChR subtype is extensively distributed in the forebrain (Flores et al., 1992), including the frontal cortex (Perry et al., 2002). Cortically projecting basal forebrain neurons express α4β receptors (Azam et al., 2003), thus supporting a central role of this receptor confirmation in regulating cholinergic projections. The advent of enzyme-selective microelectrodes, developed for amperometric measurement of changes in extracellular choline concentrations at high temporal resolution, has provided further insight into pro-cholinergic mechanisms controlled by α4β nAChRs. Compared with nicotine, α4β-selective agonists produce more potent and temporally “sharper” increases in ACh release in PFC (Man et al., 2006).
Nicotinic receptor-mediated modulation of ACh transmission may also occur via intermediary neurotransmitter systems. For example, nicotine-evoked ACh release, through activation of nicotinic receptors, is inhibited in the presence of AMPA receptor antagonists (Man et al., 2006; see also Fadel et al., 2001). One potential explanation for these results is that stimulation of glutamate release through nicotinic receptors located on glutamatergic neurons may lead to an increase in ACh release through AMPA receptors located on cholinergic cells (Giovanni et al., 1999).
Another likely intermediary system involves dopaminergic afferents. In the PFC, nicotine binding at the α4β and α7 receptors enhances amphetamine-stimulated DA release (Drew et al., 2000; George et al., 2000). In turn, pharmacological elevation of DA results in increases of cortical ACh (Day and Fibiger, 1992). Thus, it may be possible that these interactions between dopaminergic and cholinergic neurons constitute a regulatory feedback loop involved in the maintenance of a homeostatic balance between these neurotransmitter systems in prefrontal regions. Specifically, the slow temporal dynamics of nicotine-induced increases in cholinergic activity, when compared with the effects of α4β-selective agonists, are hypothesized to be due, at least in part, to dopamine release and metabotropic DA receptor stimulation at cholinergic terminals.
Finally, muscarinic receptor activation was also demonstrated to stimulate ACh efflux (Douglas et al., 2001; Quirion et al., 1994). It is possible that binding at metabotropic homoreceptors located on cholinergic neurons may invoke changes in intracellular processes that, in turn, affect m2 autoreceptor function; however, no convincing evidence demonstrating this phenomenon has yet surfaced.
5. Cholinergic modulation of prefrontal DA, 5-HT, and NE release and function
Although the discussion above focused on the modulation of cholinergic function, it is important to note that this is only one perspective. It is clear from the interconnectedness and parallel organization of the modulator systems (Figures 1 and 2) that each of these neurotransmitters modulates, and is modulated by, each of the others. Therefore, in this section we will point out some of the examples indicating cholinergic modulation of the activity of other modulator systems.
Immunohistological studies have identified several nAChR subtypes expressed in the nuclei of ascending modulator systems. Serotonergic neurons of the MRN and the DRN, noradrenergic neurons of the LC, and dopaminergic neurons of the SN and the VTA all express α4-containing nAChRs (Bitner et al., 1998; Cucchiaro and Commons, 2003; Fiorillo and Williams, 2000). Additionally, α7-containing nAChRs have been identified in the rat DRN and the LC (Bitner et al., 1998; see also Centeno et al., 2006).
Electrophysiological studies examined the effects of nicotine on the excitability of midbrain dopamine neurons. Single cell electrophysiology has demonstrated that systemic nicotine administration leads to an increase in burst firing in the dopaminergic cells of the VTA (Grenhoff et al., 1986; Mereu et al., 1987; see also Memeli-Engvall et al., 2006). These effects of nicotine or ACh on midbrain dopaminergic activity may be mediated by glutamatergic mechanisms (Grillner and Svensson, 2000).
The majority of experiments on nAChR-induced changes in the release of neuromodulators has focused on the role of presynaptic nicotinic receptors in regulating the release of NE (Cucchiaro and Commons, 2003; Yoshida et al., 1980; Anderson et al., 2000), 5-HT (Seth et al., 2002, Shearman et al., 2005; Singer et al., 2004), DA (Champtiaux et al., 2003) and ACh (MacDermott et al., 1999; see also Rao et al., 2003). Muscarinic acetylcholine receptors also influence the activity and release of other neuromodulators. For example, stimulation of muscarinic receptor dose-dependently increase DA release in the rat medial PFC, while minimally affecting release in the NAc (Ichikawa et al., 2002).
Taken together, findings from electrophysiologcal and neurochemical experiments demonstrated that ACh, either based on nicotinic and muscarinic receptor-mediated effects at the level of neuromodulator nuclei or via modulating release via terminal-based mechanisms modify neurotransmission levels of NE, 5-HT, and DA.
6. Modulator interactions in the PFC: global gating functions versus specific information processing
The traditional conception of ascending systems as modulators has carried with it the assumption that they function primarily as gating mechanisms. Alterations in the activity of, and neurotransmitter release by, modulatory systems occur in a global manner and act to enhance or effectively reduce the responsiveness of neurons in target regions. Such changes in the activity of ascending projections occur over relatively extended periods of time. In contrast to this view, emerging evidence suggests that, in addition to these prolonged and relatively slow alterations in neuromodulator activity, fast-acting, transient increases are observed in the context of specific cognitive functions. The discernment between these transient, or phasic, and protracted, or tonic, changes within each modulatory system implies that along with the ability to modulate changes on a global scale, these ascending systems can contribute to specific processes normally attributed to non-modulatory systems. The description of these two modes of activity has been approached from several different levels of analysis. Therefore, although the terminology used to delineate two discreet modes of transmission is consistent, the characteristics that define phasic and tonic activity require attention.
Phasic and tonic dopaminergic activity has been predominantly described by the measurement of changes in local field potentials. Characterization of changes in tonic and phasic activity is dependent upon two variables; basal firing rate and time. Tonic changes reflect alterations in activity occurring over the course of several minutes, while phasic variation is measured in terms of seconds or even milliseconds. These two modes of activity have been extensively studied with respect to the dopaminergic system. DA reaches the extracellular space following spillover from the synaptic cleft; thus, an increase in tonic DA levels is indicative of an overall elevation of the basal rate of neural spiking. An increase in phasic activity refers to the release of DA into the synaptic cleft via the generation of action-potentials following a transient increase in firing rate, relative to baseline (Grace, 2000).
Not only can the tonic and phasic components of DA release be distinguished temporally, but the two can be disassociated based on their functional implications. Phasic activation has been associated with an enhancement of spatial learning through the modulation of D1 receptors on NAc afferents from the hippocampus. Carelli and colleagues (2004) utilized fast-scan cyclic voltammetry to measure DA release in the NAc of rats presented with a reward-predicting cue. While no release of DA was found in naïve controls in response to the cue, trained rats showed an increase in DA that quickly subsided to baseline levels after cue presentation. The tonic element of DA release, on the other hand, acts via the D2 receptors to modulate PFC-NAcc circuitry, which are implicated as necessary for set-shifting tasks (Goto and Grace, 2005). Due to the heterogeneity of the VTA, the major dopaminergic input to the PFC, electrochemical techniques used to parse out DA release have thus far been unsuccessful, although phasic burst activity in this region has been linked to the initation of goal-direct behavior (Kiyatkin and Rebec, 2001). Therefore, the possibility remains that the cortically projecting neurons of the VTA, as with cells of the NAc, exhibit phasic and tonic modes of activity, which uniquely contribute to cognitive functions.
The delineation of the tonic and phasic components of noradrenergic activity has been approached in a similar manner. Neurophysiological recordings of neurons in the LC have differentiated the burst-firing of neurons during phasic activation from the overall firing pattern which defines tonic activity. Phasic increases in firing patterns have been demonstrated to be associated with the presentation of a cue or target stimulus and the execution of responses (Aston-Jones and Cohen, 2005b). In accordance with the more classical view of the role of modulatory systems, the relative base firing rate, or tonic level, has been correlated with arousal states and exploratory cognitive activity (Aston-Jones and Cohen, 2005b; Aston-Jones et al., 1999). Additionally, tonic release has also been hypothesized to subserve an “adaptive gain” function, wherein an elevation of basal firing facilitates the disengagement of attention from stimuli that have diminished in goal-directed relevance (Aston-Jones and Cohen, 2005a).
The disparity in the functions of phasic and tonic LC neuronal activity highlights the importance of circuitry connecting this nucleus with frontal cortical structures. Specifically, Aston-Jones and colleagues hypothesized that inputs from the anterior cingulate and orbitofrontal cortices are able to elicit a phasic or tonic firing pattern within the LC. These inputs create a pattern of neural response in the LC that is characterized by cue-evoked increases in spike-rate by stimuli that remain reliable predictors of reward. Conversely, in such a case where a stimulus becomes a less effective predictor of reward, inputs from the OFC and ACC that innervate the LC may enhance tonic rates of neural spiking. Such increases in base firing rate have been shown to increase animals’ responses to goal-irrelevant stimuli. Thus, a frontally mediated shift to a tonic firing mode may sensitize the subject to non-target, environmental stimuli, and may actually represent an adaptive function of facilitating the identification of new cues that prove more useful for goal acquisition (Aston-Jones and Cohen, 2005a; 2005b).
Emerging evidence has also allowed for the identification of tonic and phasic modes of cholinergic activity. Tonic cholinergic activity refers to the general pattern of fluctuations in extracellular ACh concentrations over the course of several minutes, while phasic activity refers to transient changes in this concentration on a second-by-second time scale. Microdialysis techniques, coupled with high performance liquid chromatography, have served as the primary means with which to describe tonic changes in ACh release. These studies have revealed that performance in operant tasks designed to test cognitive constructs such as attention is associated with session-related increases in extracellular ACh, a finding which may be indicative of an overall increase in tonic levels coinciding with an enhancement of arousal or readiness for input processing (Kozak et al., 2006; Parikh, 2006).
In recent years, a new technique utilizing constant voltage amperometry has been developed for the estimation of changes in ACh via fluctuations in extracellular choline concentrations. Microelectrodes are placed into the brain with an enzyme (choline oxidase) immobilized on the surface of the electrodes. Released ACh is converted to choline and betain by acetylcholinesterase (AChE), and when the resultant choline comes into contact with this enzyme, it is converted to hydrogen peroxide. A constantly applied voltage then oxidizes the hydrogen peroxide, leading to the release of electrons which are detected as changes in current. The changes in current that are observed with this are abolished by AChE inhibitors and are tetrodotoxin-sensitive, thus validating that the changes in choline detected by this method are derived from the hydrolysis of newly released ACh by AChE, and can be examined at a temporal resolution of less than one second (Parikh et al., 2004).
Parikh and colleagues (2006) examined the tonic and phasic activity in the prefrontal and motor cortices of task-performing rats utilizing choline sensitive microelectrodes. Tonic increases were observed in both regions following initiation of an experimental session, supporting hypotheses that posit changes in tonic activity are cortex-wide and indicative of a global state-setting function of the cholinergic system. Phasic ACh release, however, was evoked by attended, but not missed target stimuli. Furthermore, this phasic response was confined to the PFC. Thus, the selectivity of transient increases in ACh release appears to reflect the encoding of target detection in regions engaged in cognitive processes. The demonstration of regional specificity in phasic cholinergic signals corresponds with theories proposing segregation of the functional significance of cholinergic inputs in separate brain regions, as well as those that ascribe a central role to PFC inputs in the neurobiological network underlying attentional processing (Parikh et al., 2006; Sarter et al., 2005a; Zaborszky, 2002)
Previous research has demonstrated that basal serotonergic concentrations can be altered by salient stimuli and stimuli with affective significance (Gonzalez et al., 2004). Unlike the previously mentioned neurotransmitter systems, descriptions of serotonergic activity have generally referred to tonic modes of firing, such as that of neurons in the MRN associated with hippocampal theta rhythms (Viana Di Prisco et al., 2002). Evidence for temporally-distinct, phasic modes of serotonergic release does not appear to be available, due in part to the absence of appropriate methods.
Taken together, accumulating data detracts from the conception of ascending systems as primarily performing slow and global modulatory functions, and instead supports a more direct role in information processing. This attribution is particularly well supported by research demonstrating cue-evoked, transient increases in activity within the noradrenergic and cholinergic systems. Thus, the activity of these modulators appears to represent a necessary component of the process of incorporating stimuli into ongoing cognitive processes. The ability of the neuromodulators to display two or more modes of activity demands the re-conceptualization of these systems as necessary components in specific cognitive functions. The parallel organization of the ascending systems suggests that they may be activated in concert, and their overlapping terminal fields imply that the interactions between these systems most certainly have functional significance. Therefore, studies designed to examine how tonic and phasic modes of activity within one neuromodulator system may influence the same modes of another are needed.
7. Impact for theories of neuropsychiatric disorders
Theories describing abnormal regulation or disintegration of neuronal systems as a major basis for the development of the symptoms of schizophrenia, depression, anxiety disorders, age-related cognitive impairments, and Alzheimer’s disease have consistently focused on individual neuromodulator systems. To illustrate the issue, contemporary hypotheses concerning the cognitive symptoms of schizophrenia propose that abnormal reactivity of mesolimbic DA projections in ventral striatal regions mediate the expression of psychotic symptoms, including the associated or even underlying cognitive dysfunction (Breier et al., 1997; Kapur, 2003; Laruelle et al., 1999). At the same time, the more persistent cognitive impairments of schizophrenia have been hypothesized to be a result, at least in part, of hypodopaminergia in the PFC (Castner et al., 2000; Goldman-Rakic et al., 2004). There are competing hypotheses, including those that focus on the role of the abnormal regulation of cholinergic inputs into this region (Sarter et al., 2005b) and on the significance of a hypoglutamatergic state and associated glutamate receptor dysregulation (Coyle et al., 2003; Goff and Coyle, 2001; Lewis and Moghaddam, 2006; Moghaddam and Jackson, 2003), perhaps associated with, or even resulting from, disruption of insufficient GABAergic inhibition in prefrontal regions (Lewis and Moghaddam, 2006). However, the hypotheses concerning abnormally regulated neuromodulator systems rarely made explicit contact with each other, or on the basis of very limited evidence (e.g., the role of NAc dopaminergic abnormalities in the manifestation of cortical cholinergic dysfunction in Sarter et al., 2005b).
This review aims at suggesting that the largely isolated investigation of the role of individual neuromodulator systems is likely to fail in identifying the central role of interactions between multiple dysregulated neuromodulatory afferents of forebrain system in the development of the cognitive symptoms of schizophrenia or other neuropsychiatric and neurodegenerative disorders. Although research has begun addressing interactions between abnormally regulated glutamatergic and dopaminergic systems in models of schizophrenia (Jentsch et al., 1998a; Jentsch et al., 1998b; Kegeles et al., 2000), a hypothesis that would describe the role of interactions between abnormally regulated dopaminergic, glutamatergic, GABAergic, cholinergic, serotonergic (Pehek et al., 2006), and noradrenergic (Marrs et al., 2005) projection systems remains out of reach.
The long list of neuromodulator systems directly or indirectly involved in cognitive disorders, and the enormous number of resulting interactions, demands comment on potentially productive research strategies. First, conventional approaches to studying synergistic interactions between these systems, by assessing the effects of the removal of one, two or more systems on behavioral and cognitive functions, appear to generate relatively limited insights into these interactions and their functional significance, as such approaches merely reveal the mounting deficits resulting from the removal or inactivation of an accumulating number of neuromodulatory systems. Second, and as frequently mentioned above, neurotransmitter interactions studied with respect to basal release and in intact animals not engaging in behavioral or cognitive activities that recruit some of the systems of interest are unlikely to generate informative insights concerning the impact of such interactions (see also Sarter et al., 2007).
In order to constrain research on a theoretically high number of interactions between four or more modulator systems, hypotheses that describe the specific functional impact of such interactions, based on the available evidence summarized above, may provide the basis for more informative and also feasible research approaches. For example, as discussed, there is relatively solid evidence in support of the attentional functions mediated via the cortical cholinergic input system, and there is also extensive evidence for the role of dopaminergic inputs to the PFC in learning. The two cognitive domains are, of course, interrelated, as most forms of learning require the attentional processing of conditioned stimuli (Sarter et al., 2003; Sarter and Lustig, 2006). Furthermore, hypotheses are available that describe the state of these two systems in the PFC of schizophrenics (references above). Consequently, it appears feasible to investigate - as a first step - whether one neuromodulator abnormality, be it an abnormally low level of dopaminergic neurotransmission and insufficient D1 receptor stimulation or abnormally reactive cholinergic responsivity, is sufficient to cause the other, and then to define the cognitive challenges that reveal such interactions, such as the learning about stimuli that are difficult to detect and discriminate from “noise”. Similar hypotheses, albeit on a somewhat more speculative level, can be generated with respect to other neuromodulator combinations and therefore begin guiding this research.
Finally, our perspective has implications for the treatment of cognitive disorders that go beyond descriptive calls for poly-psychopharmacological treatment approaches. Once we arrive at hypotheses describing the interactions between the dysregulatory effects of multiple neuromodulators, stabilization of such interactive networks (Carlsson et al., 2000) can be defined as a new target for treatments. The definition of such a target, combined with biopsychological approaches toward the identification and characterization of treatments targeting abnormal neuromodulator interactions (Sarter, 2006), would assist shifting the present focus away from attempts to normalize a single hypodopaminergic or a hypoglutamatergic state to treatments designed to return the disjointed interactions between multiple neuromodulator systems acting in concert.
Acknowledgments
Our research was supported by PHS Grants MH063114, MH057436, NS37026, KO2MH01072 and MH 073600, as well as grants from Abbott Laboratories and Pfizer Inc. (MS). Damon Young was supported by a Ford Foundation Diversity Fellowship.
List of Abbreviations
- 5-HT
serotonin
- ACh
acetylcholine
- AChE
acetylcholinesterase
- BFCS
basal forebrain cholinergic system
- DA
dopamine, dlPFC, dorsolateral prefrontal cortex
- DRN
dorsal raphe nucleus
- GP
globus pallidus
- LC
locus coeruleus
- MD
mediodorsal nucleus of the thalamus
- MFB
medial forebrain bundle
- MRN
medial raphe nuclei
- NAc
nucleus accumbens
- nAChR
nicotinic acetylcholine receptor
- nbM
nucleus basalis of Meynert, NE, norepinephrine
- PFC
prefrontal cortex
- RN
raphe nuclei
- SI
substantia innominata
- SN
substantia nigra
- vlPFC
ventrolateral prefrontal cortex
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–19. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acquas E, Day JC, Fibiger HC. The potent and selective dopamine D1 receptor agonist A-77636 increases cortical and hippocampal acetylcholine release in the rat. Eur J Pharmacol. 1994;260:85–7. doi: 10.1016/0014-2999(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Foote WE, Sheard MH. Lysergic acid diethylamide: sensitive neuronal units in the midbrain raphe. Science. 1968;161:706–8. doi: 10.1126/science.161.3842.706. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Wang RY. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 1977;122:229–42. doi: 10.1016/0006-8993(77)90291-8. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Leo G, Zanardi A, Genedani S, Rivera A, Fuxe K, Guidolin D. Volume transmission and wiring transmission from cellular to molecular networks: history and perspectives. Acta Physiol (Oxf) 2006;187:329–44. doi: 10.1111/j.1748-1716.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Braga MF, Pereira EF, Maelicke A, Albuquerque EX. alpha7 nicotinic acetylcholine receptors and modulation of gabaergic synaptic transmission in the hippocampus. Eur J Pharmacol. 2000;393:59–67. doi: 10.1016/s0014-2999(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–96. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–72. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anden NE, Dahlstrom A, Fuxe K, Olson L, Ungerstedt U. Ascending noradrenaline neurons from the pons and the medulla oblongata. Experientia. 1966;22:44–5. doi: 10.1007/BF01897761. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Puttfarcken PS, Jacobs I, Faltynek C. Assessment of nicotinic acetylcholine receptor-mediated release of [(3)H]-norepinephrine from rat brain slices using a new 96-well format assay. Neuropharmacology. 2000;39:2663–72. doi: 10.1016/s0028-3908(00)00143-x. [DOI] [PubMed] [Google Scholar]
- Aoki C. Beta-adrenergic receptors: astrocytic localization in the adult visual cortex and their relation to catecholamine axon terminals as revealed by electron microscopic immunocytochemistry. J Neurosci. 1992;12:781–92. doi: 10.1523/JNEUROSCI.12-03-00781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apparsundaram S, Martinez V, Parikh V, Kozak R, Sarter M. Increased capacity and density of choline transporters situated in synaptic membranes of the right medial prefrontal cortex of attentional task-performing rats. J Neurosci. 2005;25:3851–6. doi: 10.1523/JNEUROSCI.0205-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983;216:53–68. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, et al. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–53. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience. 2002;114:451–60. doi: 10.1016/s0306-4522(02)00292-0. [DOI] [PubMed] [Google Scholar]
- Arnold HM, Nelson CL, Neigh GN, Sarter M, Bruno JP. Systemic and intra-accumbens administration of amphetamine differentially affects cortical acetylcholine release. Neuroscience. 2000;96:675–85. doi: 10.1016/s0306-4522(99)00590-4. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–62. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Through the looking glass: differential noradrenergic modulation of prefrontal cortical function. Neural Plast. 2000;7:133–46. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J Neurosci. 1988;8:4287–98. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res. 1984;306:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–6. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–84. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005a;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005b;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234:734–7. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. Role of the locus coeruleus in emotional activation. Prog Brain Res. 1996;107:379–402. doi: 10.1016/s0079-6123(08)61877-4. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience. 1997;80:697–715. doi: 10.1016/s0306-4522(97)00060-2. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–20. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res. 2000;126:165–82. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Aultman JM, Moghaddam B. Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology (Berl) 2001;153:353–64. doi: 10.1007/s002130000590. [DOI] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan U, Leslie FM. Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience. 2003;119:965–77. doi: 10.1016/s0306-4522(03)00220-3. [DOI] [PubMed] [Google Scholar]
- Azmitia E, Gannon P. The ultrastructural localization of serotonin immunoreactivity in myelinated and unmyelinated axons within the medial forebrain bundle of rat and monkey. J Neurosci. 1983;3:2083–90. doi: 10.1523/JNEUROSCI.03-10-02083.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Grodzinsky G, DuPaul GJ. Frontal lobe functions in attention deficit disorder with and without hyperactivity: a review and research report. J Abnorm Child Psychol. 1992;20:163–188. doi: 10.1007/BF00916547. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Fleming DL. Aging in the rhesus monkey: effects on visual discrimination learning and reversal learning. J Gerontol. 1979;34:209–219. doi: 10.1093/geronj/34.2.209. [DOI] [PubMed] [Google Scholar]
- Bates JF, Goldman-Rakic PS. Prefrontal connections of medial motor areas in the rhesus monkey. J Comp Neurol. 1993;336:211–228. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- Beani L, Bianchi C, Giacomelli A, Tamberi F. Noradrenaline inhibition of acetylcholine release from guinea-pig brain. Eur J Pharmacol. 1978;48:179–93. doi: 10.1016/0014-2999(78)90327-8. [DOI] [PubMed] [Google Scholar]
- Berger B, Trottier S, Verney C, Gaspar P, Alvarez C. Regional and laminar distribution of the dopamine and serotonin innervation in the macaque cerebral cortex: a radioautographic study. J Comp Neurol. 1988;273:99–119. doi: 10.1002/cne.902730109. [DOI] [PubMed] [Google Scholar]
- Berlanga ML, Simpson TK, Alcantara AA. Dopamine D5 receptor localization on cholinergic neurons of the rat forebrain and diencephalon: a potential neuroanatomical substrate involved in mediating dopaminergic influences on acetylcholine release. J Comp Neurol. 2005;492:34–49. doi: 10.1002/cne.20684. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Arnsten AF, Foote SL. Noradrenergic modulation of cognitive function: clinical implications of anatomical, electrophysiological and behavioural studies in animal models. Psychol Med. 1993;23:557–64. doi: 10.1017/s0033291700025332. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Stratford TL, Foote SL, Kelley AE. Distribution of dopamine beta-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse. 1997;27:230–41. doi: 10.1002/(SICI)1098-2396(199711)27:3<230::AID-SYN8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Siniscalchi A, Beani L. 5-HT1A agonists increase and 5-HT3 agonists decrease acetylcholine efflux from the cerebral cortex of freely-moving guinea-pigs. Br J Pharmacol. 1990;101:448–52. doi: 10.1111/j.1476-5381.1990.tb12728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Nikkel AL, Curzon P, Arneric SP, Bannon AW, Decker MW. Role of the nucleus raphe magnus in antinociception produced by ABT-594: immediate early gene responses possibly linked to neuronal nicotinic acetylcholine receptors on serotonergic neurons. J Neurosci. 1998;18:5426–32. doi: 10.1523/JNEUROSCI.18-14-05426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondareff W, Mountjoy CQ, Roth M. Selective loss of neurones of origin of adrenergic projection to cerebral cortex (nucleus locus coeruleus) in senile dementia. Lancet. 1981;1:783–4. doi: 10.1016/s0140-6736(81)92657-x. [DOI] [PubMed] [Google Scholar]
- Book AA, Wiley RG, Schweitzer JB. Specificity of 192 IgG-saporin for NGF receptor-positive cholinergic basal forebrain neurons in the rat. Brain Res. 1992;590:350–5. doi: 10.1016/0006-8993(92)91121-t. [DOI] [PubMed] [Google Scholar]
- Bramwell GJ. Factors affecting the activity of 5-HT-containing neurons. Brain Res. 1974;79:515–9. doi: 10.1016/0006-8993(74)90450-8. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA. 1997;94:2569–74. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broersen LM, Heinsbroek RP, de Bruin JP, Joosten RN, van Hest A, Olivier B. Effects of local application of dopaminergic drugs into the dorsal part of the medial prefrontal cortex of rats in a delayed matching to position task: comparison with local cholinergic blockade. Brain Res. 1994;645:113–22. doi: 10.1016/0006-8993(94)91644-6. [DOI] [PubMed] [Google Scholar]
- Brown RM, Crane AM, Goldman PS. Regional distribution of monoamines in the cerebral cortex and subcortical structures of the rhesus monkey: concentrations and in vivo synthesis rates. Brain Res. 1979;168:133–50. doi: 10.1016/0006-8993(79)90132-x. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Buhot MC. Serotonin receptors in cognitive behaviors. Curr Opin Neurobiol. 1997;7:243–54. doi: 10.1016/s0959-4388(97)80013-x. [DOI] [PubMed] [Google Scholar]
- Burgess PW. Strategy application disorder: the role of the frontal lobes in human multitasking. Psychol Res. 2000;63:279–288. doi: 10.1007/s004269900006. [DOI] [PubMed] [Google Scholar]
- Cai JX, Ma YY, Xu L, Hu XT. Reserpine impairs spatial working memory performance in monkeys: reversal by the alpha 2-adrenergic agonist clonidine. Brain Res. 1993;614:191–6. doi: 10.1016/0006-8993(93)91034-p. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Waters S, Carlsson ML. Network interactions in schizophrenia - therapeutic implications. Brain Res Brain Res Rev. 2000;31:342–9. doi: 10.1016/s0165-0173(99)00050-8. [DOI] [PubMed] [Google Scholar]
- Campbell CB, Hodos W. The concept of homology and the evolution of the nervous system. Brain Behav Evol. 1970;3:353–367. doi: 10.1159/000125482. [DOI] [PubMed] [Google Scholar]
- Carnes KM, Fuller TA, Price JL. Sources of presumptive glutamatergic/aspartatergic afferents to the magnocellular basal forebrain in the rat. J Comp Neurol. 1990;302:824–52. doi: 10.1002/cne.903020413. [DOI] [PubMed] [Google Scholar]
- Casamenti F, Deffenu G, Abbamondi AL, Pepeu G. Changes in cortical acetylcholine output induced by modulation of the nucleus basalis. Brain Res Bull. 1986;16:689–95. doi: 10.1016/0361-9230(86)90140-1. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287:2020–2. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Multiple visual areas in the posterior parietal cortex of primates. Prog Brain Res. 1993;95:123–137. doi: 10.1016/s0079-6123(08)60363-5. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–29. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno ML, Henderson JA, Pau KY, Bethea CL. Estradiol increases alpha7 nicotinic receptor in serotonergic dorsal raphe and noradrenergic locus coeruleus neurons of macaques. J Comp Neurol. 2006;497:489–501. doi: 10.1002/cne.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79:2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–9. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V. Serotonin axons in the supra- and subependymal plexuses and in the leptomeninges; their roles in local alterations of cerebrospinal fluid and vasomotor activity. Brain Res. 1976;102:103–30. doi: 10.1016/0006-8993(76)90578-3. [DOI] [PubMed] [Google Scholar]
- Ciombor KJ, Ennis M, Shipley MT. Norepinephrine increases rat mitral cell excitatory responses to weak olfactory nerve input via alpha-1 receptors in vitro. Neuroscience. 1999;90:595–606. doi: 10.1016/s0306-4522(98)00437-0. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–80. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–8. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G. Phasic activation of monkey locus coeruleus neurons by simple decisions in a forced-choice task. J Neurosci. 2004;24:9914–9920. doi: 10.1523/JNEUROSCI.2446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consolo S, Arnaboldi S, Giorgi S, Russi G, Ladinsky H. 5-HT4 receptor stimulation facilitates acetylcholine release in rat frontal cortex. Neuroreport. 1994;5:1230–2. doi: 10.1097/00001756-199406020-00018. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann N Y Acad Sci. 2003;1003:318–27. doi: 10.1196/annals.1300.020. [DOI] [PubMed] [Google Scholar]
- Cucchiaro G, Commons KG. Alpha 4 nicotinic acetylcholine receptor subunit links cholinergic to brainstem monoaminergic neurotransmission. Synapse. 2003;49:195–205. doi: 10.1002/syn.10218. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–9. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of an outflow of noradrenaline nerve fibres in the ventral roots of the rat spinal cord. Experientia. 1965;21:409–10. doi: 10.1007/BF02139774. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–84. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The Prefrontal Cortex: Executive and Cognitive Functions. Oxford Univ Press; Oxford: 1998. [Google Scholar]
- Day J, Fibiger HC. Dopaminergic regulation of cortical acetylcholine release. Synapse. 1992;12:281–6. doi: 10.1002/syn.890120405. [DOI] [PubMed] [Google Scholar]
- Day J, Fibiger HC. Dopaminergic regulation of cortical acetylcholine release: effects of dopamine receptor agonists. Neuroscience. 1993;54:643–8. doi: 10.1016/0306-4522(93)90235-8. [DOI] [PubMed] [Google Scholar]
- Decker MW, McGaugh JL. The role of interactions between the cholinergic system and other neuromodulatory systems in learning and memory. Synapse. 1991;7:151–168. doi: 10.1002/syn.890070209. [DOI] [PubMed] [Google Scholar]
- Dekker AJ, Thal LJ. Independent effects of cholinergic and serotonergic lesions on acetylcholine and serotonin release in the neocortex of the rat. Neurochem Res. 1993;18:277–83. doi: 10.1007/BF00969083. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Fuxe K, Mora F. Changes in dialysate concentrations of glutamate and GABA in the brain: an index of volume transmission mediated actions? J Neurochem. 2003;85:23–33. doi: 10.1046/j.1471-4159.2003.01692.x. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res. 1998;806:127–40. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- Descarries L, Beaudet A, Watkins KC. Serotonin nerve terminals in adult rat neocortex. Brain Res. 1975;100:563–88. doi: 10.1016/0006-8993(75)90158-4. [DOI] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J Comp Neurol. 1982;207:239–54. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- Di Cara B, Panayi F, Gobert A, Dekeyne A, Sicard D, De Groote L, et al. Activation of dopamine D1 receptors enhances cholinergic transmission and social cognition: a parallel dialysis and behavioural study in rats. Int J Neuropsychopharmacol. 2006;22:1–17. doi: 10.1017/S1461145706007103. [DOI] [PubMed] [Google Scholar]
- Dinopoulos A, Dori I, Parnavelas JG. The serotonin innervation of the basal forebrain shows a transient phase during development. Brain Res Dev Brain Res. 1997;99:38–52. doi: 10.1016/s0165-3806(96)00198-8. [DOI] [PubMed] [Google Scholar]
- Douglas CL, Baghdoyan HA, Lydic R. M2 muscarinic autoreceptors modulate acetylcholine release in prefrontal cortex of C57BL/6J mouse. J Pharmacol Exp Ther. 2001;299:960–6. [PubMed] [Google Scholar]
- Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry. 1998;3:220–226. 190–221. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- Drew AE, Derbez AE, Werling LL. Nicotinic receptor-mediated regulation of dopamine transporter activity in rat prefrontal cortex. Synapse. 2000;38:10–6. doi: 10.1002/1098-2396(200010)38:1<10::AID-SYN2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Ellis JR, Ellis KA, Bartholomeusz CF, Harrison BJ, Wesnes KA, Erskine FF, Vitetta L, Nathan PJ. Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol. 2006;9:175–89. doi: 10.1017/S1461145705005407. [DOI] [PubMed] [Google Scholar]
- Emson PC, Koob GF. The origin and distribution of dopamine-containing afferents to the rat frontal cortex. Brain Res. 1978;142:249–67. doi: 10.1016/0006-8993(78)90634-0. [DOI] [PubMed] [Google Scholar]
- Espana RA, Berridge CW. Organization of noradrenergic efferents to arousal-related basal forebrain structures. J Comp Neurol. 2006;496:668–83. doi: 10.1002/cne.20946. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–84. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fadel J, Sarter M, Bruno JP. Basal forebrain glutamatergic modulation of cortical acetylcholine release. Synapse. 2001;39:201–12. doi: 10.1002/1098-2396(20010301)39:3<201::AID-SYN1001>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–28. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Cholinergic inhibition of ventral midbrain dopamine neurons. J Neurosci. 2000;20:7855–60. doi: 10.1523/JNEUROSCI.20-20-07855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch TE, Sahr RN, Eastwood BJ, Zhou FC, Yang CR. Dopamine D1/5 receptor modulation of firing rate and bidirectional theta burst firing in medial septal/vertical limb of diagonal band neurons in vivo. J Neurophysiol. 2006;95:2808–2820. doi: 10.1152/jn.01210.2005. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–7. [PubMed] [Google Scholar]
- Fluxe K, Hokfelt T, Johansson O, Jonsson G, Lidbrink P, Ljungdahl A. The origin of the dopamine nerve terminals in limbic and frontal cortex. Evidence for mesocortico dopamine neurons. Brain Res. 1974;82:349–55. doi: 10.1016/0006-8993(74)90618-0. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77:3033–7. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Fornal CA, Metzler CW, Marrosu F, Ribiero-do-Valle LE, Jacobs BL. A subgroup of dorsal raphe serotonergic neurons in the cat is strongly activated during oral-buccal movements. Brain Res. 1996;716:123–33. doi: 10.1016/0006-8993(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Fort P, Khateb A, Pegna A, Muhlethaler M, Jones BE. Noradrenergic modulation of cholinergic nucleus basalis neurons demonstrated by in vitro pharmacological and immunohistochemical evidence in the guinea-pig brain. Eur J Neurosci. 1995;7:1502–11. doi: 10.1111/j.1460-9568.1995.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Freedman R, Foote SL, Bloom FE. Histochemical characterization of a neocortical projection of the nucleus locus coeruleus in the squirrel monkey. J Comp Neurol. 1975;164:209–31. doi: 10.1002/cne.901640205. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Visuospatial coding in primate prefrontal neurons revealed by oculomotor paradigms. J Neurophysiol. 1990;63:814–831. doi: 10.1152/jn.1990.63.4.814. [DOI] [PubMed] [Google Scholar]
- Fuster J. The Prefrontal Cortex. Raven; New York: 1980. [Google Scholar]
- Fuster JM. Executive frontal functions. Exp Brain Res. 2000;133:66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Hamberger B, Hokfelt T. Distribution of noradrenaline nerve terminals in cortical areas of the rat. Brain Res. 1968;8:125–31. doi: 10.1016/0006-8993(68)90175-3. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Zaborszky L. Direct catecholaminergic-cholinergic interactions in the basal forebrain. II. Substantia nigra-ventral tegmental area projections to cholinergic neurons. J Comp Neurol. 1996;374:555–77. doi: 10.1002/(SICI)1096-9861(19961028)374:4<555::AID-CNE6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- George TP, Verrico CD, Picciotto MR, Roth RH. Nicotinic modulation of mesoprefrontal dopamine neurons: pharmacologic and neuroanatomic characterization. J Pharmacol Exp Ther. 2000;295:58–66. [PubMed] [Google Scholar]
- Geula C, Slevin JT. Substantia nigra 6-hydroxydopamine lesions alter dopaminergic synaptic markers in the nucleus basalis magnocellularis and striatum of rats. Synapse. 1989;4:248–253. doi: 10.1002/syn.890040310. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Serotonergic functions in arousal and motor activity. Behav Brain Res. 1996;73:31–5. doi: 10.1016/0166-4328(96)00065-4. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Neural interaction between the basal forebrain and functionally distinct prefrontal cortices in the rhesus monkey. Neuroscience. 2001;103:593–614. doi: 10.1016/s0306-4522(00)00585-6. [DOI] [PubMed] [Google Scholar]
- Gioanni Y, Rougeot C, Clarke PB, Lepouse C, Thierry AM, Vidal C. Nicotinic receptors in the rat prefrontal cortex: increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. Eur J Neurosci. 1999;11:18–30. doi: 10.1046/j.1460-9568.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Anatomical segregation of component processes in an inductive inference task. J Cogn Neurosci. 2000;12:110–119. doi: 10.1162/08989290051137639. [DOI] [PubMed] [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–77. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Nauta WJ. Autoradiographic demonstration of a projection from prefrontal association cortex to the superior colliculus in the rhesus monkey. Brain Res. 1976;116:145–149. doi: 10.1016/0006-8993(76)90256-0. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. Am Physiol Soc. Bethesda: 1987. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. [Google Scholar]
- Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci U S A. 1989;86:9015–9. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Gallager DW. Overlap of dopaminergic, adrenergic, and serotonergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci. 1990;10:2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Schwartz ML. Interdigitation of contralateral and ipsilateral columnar projections to frontal association cortex in primates. Science. 1982;216:755–757. doi: 10.1126/science.6177037. [DOI] [PubMed] [Google Scholar]
- Golmayo L, Nunez A, Zaborszky L. Electrophysiological evidence for the existence of a posterior cortical-prefrontal-basal forebrain circuitry in modulating sensory responses in visual and somatosensory rat cortical areas. Neuroscience. 2003;119:597–609. doi: 10.1016/s0306-4522(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Quinonez B, Rangel A, Pino S, Hernandez L. Tonic and phasic alteration in amygdala 5-HT, glutamate and GABA transmission after prefrontal cortex damage in rats. Brain Res. 2004;1005:154–63. doi: 10.1016/j.brainres.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–12. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–28. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984;4:2866–76. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology. 1996;46:1231–1238. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–8. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Grillner P, Svensson TH. Nicotine-induced excitation of midbrain dopamine neurons in vitro involves ionotropic glutamate receptor activation. Synapse. 2000;38:1–9. doi: 10.1002/1098-2396(200010)38:1<1::AID-SYN1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458:11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. The basal ganglia and motor control. Neural Plast. 2003;10:107–20. doi: 10.1155/NP.2003.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–35. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Zaborszky L. Direct catecholaminergic-cholinergic interactions in the basal forebrain. III. Adrenergic innervation of choline acetyltransferase-containing neurons in the rat. J Comp Neurol. 2002;449:141–57. doi: 10.1002/cne.10279. [DOI] [PubMed] [Google Scholar]
- Hartley LR, Adams RG. Effect of noise on the Stroop Test. J Exp Psychol. 1974;102:62–66. doi: 10.1037/h0035695. [DOI] [PubMed] [Google Scholar]
- Harvey JA. Serotonergic regulation of associative learning. Behav Brain Res. 1996;73:47–50. doi: 10.1016/0166-4328(96)00068-x. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Progr Brain Res. 2004;145:207–31. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Hersi AI, Kitaichi K, Srivastava LK, Gaudreau P, Quirion R. Dopamine D-5 receptor modulates hippocampal acetylcholine release. Brain Res. 2000;76:336–340. doi: 10.1016/s0169-328x(00)00015-2. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. Increases in cortical acetylcholine release during sustained attention performance in rats. Brain Res Cogn Brain Res. 2000;9:313–25. doi: 10.1016/s0926-6410(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–8. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- Hu XJ, Wang FH, Stenfors C, Ogren SO, Kehr J. Effects of the 5-HT(1B) receptor antagonist NAS-181 on extracellular levels of acetylcholine, glutamate and GABA in the frontal cortex and ventral hippocampus of awake rats: A microdialysis study. Eur Neuropsychopharmacol. 2007 doi: 10.1016/j.euroneuro.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Li Z, Dai J, Meltzer HY. Atypical antipsychotic drugs, quetiapine, iloperidone, and melperone, preferentially increase dopamine and acetylcholine release in rat medial prefrontal cortex: role of 5-HT1A receptor agonism. Brain Res. 2002;956:349–57. doi: 10.1016/s0006-8993(02)03570-9. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol. 2000;417:337–48. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Dazzi L, Chhatwal JP, Verrico CD, Roth RH. Reduced prefrontal cortical dopamine, but not acetylcholine, release in vivo after repeated, intermittent phencyclidine administration to rats. Neurosci Lett. 1998a;258:175–8. doi: 10.1016/s0304-3940(98)00879-9. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, Roth RH. Subchronic phencyclidine administration increases mesolimbic dopaminergic system responsivity and augments stress- and psychostimulant-induced hyperlocomotion. Neuropsychopharmacology. 1998b;19:105–13. doi: 10.1016/S0893-133X(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Jodo E, Chiang C, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83:63–79. doi: 10.1016/s0306-4522(97)00372-2. [DOI] [PubMed] [Google Scholar]
- Jones BE, Cuello AC. Afferents to the basal forebrain cholinergic cell area from pontomesencephalic--catecholamine, serotonin, and acetylcholine-neurons. Neuroscience. 1989;31:37–61. doi: 10.1016/0306-4522(89)90029-8. [DOI] [PubMed] [Google Scholar]
- Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res. 1977;127:25–53. [PubMed] [Google Scholar]
- Kalaria RN, Andorn AC. Adrenergic receptors in aging and Alzheimer’s disease: decreased alpha 2-receptors demonstrated by [3H]p-aminoclonidine binding in prefrontal cortex. Neurobiol Aging. 1991;12:131–136. doi: 10.1016/0197-4580(91)90051-k. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Katz PS. Beyond neurotransmission: neuromodulation and its importance for information processing. Oxford University Press; Oxford, UK: 1999. [Google Scholar]
- Kawahara H, Kawahara Y, Westerink BH. The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of noradrenaline in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur J Pharmacol. 2000;387:279–86. doi: 10.1016/s0014-2999(99)00793-1. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Beaulieu M, Itoh Y. Pharmacological and biochemical evidence for the existence of two categories of dopamine receptor. Can J Neurol Sci. 1984;11:114–7. doi: 10.1017/s0317167100046254. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL, Cooper TB, Carlsson A, Laruelle M. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48:627–40. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–15. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kinberg DYaFMJ. A unified account of cognitive impairments following frontal lobe damage: The role of woring memory in complex organised behaviour. J Expl Psychol. 1993;122:411–428. doi: 10.1037//0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]
- Kim MA, Lee HS, Lee BY, Waterhouse BD. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res. 2004;1026:56–67. doi: 10.1016/j.brainres.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Impulse activity of ventral tegmental area neurons during heroin self-administration in rats. Neuroscience. 2001;102:565–80. doi: 10.1016/s0306-4522(00)00492-9. [DOI] [PubMed] [Google Scholar]
- Knox D, Sarter M, Berntson GG. Visceral afferent bias on cortical processing: role of adrenergic afferents to the basal forebrain cholinergic system. Behav Neurosci. 2004;118:1455–9. doi: 10.1037/0735-7044.118.6.1455. [DOI] [PubMed] [Google Scholar]
- Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Res. 1984;320:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Kolb B. Prefrontal Cortex. MIT Press: Cambridge, MA; 1990. [Google Scholar]
- Kosofsky BE, Molliver ME. The serotoninergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse. 1987;1:153–68. doi: 10.1002/syn.890010204. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Molliver ME, Morrison JH, Foote SL. The serotonin and norepinephrine innervation of primary visual cortex in the cynomolgus money (Macaca fascicularis) J Com Neurol. 1984;230:168–78. doi: 10.1002/cne.902300203. [DOI] [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex. 2006;16:9–17. doi: 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- Laplante F, Sibley DR, Quirion R. Reduction in acetylcholine release in the hippocampus of dopamine D5 receptor-deficient mice. Neuropsychopharmacology. 2004;29:1620–1627. doi: 10.1038/sj.npp.1300467. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Waterhouse BD. Retrograde double-labeling study of common afferent projections to the dorsal raphe and the nuclear core of the locus coeruleus in the rat. J Comp Neurol. 2005;481:179–93. doi: 10.1002/cne.20365. [DOI] [PubMed] [Google Scholar]
- Leger L, Charnay Y, Hof PR, Bouras C, Cespuglio R. Anatomical distribution of serotonin-containing neurons and axons in the central nervous system of the cat. J Comp Neurol. 2001;433:157–82. [PubMed] [Google Scholar]
- Leonard CM. The prefrontal cortex of the rat. I. Cortical projection of the mediodorsal nucleus. II. Efferent connections. Brain Res. 1969;12:321–343. doi: 10.1016/0006-8993(69)90003-1. [DOI] [PubMed] [Google Scholar]
- Levin E, Icenogle L, Farzad A. Ketanserin attenuates nicotine-induced working memory improvement in rats. Pharmacol Biochem Behav. 2005;82:289–92. doi: 10.1016/j.pbb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Levitt P, Moore RY. Noradrenaline neuron innervation of the neocortex in the rat. Brain Res. 1978;139:219–31. doi: 10.1016/0006-8993(78)90925-3. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hayes TL, Lund JS, Oeth KM. Dopamine and the neural circuitry of primate prefrontal cortex: Implications for schizophrenia research. Neuropsychopharmacology. 1992;6:127–34. [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–6. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Lidov HG, Molliver ME, Zecevic NR. Characterization of the monoaminergic innervation of immature rat neocortex: a histofluorescence analysis. J Comp Neurol. 1978;181:663–79. doi: 10.1002/cne.901810311. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl. 1974;412:1–48. [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Fallon JH. Locus coeruleus projections to cortex: topography, morphology and collateralization. Brain Res Bull. 1982;9:287–94. doi: 10.1016/0361-9230(82)90142-3. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Grzanna R. Efferent projections of nucleus locus coeruleus: morphologic subpopulations have different efferent targets. Neuroscience. 1986;18:307–19. doi: 10.1016/0306-4522(86)90156-9. [DOI] [PubMed] [Google Scholar]
- Lu MT, Preston JB, Strick PL. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J Comp Neurol. 1994;341:375–392. doi: 10.1002/cne.903410308. [DOI] [PubMed] [Google Scholar]
- Luiten PG, Gaykema RP, Traber J, Spencer DG., Jr Cortical projection patterns of magnocellular basal nucleus subdivisions as revealed by anterogradely transported Phaseolus vulgaris leucoagglutinin. Brain Res. 1987;413:229–50. doi: 10.1016/0006-8993(87)91014-6. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Ann Rev Neurosci. 1999;22:443–85. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Kobilka BK, Scheinin M. Gene targeting--homing in on alpha 2-adrenoceptor-subtype function. Trends Pharmacol Sci. 1997;18:211–9. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- Malone SG, Rasmusson DD, Semba K. Cortical projections of rat basal forebrain neurons: lack of axonal collateralization and similar topography of cholinergic and noncholinergic projections. J Sleep & Sleep Dis Res. 2004;27:A10–A11. [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. Differential c-Fos expression in cholinergic, monoaminergic, and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci. 1999;19:3057–72. doi: 10.1523/JNEUROSCI.19-08-03057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas LA, Mullen CA, O’Hearn E, Molliver ME. Dual serotoninergic projections to forebrain in the rat: morphologically distinct 5-HT axon terminals exhibit differential vulnerability to neurotoxic amphetamine derivatives. J Comp Neurol. 1991;314:558–86. doi: 10.1002/cne.903140312. [DOI] [PubMed] [Google Scholar]
- Man Z, Wu HR, Liu S, Yu X. A new adaptive backpropagation algorithm based on Lyapunov stability theory for neural networks. IEEE Trans Neural Netw. 2006;17:1580–91. doi: 10.1109/TNN.2006.880360. [DOI] [PubMed] [Google Scholar]
- Mao ZM, Arnsten AF, Li BM. Local infusion of an alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol Psychiatry. 1999;46:1259–65. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- Marrs W, Kuperman J, Avedian T, Roth RH, Jentsch JD. Alpha-2 adrenoceptor activation inhibits phencyclidine-induced deficits of spatial working memory in rats. Neuropsychopharmacology. 2005;30:1500–10. doi: 10.1038/sj.npp.1300700. [DOI] [PubMed] [Google Scholar]
- Mason ST, Fibiger HC. Regional topography within noradrenergic locus coeruleus as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979;187:703–24. doi: 10.1002/cne.901870405. [DOI] [PubMed] [Google Scholar]
- Matrenza C, Hughes JM, Kemp AH, Wesnes KA, Harrison BJ, Nathan PJ. Simultaneous depletion of serotonin and catecholamines impairs sustained attention in healthy female subjects without affecting learning and memory. J Psychopharmacol. 2004;18:21–31. doi: 10.1177/0269881104040215. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Hirano H, Watanabe Y. Effects of estrogen on acetylcholine release in frontal cortex of female rats: involvement of serotonergic neuronal systems. Brain Res. 2002;937:58–65. doi: 10.1016/s0006-8993(02)02465-4. [DOI] [PubMed] [Google Scholar]
- Mattes JA. The role of frontal lobe dysfunction in childhood hyperkinesis. Compr Psychiatry. 1980;21:358–369. doi: 10.1016/0010-440x(80)90017-6. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psychopharmacology (Berl) 1999;144:175–82. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–65. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Sustained attention performance in rats with intracortical infusions of 192 IgG-saporin-induced cortical cholinergic deafferentation: effects of physostigmine and FG 7142. Behav Neurosci. 1998;112:1519–25. doi: 10.1037//0735-7044.112.6.1519. [DOI] [PubMed] [Google Scholar]
- Mechawar N, Cozzari C, Descarries L. Cholinergic innervation in adult rat cerebral cortex: a quantitative immunocytochemical description. J Comp Neurol. 2000;428:305–18. doi: 10.1002/1096-9861(20001211)428:2<305::aid-cne9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Memeli-Engvall MM, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, Faure P. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–21. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Mereu G, Yoon KW, Boi V, Gessa GL, Naes L, Westfall TC. Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur J Pharmacol. 1987;141:395–9. doi: 10.1016/0014-2999(87)90556-5. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Basal forebrain stimulation modifies auditory cortex responsiveness by an action at muscarinic receptors. Brain Res. 1991;559:163–7. doi: 10.1016/0006-8993(91)90301-b. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH, Weinberger NM. Acetylcholine modifies neuronal acoustic rate-level functions in guinea pig auditory cortex by an action at muscarinic receptors. Synapse. 1990;6:364–8. doi: 10.1002/syn.890060409. [DOI] [PubMed] [Google Scholar]
- Metherate R, Weinberger NM. Acetylcholine produces stimulus-specific receptive field alterations in cat auditory cortex. Brain Res. 1989;480:372–7. doi: 10.1016/0006-8993(89)90210-2. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Di Cara B, Dekeyne A, Panayi F, De Groote L, Sicard D, et al. Selective blockade of dopamine D(3) versus D(2) receptors enhances frontocortical cholinergic transmission and social memory in rats: a parallel neurochemical and behavioural analysis. J Neurochem. 2007;100:1047–1061. doi: 10.1111/j.1471-4159.2006.04262.x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Gobert A, Roux S, Porsolt R, Meneses A, Carli M, et al. The serotonin1A receptor partial agonist S15535 [4-(benzodioxan-5-yl)1-(indan-2-yl)piperazine] enhances cholinergic transmission and cognitive function in rodents: a combined neurochemical and behavioral analysis. J Pharmacol Exp Ther. 2004;311:190–203. doi: 10.1124/jpet.104.069625. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 2000;152:390–7. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Jackson ME. Glutamatergic animal models of schizophrenia. Ann N Y Acad Sci. 2003;1003:131–7. doi: 10.1196/annals.1300.065. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Ann Rev Neurosci. 1979;2:113–68. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Moroni F, Tanganelli S, Antonelli T, Carla V, Bianchi C, Beani L. Modulation of cortical acetylcholine and gamma-aminobutyric acid release in freely moving guinea pigs: effects of clonidine and other adrenergic drugs. J Pharmacol Exp Ther. 1983;227:435–40. [PubMed] [Google Scholar]
- Morrison JH, Grzanna R, Molliver ME, Coyle JT. The distribution and orientation of noradrenergic fibers in neocortex of the rat: an immunofluorescence study. J Comp Neurol. 1978;181:17–39. doi: 10.1002/cne.901810103. [DOI] [PubMed] [Google Scholar]
- Mosko SS, Jacobs BL. Midbrain raphe neurons: spontaneous activity and response to light. Physiol Behav. 1974;13:589–93. doi: 10.1016/0031-9384(74)90292-3. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Goldman-Rakic PS. Acetylcholinesterase reactivity in the frontal cortex of human and monkey: contribution of AChE-rich pyramidal neurons. J Comp Neurol. 1992;324:261–81. doi: 10.1002/cne.903240208. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Levey AI, Goldman-Rakic PS. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc Natl Acad Sci USA. 1993;90:5194–8. doi: 10.1073/pnas.90.11.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Pappy M, Leranth C, Goldman-Rakic PS. Cholinergic synaptic circuitry in the macaque prefrontal cortex. J Comp Neurol. 1995;357:603–17. doi: 10.1002/cne.903570409. [DOI] [PubMed] [Google Scholar]
- Muller U, von Cramon DY, Pollmann S. D1- versus D2-receptor modulation of visuospatial working memory in humans. J Neurosci. 1998;18:2720–8. doi: 10.1523/JNEUROSCI.18-07-02720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Wachter T, Barthel H, Reuter M, von Cramon DY. Striatal [123I]beta-CIT SPECT and prefrontal cognitive functions in Parkinson’s disease. J Neural Transm. 2000;107:303–319. doi: 10.1007/s007020050025. [DOI] [PubMed] [Google Scholar]
- Munk MH, Roelfsema PR, Konig P, Engel AK, Singer W. Role of reticular activation in the modulation of intracortical synchronization. Science. 1996;272:271–4. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- Nair SG, Gudelsky GA. Activation of 5-HT2 receptors enhances the release of acetylcholine in the prefrontal cortex and hippocampus of the rat. Synapse. 2004;53:202–7. doi: 10.1002/syn.20054. [DOI] [PubMed] [Google Scholar]
- Nair SG, Gudelsky GA. Effect of a serotonin depleting regimen of 3,4-methylenedioxymethamphetamine (MDMA) on the subsequent stimulation of acetylcholine release in the rat prefrontal cortex. Brain Res Bull. 2006;69:382–7. doi: 10.1016/j.brainresbull.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Nauta WJ. Neural associations of the amygdaloid complex in the monkey. Brain. 1962;85:505–520. doi: 10.1093/brain/85.3.505. [DOI] [PubMed] [Google Scholar]
- O’Hearn E, Molliver ME. Organization of raphe-cortical projections in rat: a quantitative retrograde study. Brain Res Bull. 1984;13:709–26. doi: 10.1016/0361-9230(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J Comp Neurol. 2006;494:863–75. doi: 10.1002/cne.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ. Catecholamines in the central nervous system. Hdbk Exp Pharmacol. 1988;89:1–26. [Google Scholar]
- Papadopoulos GC, Parnavelas JG, Buijs R. Monoaminergic fibers form conventional synapses in the cerebral cortex. Neurosci Lett. 1987;76:275–9. doi: 10.1016/0304-3940(87)90414-9. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Phasic and tonic changes in cortical cholinergic neurotransmission evoked by attention-demanding cues and associated cognitive operations. Poster presented at the Society for Neuroscience Meeting; Atlanta, Georgia. 2006. [Google Scholar]
- Parsons LH, Justice JB., Jr Extracellular concentration and in vivo recovery of dopamine in the nucleus accumbens using microdialysis. J Neurochem. 1992;58:212–8. doi: 10.1111/j.1471-4159.1992.tb09298.x. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology. 2006;31:265–77. doi: 10.1038/sj.npp.1300819. [DOI] [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–81. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–68. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Pribram KH, McGuinness D. Arousal, activation, and effort in the control of attention. Psychol Rev. 1975;82:116–49. doi: 10.1037/h0076780. [DOI] [PubMed] [Google Scholar]
- Price DL, Sisodia SS, Koo EH, Muma NA, Kitt CA, Walker LC, Martin LJ, Troncoso JC, Griffin JW, Hoffman PN, et al. Neuronal disorders: studies of animal models and human diseases. Toxicol Pathol. 1990;18:128–37. doi: 10.1177/019262339001800118. [DOI] [PubMed] [Google Scholar]
- Price JL, Stern R. Individual cells in the nucleus basalis--diagonal band complex have restricted axonal projections to the cerebral cortex in the rat. Brain Res. 1983;269:352–6. doi: 10.1016/0006-8993(83)90145-2. [DOI] [PubMed] [Google Scholar]
- Quirion R, Richard J, Wilson A. Muscarinic and nicotinic modulation of cortical acetylcholine release monitored by in vivo microdialysis in freely moving adult rats. Synapse. 1994;17:92–100. doi: 10.1002/syn.890170205. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Kesner RP. The effects of muscarinic cholinergic receptor blockade in the rat anterior cingulate and Prelimbic/Infralimbic cortices on spatial working memory. Neurobiol Learn Mem. 1998;69:241–57. doi: 10.1006/nlme.1998.3823. [DOI] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci USA. 1984;81:1585–9. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao TS, Correa LD, Adams P, Santori EM, Sacaan AI. Pharmacological characterization of dopamine, norepinephrine and serotonin release in the rat prefrontal cortex by neuronal nicotinic acetylcholine receptor agonists. Brain Res. 2003;990:203–8. doi: 10.1016/s0006-8993(03)03532-7. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997;76:707–14. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J Neurosci. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Aghajanian GK. Activation of lateral geniculate neurons by norepinephrine: mediation by an alpha-adrenergic receptor. Brain Res. 1980a;182:345–59. doi: 10.1016/0006-8993(80)91193-2. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Aghajanian GK. Modulation of lateral geniculate neurone excitability by noradrenaline microiontophoresis or locus coeruleus stimulation. Nature. 1980b;287:731–4. doi: 10.1038/287731a0. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–71. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter A, Jacobowitz DM. Neurochemical identification of cholinergic forebrain projection sites of the nucleus tegmentalis dorsalis lateralis. Brain Res Bull. 1981;6:525–9. doi: 10.1016/s0361-9230(81)80027-5. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Russell GV. The nucleus locus coeruleus (dorsolateralis tegmenti) Tex Rep Biol Med. 1955;13:939–88. [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol. 1987;259:483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J Comp Neurol. 1984;222:313–42. doi: 10.1002/cne.902220302. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Devauges V, Biegon A, Blizard DA. The Maudsley rat strains as a probe to investigate noradrenergic-cholinergic interaction in cognitive function. J Physiol Paris. 1994;88:337–45. doi: 10.1016/0928-4257(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Sarter M. Preclinical research into cognition enhancers. Trends Pharmacol Sci. 2006;27:602–8. doi: 10.1016/j.tips.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for memory? Neurobiol Learn Mem. 2003;80:245–256. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Parikh V. Abnormal neurotransmitter release in behavioral and cognitive disorders: toward concepts of dynamic and function-specific dysregulation. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301285. in press. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: The neurobiology of attentional effort. Brain Res Rev. 2006;51:155–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and top-down cholinergic modulation of signal detection. Brain Res Rev. 2005a;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C. Attention and memory. In: Squire L, Albright F, Bloom F, Gage F, Spitzer N, editors. New Encyclopedia of Neuroscience. Elsevier; Oxford: 2006. in press. [Google Scholar]
- Sarter M, Nelson CL, Bruno JP. Cortical cholinergic transmission and cortical information processing following psychostimulant-sensitization: implications for models of schizophrenia. Schizophren Bull. 2005b;31:117–138. doi: 10.1093/schbul/sbi006. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–50. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci. 1997a;17:438–458. doi: 10.1523/JNEUROSCI.17-01-00438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. Int Rev Neurobiol. 1997b;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Jorissen BL, Sobczak S, van Boxtel MP, Hogervorst E, Deutz NE, Riedel WJ. Tryptophan depletion impairs memory consolidation but improves focussed attention in healthy young volunteers. J Psychopharmacol. 2000;14:21–9. doi: 10.1177/026988110001400102. [DOI] [PubMed] [Google Scholar]
- Schroder N, O’Dell SJ, Marshall JF. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse. 2003;49:89–96. doi: 10.1002/syn.10210. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–7. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav. 2002;77:513–7. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–60. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Seth P, Cheeta S, Tucci S, File SE. Nicotinic--serotonergic interactions in brain and behaviour. Pharmacol Biochem Behav. 2002;71:795–805. doi: 10.1016/s0091-3057(01)00715-8. [DOI] [PubMed] [Google Scholar]
- Shearman E, Rossi S, Sershen H, Hashim A, Lajtha A. Locally administered low nicotine-induced neurotransmitter changes in areas of cognitive function. Neurochem Res. 2005;30:1055–66. doi: 10.1007/s11064-005-7132-9. [DOI] [PubMed] [Google Scholar]
- Singer S, Rossi S, Verzosa S, Hashim A, Lonow R, Cooper T, Sershen H, Lajtha A. Nicotine-induced changes in neurotransmitter levels in brain areas associated with cognitive function. Neurochem Res. 2004;29:1779–92. doi: 10.1023/b:nere.0000035814.45494.15. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Goldman-Rakic PS. Heterogeneous targets of dopamine synapses in monkey prefrontal cortex demonstrated by serial section electron microscopy: a laminar analysis using the silver-enhanced diaminobenzidine sulfide (SEDS) immunolabeling technique. Cereb Cortex. 1993;3:223–38. doi: 10.1093/cercor/3.3.223. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Subramanian M, Mesulam MM. Monoaminergic-cholinergic interactions in the primate basal forebrain. Neuroscience. 1999;93:817–29. doi: 10.1016/s0306-4522(99)00116-5. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Williams SM, Szigeti K, Goldman-Rakic PS. Light and electron microscopic characterization of dopamine-immunoreactive axons in human cerebral cortex. J Comp Neurol. 1992;321:325–35. doi: 10.1002/cne.903210302. [DOI] [PubMed] [Google Scholar]
- Somboonthum P, Matsuda T, Asano S, Sakaue M, Baba A. MKC-242, a novel 5-HT1A receptor agonist, facilitates cortical acetylcholine release by a mechanism different from that of 8-OH-DPAT in awake rats. Neuropharmacology. 1997;36:1733–9. doi: 10.1016/s0028-3908(97)00174-3. [DOI] [PubMed] [Google Scholar]
- Spencer DG, Jr, Horvath E, Traber J. Direct autoradiographic determination of M1 and M2 muscarinic acetylcholine receptor distribution in the rat brain: relation to cholinergic nuclei and projections. Brain Res. 1986;380:59–68. doi: 10.1016/0006-8993(86)91429-0. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Rule learning and reward contingency are associated with dissociable patterns of dopamine activation in the rat prefrontal cortex, nucleus accumbens, and dorsal striatum. J Neurosci. 2006;26:8810–8. doi: 10.1523/JNEUROSCI.1656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981;294:366–8. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- Storey JD, Robertson DA, Beattie JE, Reid IC, Mitchell SN, Balfour DJ. Behavioural and neurochemical responses evoked by repeated exposure to an elevated open platform. Behav Brain Res. 2006;166:220–9. doi: 10.1016/j.bbr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The locus coeruleus: a cytoarchitectonic, Golgi and immunohistochemical study in the albino rat. Brain Res. 1976;110:39–56. doi: 10.1016/0006-8993(76)90207-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975;163:467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Tani Y, Saito K, Imoto M, Ohno T. Pharmacological characterization of nicotinic receptor-mediated acetylcholine release in rat brain--an in vivo microdialysis study. Eur J Pharmacol. 1998;351:181–8. doi: 10.1016/s0014-2999(98)00314-8. [DOI] [PubMed] [Google Scholar]
- Tayebati SK, Di Tullio MA, Amenta F. Muscarinic cholinergic receptor subtypes in cerebral cortex of Fisher 344 rats: a light microscope autoradiography study of age-related changes. Mech Ageing Dev. 2006;127:115–22. doi: 10.1016/j.mad.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Tellez S, Colpaert F, Marien M. The alpha 2-adrenoceptor antagonist, (+)-efaroxan, enhances acetylcholine release in the rat cortex in vivo. Eur J Pharmacol. 1995;277:113–6. doi: 10.1016/0014-2999(95)00110-7. [DOI] [PubMed] [Google Scholar]
- Tellez S, Colpaert F, Marien M. Acetylcholine release in the rat prefrontal cortex in vivo: modulation by alpha 2-adrenoceptor agonists and antagonists. J Neurochem. 1997;68:778–85. doi: 10.1046/j.1471-4159.1997.68020778.x. [DOI] [PubMed] [Google Scholar]
- Turchi J, Holley LA, Sarter M. Effects of nicotinic acetylcholine receptor ligands on behavioral vigilance in rats. Psychopharmacology (Berl) 1995;118:195–205. doi: 10.1007/BF02245840. [DOI] [PubMed] [Google Scholar]
- Turchi J, Sarter M. Cortical acetylcholine and processing capacity: effects of cortical cholinergic deafferentation on crossmodal divided attention in rats. Cogn Brain Res. 1997;6:147–58. doi: 10.1016/s0926-6410(97)00027-x. [DOI] [PubMed] [Google Scholar]
- Turchi J, Sarter M. Cortical cholinergic inputs mediate processing capacity: effects of 192 IgG-saporin-induced lesions on olfactory span performance. Eur J Neurosci. 2000;12:4505–14. [PubMed] [Google Scholar]
- Turrini P, Casu MA, Wong TP, De Koninck Y, Ribeiro-da-Silva A, Cuello AC. Cholinergic nerve terminals establish classical synapses in the rat cerebral cortex: synaptic pattern and age-related atrophy. Neuroscience. 2001;105:277–85. doi: 10.1016/s0306-4522(01)00172-5. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Overshiner CD, Davis RJ, Perry KW, Wolff M, McKinzie DL, Witkin JM, Nomikos GG. Procholinergic and memory enhancing properties of the selective norepinephrine uptake inhibitor atomoxetine. Mol Psychiatry. 2006;11:187–95. doi: 10.1038/sj.mp.4001763. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- Viana Di Prisco G, Albo Z, Vertes RP, Kocsis B. Discharge properties of neurons of the median raphe nucleus during hippocampal theta rhythm in the rat. Exp Brain Res. 2002;145:383–94. doi: 10.1007/s00221-002-1123-8. [DOI] [PubMed] [Google Scholar]
- Vijayashankar N, Brody H. A quantitative study of the pigmented neurons in the nuclei locus coeruleus and subcoeruleus in man as related to aging. J Neuropathol Exp Neurol. 1979;38:490–7. doi: 10.1097/00005072-197909000-00004. [DOI] [PubMed] [Google Scholar]
- Walker AE. A cytoarchitectural study of the prefrontal cortex of the macaque monkey. J Comp Neurol. 1940;73:59–86. [Google Scholar]
- Walker LC, Kitt CA, DeLong MR, Price DL. Noncollateral projections of basal forebrain neurons to frontal and parietal neocortex in primates. Brain Res Bull. 1985;15:307–14. doi: 10.1016/0361-9230(85)90156-x. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Border B, Wahl L, Mihailoff GA. Topographic organization of rat locus coeruleus and dorsal raphe nuclei: distribution of cells projecting to visual system structures. J Comp Neurol. 1993;336:345–61. doi: 10.1002/cne.903360304. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Lin CS, Burne RA, Woodward DJ. The distribution of neocortical projection neurons in the locus coeruleus. J Comp Neurol. 1983;217:418–31. doi: 10.1002/cne.902170406. [DOI] [PubMed] [Google Scholar]
- Wiley RG, Oeltmann TN, Lappi DA. Immunolesioning: selective destruction of neurons using immunotoxin to rat NGF receptor. Brain Res. 1991;562:149–53. doi: 10.1016/0006-8993(91)91199-b. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Molliver ME. The organization of serotonergic projections to cerebral cortex in primates: regional distribution of axon terminals. Neuroscience. 1991;44:537–53. doi: 10.1016/0306-4522(91)90076-z. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Irons J, Rapier C, Thorne B, Lunt GG. Presynaptic modulation of transmitter release by nicotinic receptors. Progr Brain Res. 1989;79:157–63. doi: 10.1016/s0079-6123(08)62475-9. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986;16:603–37. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Eckenstein F, Butcher LL. Cholinergic projections from the basal forebrain to the frontal cortex: a combined fluorescent tracer and immunohistochemical analysis in the rat. Neurosci Lett. 1983;40:93–8. doi: 10.1016/0304-3940(83)90285-9. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–8. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kato Y, Imura H. Nicotine-induced release of noradrenaline from hypothalamic synaptosomes. Brain Res. 1980;182:361–8. doi: 10.1016/0006-8993(80)91194-4. [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–92. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Zaborszky L. The modular organization of brain systems. Basal forebrain: the last frontier. Prog Brain Res. 2002;136:359–72. doi: 10.1016/s0079-6123(02)36030-8. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience. 1997;79:1051–78. doi: 10.1016/s0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–35. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]