Abstract
In primates, including humans, scratching and other self-directed behaviors (SDBs) have recently been reported to be differentially displayed as a function of social interactions, anxiety-related drugs, and response outcomes during learning tasks. Yet few studies have focused on the factors influencing SDBs in our closest living relatives, the chimpanzees (Pan troglodytes). Furthermore, no previous experimental study has examined handedness of SDBs as a function of changes in task difficulty. Using matching-to-sample tasks of varying difficulty, the present study examines the effect of manipulations of task difficulty on rates, handedness, and type of SDBs in an experimental study of eight chimpanzees. SDBs were categorized as rubs, gentle scratches, and rough scratches. SDBs increased during difficult discriminations, but only for subjects who started the experiment on an easy discrimination; subjects who started on a difficult discrimination exhibited no differential rates of SDBs as a function of task difficulty. There was a tendency to exhibit relatively more SDBs with the right hand in the more difficult task. Rates of SDBs decreased after auditory feedback signals, suggesting a link between SDBs and uncertainty. Rubs were directed more to the face (trigeminal), and gentle and rough scratches more to the body (spinothalamic), suggesting that face-directed SDBs may index a different motivational basis than scratches. Taken together, these results extend previous research on SDBs to the domain of cognitive stress in nonsocial contexts, demonstrating that SDBs are sensitive to manipulations of task difficulty in chimpanzees.
Keywords: cognitive stress, emotions, chimpanzees (Pan troglodytes), scratching, self-directed behaviors, displacement activities
INTRODUCTION
From the earliest extensive field observations of chimpanzees, scratching has been associated with conflict, frustration, and anxiety [van Lawick-Goodall, 1972]. According to van Lawick-Goodall, “[t]he more intense the anxiety or conflict situation, the more vigorous the scratching becomes” [1972, p. 40]. More recently, scratching and other self-directed behaviors (SDBs) have been associated with frustration, uncertainty, and anxiety in social conflict situations in a variety of primate species [reviewed by Maestripieri et al., 1992].
Scratching is differentially displayed in cercopithecines as a function of dominance rank (Macaca fascicularis [Pavani et al., 1991], M. fuscata [Troisi et al., 1991], M. mulatta [Diezinger & Anderson, 1986; Maestripieri, 1993], Papio cynocephalus anubis [Easley et al., 1987], and Papio anubis [Castles et al., 1999]). Although the majority of these studies have focused on Old World monkeys (especially Cercopithecinae), several studies have demonstrated increased SDBs under differential social conditions by chimpanzees (Pan troglodytes [Aureli & de Waal, 1997; Baker & Aureli, 1997]) and humans (Homo sapiens [Fairbanks et al., 1982; Lantz, 1979; Troisi et al., 1998; Waxer, 1977]), although the proximate mechanisms of these behaviors remain obscure. For example, Aureli and de Waal [1997] found increased scratching by chimpanzees under crowded, compared to less crowded, housing conditions. Baker and Aureli [1997] reported increased scratching after loud vocalizations by neighboring groups of chimpanzees, which are associated with increased risk of intragroup aggression. In humans, scratching was correlated with parental conflict in a 16-year-old patient who exhibited pathological scratching [Lantz, 1979]. Importantly, human patients who report feeling anxiety also exhibit high levels of SDBs [Fairbanks et al., 1982; Troisi et al., 1998; Waxer, 1977].
Several studies of unrestrained, group-living monkeys have found that scratching and other SDBs are sensitive to pharmacological manipulation of mood through anxiolytic (anxiety-reducing) and anxiogenic (anxiety-causing) substances. Anxiogenic drugs increase SDBs, and anxiolytic drugs reduce SDBs [Maestripieri et al., 1992; Schino et al., 1991, 1996]. Thus, ethopharmacological data suggest that SDBs are indicators of anxiety-like emotions in nonhuman primates, which is congruent with available data on humans.
The majority of this research has studied animals in social contexts. The present study tested the generality of the ethological model described above by investigating the influence of task difficulty on SDBs by chimpanzees, in a nonsocial context. Research with humans has demonstrated that manipulations of such factors as uncertainty and task difficulty increase sympathetic nervous system (SNS) activation [Callister et al., 1992; Esler et al., 1989; Gotthardt et al., 1995; Sausen et al., 1991]. Physiological measures such as plasma cortisol, plasma ACTH, catechoamines, heart rate, and blood pressure have been demonstrated to increase with increases in task difficulty. Thus, humans exhibit a well-documented SNS response to manipulations of task difficulty and other cognitive challenges. If SDBs, including scratching, index emotional arousal, then the effects of manipulations of task difficulty in cognitive testing contexts should affect SDBs in ways similar to those reported in social contexts [Maestripieri et al., 1992]. Itakura [1993], studying a 7-year-old female chimpanzee, found that self-scratching and self-grooming were elicited almost exclusively by a conditioned aversive stimulus (a buzzer), but not at all, or only rarely, by a secondary positive reinforcer (a chime).
Observational studies have generally reported no population asymmetries in hand use for SDBs in great apes [Aruguete et al., 1992; Hopkins & de Waal, 1995; Marchant & McGrew, 1996]. However, Dimond and Harries [1984] observed a left-hand bias for face-touching in orangutans (Pongo pygmaeus), gorillas (Gorilla gorilla), and chimpanzees (P. troglodytes), and Yanez-Gonzalez (2001, unpublished results), found a group-level right-hand bias for intense scratching in a group of eight chimpanzees. Leavens et al. [1997] reported that a male chimpanzee performing a match-to-sample (MTS) task exhibited relatively more SDBs with his right hand when 1) performance was low, and 2) immediately after a conditioned aversive stimulus (an auditory feedback signal heralding impending lack of primary reinforcement after incorrect responses). SDBs under different levels of task difficulty may, therefore, be subject to changes in laterality. Thus, we recorded hand use during SDBs under different conditions of task difficulty in chimpanzees.
Finally, because previous research had indicated that different types of self-touching were differentially associated with the face compared with the rest of the body [e.g., Leavens et al., 1997], we assessed target location of SDBs in a vertical dimension; that is, we distinguished SDBs directed to the face from SDBs directed elsewhere. The trigeminal system carries pain and itch information from the face and anterior scalp, but not the rest of the head, and the spinothalamic system carries pain and itch information from the rest of the body, including the posterior scalp regions [e.g., Pansky et al., 1988].
We report the results of an experimental study of eight chimpanzees that were exposed to two levels of task difficulty, in counterbalanced order. Rates and types of SDBs, hand use during self-directed touching behaviors, target location of touching (face or body), and performance measures were taken for each subject. We expected, based on previous findings [Itakura, 1993; Leavens et al., 1997], that: 1) the rate of SDBs would be higher in conditions of poor performance (high task difficulty); 2) the rate of SDBs would be higher after incorrect responses than after correct responses; 3) relatively more SDBs would be exhibited with the right hand in conditions of higher task difficulty; 4) relatively more SDBs would be exhibited with the right hand after incorrect, compared to after correct, responses; and 5) relatively more rubs would be directed to the face than the body and, conversely, that relatively more scratches would be directed to the body.
METHOD
Subjects
The subjects were eight chimpanzees (P. troglodytes), nursery-reared and living in small social groups at the Yerkes Regional Primate Research Center (YRPRC), Emory University, Atlanta, Georgia (Table I) [see Bard & Gardner, 1996, for details of nursery-rearing]. Subjects were tested in their home cages, and were neither food nor water deprived to elicit their participation in this experiment.
TABLE I.
Sample Characteristics
| Subjects | |||||
|---|---|---|---|---|---|
| Group A | Sex | Age | Group B | Sex | Age |
| Kengee | ♀ | 7 | Katrina | ♀ | 8 |
| Carl | ♂ | 9 | Jarred | ♂ | 7 |
| Lamar | ♂ | 7 | Winston | ♂ | 8 |
| Scott | ♂ | 8 | Columbus | ♂ | 18 |
| Mean = 7.75 | Mean = 10.25 | ||||
Mean age is not significantly different across the two groups (t(6) = −0.95, ns).
Procedure
The two female subjects were each assigned to one of two groups, and the six males were randomly assigned to these groups. All tests were administered via the Language Research Center Computerized Testing System (LRCCTS) [Washburn et al., 1989]. In the simultaneous MTS procedure used here, a sample stimulus appeared centered on the top half of the computer screen and the subject moved a cursor, via a joystick mounted on a plate affixed to the cage mesh, into this sample stimulus (Fig. 1). When the cursor contacted the sample stimulus, two comparison stimuli appeared in the bottom half of the screen. One of the two comparison stimuli (the target) was identical in appearance to the sample stimulus, whereas the other comparison stimulus (the foil) was discriminably different. Group 1 was exposed first to an easy discrimination in an MTS task (EASY condition), then to a difficult discrimination (HARD condition), and exposure to these two conditions were alternated for a total of four EASY sessions and four HARD sessions. Group 2 was exposed first to the HARD condition, then to the EASY condition, and then experienced systematic alternation of the tasks, again for a total of four EASY sessions and four HARD sessions. Thus, the order of presentation of HARD and EASY sessions was a between-subjects variable. In the EASY task, the foil (the nonrewarded stimulus) was easily discriminated from the target stimulus (which matched the sample stimulus), whereas in the HARD task, the foil differed only slightly from the target (Fig. 1). For three subjects, a scheduling error occurred in the administration of the tasks, such that the intended alternation between tasks was not achieved in some of the later sessions (i.e., two EASY or two HARD sessions were administered successively); for this reason, data analyses include the first four sessions for each subject (two EASY and two HARD), except where noted below.
Fig. 1.

Examples of EASY and HARD discriminations generated by the LRCCTS. Pluses indicate the correct responses, minuses indicate the incorrect responses.
Each correct joystick response was accompanied by a high-pitched chime (secondary positive reinforcer), and each incorrect response by a lower-pitched buzzer (conditioned aversive stimulus). After each joystick-mediated selection of a comparison stimulus, the subjects experienced a 5-sec interval with no feedback (Interval 1) terminating in the delivery of a feedback signal (a high-pitched chime for correct response, and a low-pitched buzzer for incorrect responses). This feedback tone marked the beginning of a second 5-sec interval (Interval 2), which terminated in either delivery of reinforcement (juice) or no reinforcement. A third 5-sec interval (Interval 3) served as the intertrial interval. Behavioral observations were taken in each of three 5-sec intervals after the subjects’ joystick responses.
SDBs were recorded directly on data sheets by an experimenter using a onezero sampling technique [Altmann, 1974]. SDBs were categorized as: 1) rubs (self-touches not involving the ends of the digits, presumably analogous to the face-stroking reported by Itakura [1993]); 2) gentle scratches (GSs; self-touching involving the ends of the digits, but no discernable movement of the shoulder joint); and 3) rough scratches (RSs; self-touching involving the ends of the digits, including movement of the shoulder joint) [cf., Baker & Aureli, 1997]. The hand used by the subjects in self-touching was recorded. The target location of self-touching in vertical dimension was the face or body. The face was defined as the area bounded superiorly by the supraorbital torus (browridge), laterally by the anteriormost portion of the ear, and inferiorly by the inferior margin of the mandibular corpus (i.e., the jawline); all other locations, including the scalp, were coded as “body.” This parsing captures the division of cutaneous afferent transmission of pain and itch into trigeminal (face) and spinothalamic (body) pathways [Pansky et al., 1988].
A bout of SDBs for any 5-sec interval of observation was defined as the occurrence of any of the three behaviors of interest. In rare cases (0.4% of intervals), more than one type of SDB was exhibited in a 5-sec interval. For these few observations, the data were scored in accordance with a hierarchy such that RS > GS > Rubs; thus, if a GS and an RS occurred in the same interval, it was scored as RS. This hierarchy is in the opposite direction from that of the observed frequencies of these SDBs, so scoring in this fashion very slightly increases the representation of the lower-frequency behaviors. Because one-zero sampling differs little from frequency data when sampling intervals only rarely encompass more than one behavioral event of interest [Bernstein, 1991], rates were calculated for each level of each independent variable by dividing the sum of SDBs of each type by the total number of trials in each condition. Because each trial comprised 15 sec of observation, these rates were multiplied by 4 (there were four trials per minute) to render rates per minute.
The absolute amount of primary positive reinforcement (juice) was equated across EASY and HARD conditions by limiting each session (four EASY and four HARD sessions per subject) to 25 correct responses. Reinforcement was delivered to seven of the eight subjects using an automated liquid dispenser that dispensed approximately 1 ml of juice for every correct response [Hyatt & Leavens, 1997]. The delivery of juice to the subjects was via a surgical tube held near their mouths by the experimenter after correct responses. A technical problem with an early prototype of this dispenser resulted in the reinforcement of one subject, Columbus, manually with a squeeze bottle filled with fruit juice, resulting in slightly less control of the volume of juice delivered for each correct response, relative to the other subjects.
Analyses
Performance was calculated as the percent correct in each session for each subject. In analyses of performance, data were included from all eight sessions. Paired t-tests were used to compare performance measures within subjects, and independent samples t-tests were used to compare performance across subjects. Mixed and within-subjects analyses of variance (ANOVAs) were performed as appropriate. Rates of SDBs served as dependent measures. Post hoc explorations of main effects were performed with a Bonferroni-correction procedure applied to main effects contrasts.
RESULTS
Performance
The performance by both groups was virtually identical within the EASY and HARD conditions. In the EASY condition, Groups 1 and 2 exhibited mean performances of 94.3% and 93.8%, respectively (t(3) = .104, P > .05), whereas in the HARD condition, Groups 1 and 2 exhibited mean performances of 61.8% and 65.5%, respectively (t(3) = −.330, P > .05). Performance by all subjects differed significantly between the EASY and HARD conditions (paired t(7) = 9.359, P < .001); thus, what was construed by the experimenters as a difference in task difficulty was apparently perceived similarly by the chimpanzees.
Performance and Rates of SDBs
Homogeneity of variance between groups was assumed after administration of the Brown-Forsythe test [Brown & Forsythe, 1974, as described in Keppel, 1991]: F(1,6) = 4.57, P = .410. A Group (two levels: EASY first and HARD first) by Condition (two levels: EASY and HARD) by Hand (two levels: left and right) by Type of SDB (three levels: rubs, GSs, and RSs) mixed ANOVA was performed to examine the effects of task difficulty (condition) and order of presentation (group) on hand use and SDB type. There was a main effect for type of SDB (F(2,12) = 7.41, P = .008). Post hoc contrasts revealed that there were significantly more GSs than RSs (P = .009) and a trend toward more rubs than RSs (P = .066).
There was no main effect for Group, but there was a main effect for Condition (F(1,6) = 8.31, P = .028); the HARD task elicited more SDBs (mean rate = .604 SDBs/min, SE = .105) than did the EASY task (mean rate = .370 SDBs/min, SE = .163). As shown in Fig. 2, a significant interaction between order of presentation (Group) and task difficulty (Condition) was found (F(1,6) = 8.03, P = .030). No other interaction effects involving order of presentation were found. Thus, those subjects that experienced the EASY task first exhibited more SDBs in the HARD, compared to the EASY condition, as predicted, but there was no apparent difference in rates of SDBs as a function of task difficulty for those subjects who experienced the HARD task first. Hence, the order in which task difficulty was presented was important in determining the subjects’ reactivity, as measured in SDBs, to these manipulations. That is, chimpanzees who were exposed to the EASY task first exhibited a disproportionate increase in the rate of SDBs when subsequently exposed to the HARD task, compared to those chimpanzees who were exposed to the HARD task first. There was no interaction between Condition (task difficulty) and type of SDB (F(2,12) = 1.35, P = .297).
Fig. 2.
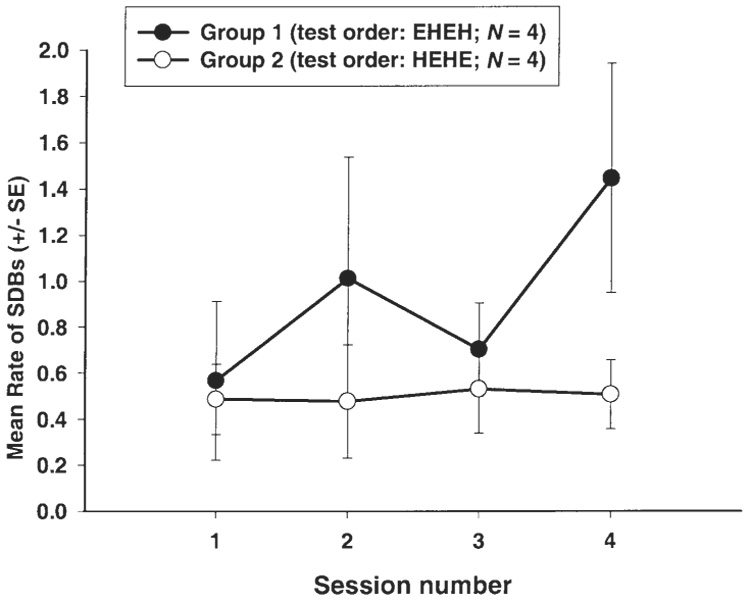
Group 1 exhibited an increase in SDBs in the HARD task, compared to the EASY task, but Group 2 exhibited no difference in rates of SDBs across the two levels of task difficulty. Data are plotted by session, for descriptive purposes. EHEH refers to the order of task presentation (EASY-HARD-EASY-HARD); HEHE is the opposite order.
Laterality
In the same ANOVA reported in the previous section, there was a trend toward a task difficulty by hand interaction (F(1,6) = 5.54, P = .057; see Fig. 3), such that there were relatively more right-handed SDBs when performance was poor than when performance was high.
Fig. 3.
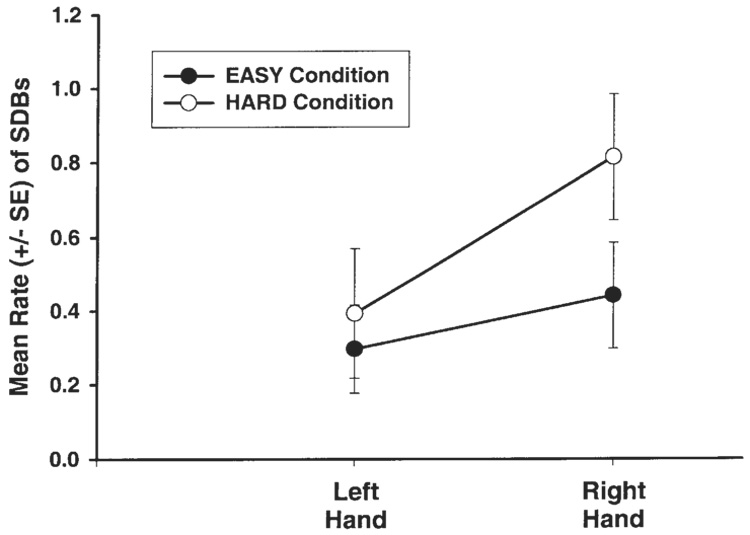
Trend toward relatively more right-handed SDBs being displayed in the HARD condition, compared to the EASY condition.
Effects of Primary Reinforcement and Feedback on SDBs
Because performance was very high in the EASY condition, there were few incorrect trials of observation in this condition. Therefore, we assessed the effects of primary reinforcement and feedback on SDBs within the HARD condition only. An Accuracy (two levels: correct or incorrect) by Interval (three levels of 5 sec each: Intervals 1, 2, and 3) by type of SDB (three levels: rubs, GSs, and RSs) ANOVA was performed to assess the temporal distributions of SDBs in relation to primary reinforcement and in relation to the feedback signals. There was a main effect for interval (F(2,14) = 10.18, P = .002) and no other main effects or interactions (Fig. 4). Post hoc analysis revealed that SDBs were distributed in Intervals 1 and 3 at significantly higher rates than in Interval 2 (Interval 1 compared to Interval 2: P = .042; Interval 2 compared to Interval 3: P = .013; and Interval 1 compared to Interval 3: ns). Thus, because the auditory feedback signals were delivered at the beginning of Interval 2, the delivery of information seemed to reduce the rate of SDBs, irrespective of whether that information signaled impending positive or negative reinforcement. This result did not confirm the prediction that rates of SDBs would increase in intervals relatively more after a conditioned aversive stimulus (the buzzer) compared to intervals following secondary positive reinforcement (the chime).
Fig. 4.
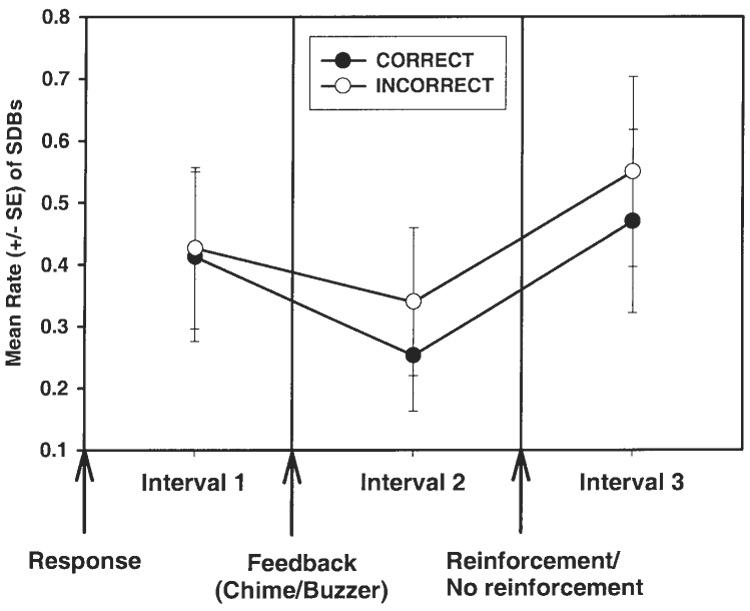
Relatively fewer SDBs were emitted in the 5-sec interval (Interval 2) following the discriminative tone than in Interval 1 or Interval 3, irrespective of whether the response was correct or incorrect. The auditory feedback tone (chime or buzzer) is the secondary reinforcer. Primary reinforcement was approximately 1 ml of grape juice.
Reinforcement Effects on Hand Use and Target Location of SDBs
To assess the spatial distribution of SDBs on the surface of the integument, a Hand (two levels: left and right) by Location (two levels: face or body) by Accuracy (two levels: correct and incorrect) by Type (three levels: rubs, GSs, and RSs) ANOVA was performed. There was a significant main effect for Hand (F(1,7) = 9.11, P = .019); there were significantly more SDBs exhibited with the right hand. This is consistent with the trend toward greater use of the right hand in the HARD condition reported above. There was a significant two-way interaction between Location and Type (F(2,14) = 6.46, P = .010) and a significant three-way interaction between Hand, Location, and Type (F(2,14) = 4.31, P = .035), and no other main effects or interactions. Figure 5 depicts the pattern evident from the three-way interaction between Hand, Location, and Type. Rubs were differentially directed to the face, compared to both types of scratches (Fig. 5a). Scratches directed to the face were exhibited without a lateral bias in hand use, whereas scratches directed to the body were strongly lateralized to the right hand (Fig. 5b).
Fig. 5.

The three-way interaction between hand use, target location of SDBs (face or body), and type of SDB. a: Difference in the mean rate of SDBs directed to the face and body. Rubs were directed primarily to the face; GSs and RSs were directed primarily to the body. b: Difference in the mean rate of SDBs exhibited with the right and left hands. That all bars are positive reflects the right-hand bias for SDBs in the HARD condition. Rubs were exhibited predominantly with the right hand whether directed to the face or body. In contrast, GSs and RSs were not strongly lateralized when directed to the face, but were exhibited overwhelmingly with the right hand when directed to the body.
DISCUSSION
The rates of SDBs increased in conditions of high-task difficulty, relative to conditions of low-task difficulty, but only for subjects initially exposed to an easy discrimination (Fig. 2). All types of SDBs exhibited increases in rates with increases in task difficulty. Thus, the level of task difficulty on initial exposure to a task seems to determine the reactivity of chimpanzee subjects, as measured in SDBs. As would be predicted from existing ethological and ethopharmacological models of SDBs as indicators of emotional arousal [Baker & Aureli, 1997; Maestripieri et al., 1992; Schino et al., 1996], rates of SDBs are sensitive to manipulations of cognitive challenge in nonsocial contexts. The level of task difficulty on initial exposure to a task seems to have a strong influence on reactivity to subsequent manipulations of task difficulty. Assuming a common underlying physiological basis for scratching in social and nonsocial contexts, we would tentatively predict that SDBs in social contexts may be differentially sensitive to baseline rates of behavioral measures of social tension in social groups of chimpanzees, and perhaps more widely among anthropoids and other mammals.
There was a tendency for an increase in right-handed SDBs in conditions of high-task relative to low-task difficulty (Fig. 3), which was confirmed by the main effect for hand use in a subsequent analysis within the HARD condition only. Interpretation of this behavioral asymmetry in SDBs is hampered by, among other things, our lack of data on 1) to which side of the body the SDBs were directed, and 2) which hands the subjects used to manipulate the joysticks. Thus, in addition to the need for replication of this finding, there are a number of possible explanations for this asymmetry, among which future research should distinguish. With respect to the replicability of an asymmetrical response in SDBs, we note that in three separate studies in our laboratory we have seen a bias toward increasing use of the right hand during conditions of poor performance, relative to conditions of high performance [Leavens et al., 1997] (Hopkins et al., 1998, unpublished results).
As to why there might be an asymmetrical behavioral response to cognitive challenge, there are two basic possibilities. There may be endogenous processing asymmetries; that is, the lateralized SDBs may be exhibited in response to asymmetries in emotional processing, with consequences for either asymmetries in cutaneous sensation or behavioral reactivity. Alternatively, there may be asymmetries in exogenous factors; that is, the SDBs may be exhibited in response to uncontrolled asymmetries in task factors, such as the preferred hand used to manipulate the joystick. For example, if one hand is preferred to manipulate the joystick, that may leave the other hand free to exhibit SDBs, or the hand preferred for manipulating the joystick may exhibit a propensity for motor activity, which is manifested in SDBs when the hand is not using the joystick. Diezinger and Anderson [1986] failed to find any evidence for a lateral bias in scratching by 14 rhesus macaques. Dimond and Harries [1984] reported no asymmetries in SDBs (face-touching) by monkeys, but strong asymmetries in great apes and humans (an overall bias towared the left hand in face-touching). Dimond and Harries [1984] suggested that the laterality bias of SDBs reflected underlying functional cerebral asymmetries present in apes and humans, but not in monkeys. There is a paucity of information in this area, and future research should investigate the phylogenetic distribution of both individual and population-level hand preferences in SDBs.
Information, in the form of auditory feedback signals (secondary positive reinforcers and conditioned aversive stimuli) seemed to cause a reduction in the rate of SDBs (Fig. 4), irrespective of whether the signal heralded the delivery or nondelivery of juice. That we did not find an interaction between Hand (left, right) and Accuracy (correct, incorrect) constitutes a failure to replicate the pattern exhibited by the male chimpanzee in the case study by Leavens et al. [1997]. We had anticipated that the conditioned aversive stimulus (the buzzer) would lead to increases in rates of SDBs. However, the introduction into the present experimental design of a 5-sec delay between response and secondary feedback may have elicited an emotional response characterized by uncertainty, whereas in the study by Leavens et al. [1997] auditory feedback was presented immediately upon the subject’s response. Hence, it may be that the subject in the study by Leavens et al. [1997] was exhibiting an emotional response characterized more by frustration than by uncertainty. Thus, it would appear that information, per se, caused a reduction in the rates of SDBs, suggesting that a component in the emotional reactions of these subjects under conditions of varying task difficulty is related to uncertainty about response outcomes. If this is true, then uncertainty about response outcome may have consequences for cutaneous sensation in both social and nonsocial contexts [e.g., Aureli, 1997; Castles et al., 1999; Maestripieri et al., 1992]. However, it remains possible that reductions in SDBs in Interval 2 would have been obtained had we delivered no feedback to the subjects (i.e., SDBs might have been under the control of elapsed time and not the feedback signal). This possibility was not tested in the present research design.
Rubs were directed primarily to the face, and GSs and RSs to the body (Fig. 5a), which replicates a finding reported by Leavens et al. [1997]. Furthermore, rubs were differentiated from scratches by a different pattern of hand use with respect to the vertical dimension of the body surface; to wit, rubs directed at the face were mostly exhibited with the right hand, whereas both GSs and RSs directed to the face were not strongly lateralized to either hand (Fig. 5b). Rubs directed to the body were also more likely to be exhibited with the right hand (though to a lesser extent than those directed to the face), whereas both GSs and RSs directed to the body were strongly lateralized to the right hand. That rubs were differentiated from both GSs and RSs is consistent with other studies that have shown scratching, particularly rough scratching, to be uniquely associated with changes in the social environments of chimpanzees that predict negative consequences [Aureli & de Waal, 1997; Baker & Aureli, 1997]. However, few previous studies have isolated rubs (or face stroking [Itakura, 1993] or face touching [Dimond & Harries, 1984]) as an analytical unit. The present pattern of results is in direct contrast to that reported by Dimond and Harries [1984]; whereas they reported a left-hand bias for face-touching in orangutans, gorillas, chimpanzees, and humans, we report a right-hand bias for SDBs of all types, including face-directed rubs. This suggests that face-touching in these experimental contexts may be subject to a different pattern of cerebral dominance than the spontaneous face-touching behaviors of apes in social contexts.
The results reported here are consistent with ethological and ethopharmacological models of displacement activities as measures of emotional reactivity [Diezinger & Anderson, 1986; Itakura, 1993; Maestripieri et al., 1992; Schino et al., 1996]. SDBs appear to be sensitive indicators of emotional reactivity in chimpanzees [Baker & Aureli, 1997], as has been suggested for humans [Fairbanks et al., 1982;Troisi et al., 1998; Waxer, 1977]. A potentially fruitful line of future inquiry would explore the interrelationships between measures of temperament and SDBs in chimpanzees and other organisms [cf., Davidson et al., 1993].
ACKNOWLEDGMENTS
The Yerkes Regional Primate Research Center is accredited by the American Association for Laboratory Animal Care. We thank Dr. Bradford N. Bunnell, Dr. Richard L. Marsh, Dr. Lori Marino, and Ms. Connie Russell for helpful commentary on these data and analyses. We also thank three anonymous reviewers, an anonymous associate editor, and Dr. Michael W. Andrews for extensive and helpful comments on this manuscript. Special thanks are due to Dr. Kim A. Bard for valuable comments on several drafts of this manuscript.
Contract grant sponsor: NIH; Contract grant numbers: RR-00165, R01-RR-09797, NS-29574; Contract grant sponsor: University of Georgia.
REFERENCES
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–265. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Aruguete MS, Ely EA, King JE. Laterality in spontaneous motor activity of chimpanzees and squirrel monkeys. Am J Primatol. 1992;27:177–188. doi: 10.1002/ajp.1350270303. [DOI] [PubMed] [Google Scholar]
- Aureli F. Post-conflict anxiety in nonhuman primates: the mediating role of emotion in conflict resolution. Aggr Behav. 1997;23:315–328. [Google Scholar]
- Aureli F, de Waal FBM. Inhibition of social behaviour in chimpanzees under high-density conditions. Am J Primatol. 1997;41:213–228. doi: 10.1002/(SICI)1098-2345(1997)41:3<213::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Baker K, Aureli F. Behavioural indicators of anxiety: an empirical test in chimpanzees. Behaviour. 1997;134:1031–1060. [Google Scholar]
- Bard KA, Gardner KH. Influences on development in infant chimpanzees: enculturation, temperament, and cognition. In: Russon AE, Bard KA, Parker ST, editors. Reaching into thought: the minds of the great apes. New York: Cambridge University Press; 1996. pp. 235–256. [Google Scholar]
- Bernstein IS. An empirical comparison of focal and ad libitum scoring with commentary on instantaneous group scans, all occurrence and one-zero techniques. Anim Behav. 1991;42:721–728. [Google Scholar]
- Brown MB, Forsythe AB. Robust tests for equality of variances. J Am Stat Assoc. 1974;69:364–367. [Google Scholar]
- Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol. 1992;454:373–387. doi: 10.1113/jphysiol.1992.sp019269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castles DL, Whiten A, Aureli F. Social anxiety, relationships and self-directed behaviour among wild female olive baboon. Anim Behav. 1999;58:1207–1215. doi: 10.1006/anbe.1999.1250. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kalin NH, Shelton SE. Lateralized response to Diazepam predicts temperamental style in rhesus monkeys. Behav Neurosci. 1993;107:1106–1110. doi: 10.1037//0735-7044.107.6.1106. [DOI] [PubMed] [Google Scholar]
- Diezinger F, Anderson JR. Starting from scratch: a first look at “displacement activity” in group-living rhesus monkeys. Am J Primatol. 1986;11:117–124. doi: 10.1002/ajp.1350110204. [DOI] [PubMed] [Google Scholar]
- Dimond S, Harries R. Face touching in monkeys, apes and man: evolutionary origins and cerebral asymmetry. Neuropsychologia. 1984;22:227–233. doi: 10.1016/0028-3932(84)90065-4. [DOI] [PubMed] [Google Scholar]
- Easley SP, Coelho AM, Taylor LL. Scratching, dominance, tension, and displacement in male baboons. Am J Primatol. 1987;13:397–411. doi: 10.1002/ajp.1350130405. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Lambert G. Measurement of overall and cardiac norepinephrine release into plasma during cognitive challenge. Psychoneuroendocrinology. 1989;14:477–481. doi: 10.1016/0306-4530(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, McGuire MT, Harris CJ. Nonverbal interaction of patients and therapists during psychiatric interviews. J Abnorm Psychol. 1982;91:109–119. doi: 10.1037//0021-843x.91.2.109. [DOI] [PubMed] [Google Scholar]
- Gotthardt U, Schweiger U, Fahrenberg J, Lauer CJ, Holsboer F, Heuser I. Cortisol, ACTH, and cardiovascular response to a cognitive challenge paradigm in aging and depression. Am J Physiol. 1995;268:R865–R873. doi: 10.1152/ajpregu.1995.268.4.R865. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, de Waal FBM. Behavioral laterality in captive bonobos (Pan paniscus): replication and extension. Int J Primatol. 1995;16:261–276. [Google Scholar]
- Hyatt CW, Leavens DA. An inexpensive liquid dispenser. Behav Res Methods Instrum Comput. 1997;29:448–449. [Google Scholar]
- Itakura S. Emotional behavior during the learning of a contingency task in a chimpanzee. Percept Mot Skills. 1993;76:563–566. doi: 10.2466/pms.1993.76.2.563. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: a researcher’s handbook. 3rd ed. Upper Saddle River, NJ: Prentice Hall; 1991. 672 pp. [Google Scholar]
- Lantz JE. Extreme itching treated by a family systems approach. Int J Fam Ther. 1979;1:244–253. [Google Scholar]
- Leavens DA, Aureli F, Hopkins WD. Scratching and cognitive stress: performance and reinforcement effects on hand use, scratch type, and afferent cutaneous pathways during computer cognitive testing by a chimpanzee (Pan troglodytes) Am J Primatol. 1997;42:126–127. [Google Scholar]
- Maestripieri D, Martel FL, Nevison CM, Simpson MJ, Keverne EB. Anxiety in rhesus monkey infants in relation to interactions with their mother and other social companions. Dev Psychobiol. 1992;24:571–581. doi: 10.1002/dev.420240805. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Schino G, Aureli F, Troisi A. A modest proposal: displacement activities as an indicator of emotions in primates. Anim Behav. 1992;44:967–979. [Google Scholar]
- Maestripieri D. Maternal anxiety in rhesus macaques (Macaca mulatta). I. Measurement of anxiety and identification of anxiety-eliciting situations. Ethology. 1993;95:19–31. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in wild chimpanzees of Gombe National Park: comprehensive study of spontaneous behaviors. J Hum Evol. 1996;30:427–443. [Google Scholar]
- Pansky B, Allen DJ, Budd GC. Review of neuroscience. 2nd ed. New York: Macmillan; 1988. 606 pp. [Google Scholar]
- Pavani S, Maestripieri D, Schino G, Giovanni Turillazzi P, Scucchi S. Factors influencing scratching behaviour in long-tailed macaques (Macaca fascicularis) Folia Primatol. 1991;57:34–38. [Google Scholar]
- Sausen KP, Lovallo WR, Wilson MF. Heart rate reactivity, behavior pattern, and parental hypertension as predictors of cardiovascular activity during cognitive challenge. Psychophysiology. 1991;28:639–647. doi: 10.1111/j.1469-8986.1991.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Schino G, Troisi A, Perretta G, Monaco V. Measuring anxiety in nonhuman primates: effect of lorazepam on macaque scratching. Pharmacol Biochem Behav. 1991;38:889–891. doi: 10.1016/0091-3057(91)90258-4. [DOI] [PubMed] [Google Scholar]
- Schino G, Perretta G, Taglioni AM, Monaco V, Troisi A. Primate displacement activities as an ethopharmacological model of anxiety. Anxiety. 1996;2:186–191. doi: 10.1002/(SICI)1522-7154(1996)2:4<186::AID-ANXI5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Troisi A, Schino G, D’Antoni M, Pandolfi N, Aureli F, D’Amato FR. Scratching as a behavioral index of anxiety in macaque mothers. Behav Neural Biol. 1991;56:307–313. doi: 10.1016/0163-1047(91)90469-7. [DOI] [PubMed] [Google Scholar]
- Troisi A, Spalletta G, Pasini A. Nonverbal behavior deficits in schizophrenia: an ethological study of drug-free patients. Acta Psych Scand. 1998;97:109–115. doi: 10.1111/j.1600-0447.1998.tb09971.x. [DOI] [PubMed] [Google Scholar]
- van Lawick-Goodall J. A preliminary report on expressive movements and communication in the Gombe Stream chimpanzees. In: Dolhinow P, editor. Primate patterns [originally published 1968] New York: Holt, Rinehart and Winston; 1972. pp. 25–84. [Google Scholar]
- Washburn DA, Hopkins WD, Rumbaugh DM. Automation of learning-set testing: the video-task paradigm. Behav Res Methods Instrum Comput. 1989;21:281–284. doi: 10.3758/bf03205596. [DOI] [PubMed] [Google Scholar]
- Waxer PH. Nonverbal cues for anxiety: an examination of emotional leakage. J Abnorm Psychol. 1977;86:306–314. doi: 10.1037//0021-843x.86.3.306. [DOI] [PubMed] [Google Scholar]


