Abstract
Behavioral laterality in head orientation while sleeping in either a supine or prone posture was examined in 43 chimpanzees (Pan troglodytes) for the first 3 months of life. An overall significant right-side lateral bias was found for head orientation in the supine posture. A trend toward greater right-side bias in females compared with males was observed but failed to reach significance. These data suggest that asymmetries in head orientation are present early in life in chimpanzees, and they may be correlated with functional asymmetries observed in adulthood.
There is evidence that hemispheric specialization is present in human infants and neonates (see Hahn, 1987; Molfese & Segalowitz, 1988; and Witelson, 1987, for reviews). Functional differences between the two cerebral hemispheres have been found in sensory systems, such as visual or auditory perception, as well as in motor systems, such as hand preference (see Molfese & Segalowitz, 1988). One of the developmentally earliest and most consistent motor biases found in human neonates involves right-head orientation during both spontaneous observations as well as during specific experimental investigations (Michel, 1987; Provin, 1992; Turkewitz, 1988). Moreover, there is some evidence that asymmetries in head orientation predict adult functional asymmetries such as hand preference or the hemisphere dominant for linguistic processing (Michel, 1981; Witelson, 1987). For example, head orientation biases during the neonatal period appear to be predictive of later lateral-hand preference (Viviani, Turkewitz, & Karp, 1978).
In contrast to studies on human infants and neonates, there are few studies examining laterality during the infancy or neonatal period in nonhuman primates (for reviews, see Fagot, 1993; Hopkins & Bard, 1993a). In great ape species, the data are extremely sparse. Some researchers (Bresard & Bresson, 1983; Chorazyna, 1976; Cunningham, Forsythe, & Ward, 1989) reported increased laterality as a function of increasing age. However, these studies combined had only 4 great ape subjects, which limits the generalization of results. Bard, Hopkins, and Fort (1990) found significant right-hand biases in hand-to-mouth behaviors in 12 infant chimpanzees. Moreover, this right-hand bias increased in strength from birth through 3 months. Because of the longitudinal aspect of this study (Hopkins & Bard, 1993b), we were able to relate neonatal variables to lateral bias at 3 months. We found that general arousal (specifically irritability) at 2 days of age predicted the degree of laterality at 3 months.
Laterality studies in neonatal and infant nonhuman primate studies are important because of their implications for current evolutionary theories of handedness and hemispheric specialization. For example, Warren (1980) has characterized nonhuman primate handedness as idiosyncratic, context specific, and entirely determined by experimental or environmental factors. Evidence of laterality in neonatal chimpanzees would certainly challenge any purely environmental explanation with the possible exception of intrauterine factors (Previc, 1991). In addition, recent evidence of population-level bias in hand preference has been reported in a number of species (see Fagot & Vauclair, 1991; Hopkins & Morris, 1993; MacNeilage, Studdert-Kennedy, & Lindblom, 1987; Marchant & McGrew, 1991; Ward & Hopkins, 1993). Evidence of laterality during the neonatal or infancy period of development may allow for assessment of ontogenetic correlates of laterality through the life span, an area severely neglected in the nonhuman primate literature. For instance, it is possible that asymmetries could exist early in life but be masked by the environmental input and thus result in a lack of population-level handedness in its adult form. Only by collecting developmental (and, especially, longitudinal) data can these issues be resolved.
In this article, we report data on lateral bias in head orientation in chimpanzees. Head orientation biases were recorded in sleeping chimpanzees from birth through 3 months of age. We chose to observe head orientation in sleeping subjects because studies with human neonates indicate that they have a spontaneous right-side bias in head orientation (Turkewitz, 1988). We reasoned that spontaneous head orientation would manifest itself asymmetrically while the chimpanzees were sleeping. Similar observations have been reported in human neonates, and we sought to adopt a similar method of data collection (Harris & Fitzgerald, 1983; Trehub, Corter, & Shosenberg, 1983). Moreover, in human infants, right biases in head orientation are found only when subjects are lying in a supine posture (Barnes, Cornwell, Fitzgerald, & Harris, 1985; Vles, Zutphen, Hasaart, Dassen, & Lodder, 1991). Thus, we hypothesized that chimpanzee infants would show a stronger lateral bias in head orientation when sleeping in a supine compared with prone posture.
Method
Subjects
Assessment of lateral bias in head orientation was conducted on 18 female and 25 male chimpanzees (Pan troglodytes). All 43 chimpanzees were raised in the Yerkes Regional Primate Research Center (Yerkes Center) nursery. The chimpanzees were placed in the nursery at the Yerkes Center because of insufficient maternal behaviors at birth (see Bard, 1994a, 1995, for information concerning maternal competence in chimpanzees). Neonate chimpanzees remain in incubators until they weigh 2.0 kg or their temperature regulates (around age 30 days). Most infants received standard nursery care, which consisted of scheduled bottles, diaper changes as needed, and social contact with adult humans typically surrounding these caregiving activities. Thirteen chimpanzees (6 males and 7 females) received an additional 4 hr per day of responsive care from human adults in a research protocol designed to foster species-typical development (Bard & Gardner, in press; Veira & Bard, 1994). Peer groups are formed as early as 30 days of age. Between the ages of 2 and 5 years, nursery-reared chimpanzees leave the nursery and are placed in social groups with adult and young adult chimpanzees.
Procedure
All chimpanzees that enter the Yerkes Center nursery are administered the Brazelton Neonatal Behavioral Assessment Scale (NBAS: Brazelton, 1984) during the first 3 months of life (for a summary, see Bard, 1994c; Bard, Platzman, Lester, & Suomi, 1992). Subjects were assessed every other day for the first 6 weeks of life. From 6 to 12 weeks, the chimpanzees were assessed once per week. Head-orientation data were collected prior to the administration of the NBAS test by recording which side of the chimpanzee’s face was resting on the sleeping mat. If the chimpanzees were lying prone (on their stomachs), then a left-face contact was scored as a right-orientation bias and a right-face contact was scored as a left-orientation bias. In the event that the chimpanzees were supine (sleeping on their backs), a right-face contact was scored as a right-orientation bias, whereas a left-face contact was scored as a left-side bias. On some occasions, the subjects were awake and moving at the beginning of the test; in these cases no head-orientation data were recorded. The number of head-orientation responses between subjects ranged from 6 to 23 (M = 13.70, SD = 4.70). This is within the range of observations reported in human subjects under comparable testing conditions (e.g., Harris & Fitzgerald, 1983). At the end of the 3-month observation period, the total frequency of observations for right- or left-side biases cross-classified with prone or supine posture was determined for each subject.
Data Analysis
For each subject, a lateral bias (LB) index (sometimes referred to as a “handedness” index; see Bard, Hopkins, & Fort, 1990) was calculated for each sleeping posture condition. Thus, for the supine and prone sleeping postures, a LB was determined by subtracting the number of right-biased observations minus the number of left-biased observations divided by the total number of observations ([R − L]/[L + R]). The resulting value ranges from −1.0 to 1.0, with negative values representing left-side bias and positive values representing right-side bias. Inferential statistics were used in the evaluation of these data.
In addition, each subject was classified as being either right or left biased on the basis of the sign of its LB score. Subjects with negative LB scores were classified as left biased, and subjects with positive LB scores were classified as right biased. Typically, nonhuman primate laterality is evaluated at the individual level by use of binomial z score to determine whether laterality deviates significantly from chance (Lehman, 1993). Unfortunately, we did not have enough observations to use this more traditional approach to the assessment of laterality. Therefore, our classification criteria for lateral bias are relatively liberal.
Results
The individual data for all subjects are listed in Table 1. In human neonates, right-side postural asymmetries are particularly evident when subjects are in a supine rather than prone position (Vles et al., 1991). Whether lateral bias was affected by the posture of the chimpanzees was assessed in this initial analysis. Subjects with no bias (i.e., LB = 0.00) were not considered in this analysis. In the prone posture, 24 chimpanzees exhibited a left-side bias and 16 exhibited a right-side bias, a proportion that does not differ from chance, χ2(1, N = 40) = 1.60, P > .10. In contrast, for the supine posture, 6 chimpanzees exhibited a left-side bias and 30 exhibited a right-side bias, a proportion that significantly differs from chance, χ2(1, N = 36) = 16.00, p < .01. For both sleeping postures, no significant sex differences were found in direction of lateral bias; however, in the supine posture, right-side biases in head orientation were higher in females (93%) compared with males (76%).
Table 1.
Individual Data for All Subjects
| Prone
|
Supine
|
|||||
|---|---|---|---|---|---|---|
| Subject | Left | Right | LB | Left | Right | LB |
| Females | ||||||
| Amanda | 3 | 2 | −0.20 | 1 | 0 | −1.00 |
| Andi | 6 | 9 | 0.20 | 0 | 0 | NA |
| Bunny | 0 | 5 | 1.00 | 2 | 9 | 0.64 |
| Callie | 4 | 2 | −0.33 | 1 | 4 | 0.60 |
| Carole | 7 | 5 | −0.17 | 0 | 2 | 1.00 |
| Debbie | 11 | 6 | −0.29 | 1 | 2 | 0.33 |
| Edwina | 5 | 2 | −0.43 | 1 | 1 | 0.00 |
| Evelyne | 5 | 6 | 0.09 | 1 | 2 | 0.33 |
| Faye | 4 | 1 | −0.60 | 2 | 3 | 0.20 |
| Frannie | 7 | 3 | −0.40 | 0 | 1 | 1.00 |
| Janice | 8 | 5 | −0.23 | 1 | 3 | 0.50 |
| Katrina | 2 | 4 | 0.33 | 4 | 8 | 0.33 |
| Lillie | 5 | 6 | 0.09 | 0 | 0 | NA |
| Lizzie | 4 | 2 | −0.33 | 5 | 7 | 0.17 |
| Rebecca | 4 | 1 | −0.60 | 2 | 3 | 0.20 |
| Sheena | 4 | 3 | −0.14 | 1 | 6 | 0.71 |
| Sierra | 11 | 3 | −0.57 | 0 | 2 | 1.00 |
| Zana | 3 | 2 | −0.20 | 3 | 7 | 0.40 |
| Males | ||||||
| Artemus | 1 | 4 | 0.60 | 7 | 3 | −0.40 |
| Arthur | 2 | 3 | 0.20 | 1 | 3 | 0.50 |
| Barney | 2 | 3 | 0.20 | 2 | 7 | 0.56 |
| Brooks | 8 | 6 | −0.14 | 1 | 3 | 0.50 |
| Chip | 9 | 10 | 0.05 | 1 | 3 | 0.50 |
| Donald | 0 | 3 | 1.00 | 2 | 7 | 0.56 |
| Drew | 4 | 1 | −0.60 | 2 | 4 | 0.33 |
| Duff | 5 | 4 | −0.11 | 3 | 6 | 0.33 |
| Elwood | 4 | 2 | −0.33 | 3 | 11 | 0.57 |
| Fritz | 6 | 11 | 0.29 | 0 | 0 | NA |
| Jarred | 1 | 1 | 0.00 | 0 | 4 | 1.00 |
| Jason | 5 | 1 | −0.67 | 1 | 3 | 0.50 |
| Jones | 5 | 4 | −0.11 | 0 | 0 | NA |
| Josh | 4 | 3 | −0.14 | 3 | 12 | 0.60 |
| Lamar | 1 | 3 | 0.50 | 4 | 0 | −1.00 |
| Landrum | 6 | 8 | 0.14 | 2 | 2 | 0.00 |
| Lewis | 4 | 4 | 0.00 | 0 | 2 | 1.00 |
| Lucas | 2 | 5 | 0.43 | 0 | 3 | 1.00 |
| Luther | 6 | 9 | 0.20 | 4 | 3 | −0.14 |
| Magnum | 1 | 2 | 0.33 | 1 | 2 | 0.33 |
| Merlin | 4 | 2 | −0.33 | 1 | 4 | 0.60 |
| Moses | 1 | 1 | 0.00 | 3 | 1 | −0.50 |
| Patrick | 10 | 4 | −0.43 | 1 | 2 | 0.33 |
| Prescott | 5 | 2 | −0.43 | 8 | 6 | −0.14 |
| Scott | 3 | 2 | −0.20 | 3 | 3 | 0.00 |
Note. LB = lateral bias; NA = not available; Negative values = left bias; positive values = right bias.
To further assess the change in lateral bias as a function of posture, each chimpanzee was characterized as exhibiting a left-to-right (L → R) or right-to-left (R → L) shift in bias for the prone and supine postures. If lateral bias was randomly determined, then equal numbers of chimpanzees should exhibit L → R and R → L shifts in head orientation. Five subjects exhibited a R → L shift, whereas 23 subjects exhibited a L → R bias, a difference that statistically differed from chance, χ2 (1, N = 28) = 10.32, p < .01.
As previously noted, there was considerable individual variability in the frequency of head orientation (range = 6 to 23 observations). To determine whether the variability in frequency of head orientation effected our laterality results, we performed a subsequent analysis. The LB scores in each sleeping posture condition (prone, supine) were the dependent measures in a mixed-design analysis of covariance with posture serving as a repeated measure and sex as a between-groups factor. The number of observations in each postural condition served as covariates. Data for both conditions were not available in 4 of the original 43 subjects. Thus, in this analysis, there were 23 male and 16 female subjects.
The mean LB as a function of sex and posture can be seen in Figure 1. A significant main effect for posture was found, F (1.36) = 6.40, p < .02. No other main effects or interactions were found. The LB score in the prone posture did not deviate significantly from chance. t(41) = −.907, p > .10; however, lateral bias in the supine posture significantly differed from chance, t(37) = −4.45, p < .01, a finding similar to those reported with the classification data. In summary, the average LB score of subjects sleeping in a supine posture (M = .34) was significantly shifted to the right side in contrast to LB scores of subjects sleeping in a prone posture (M = −0.05).
Figure 1.
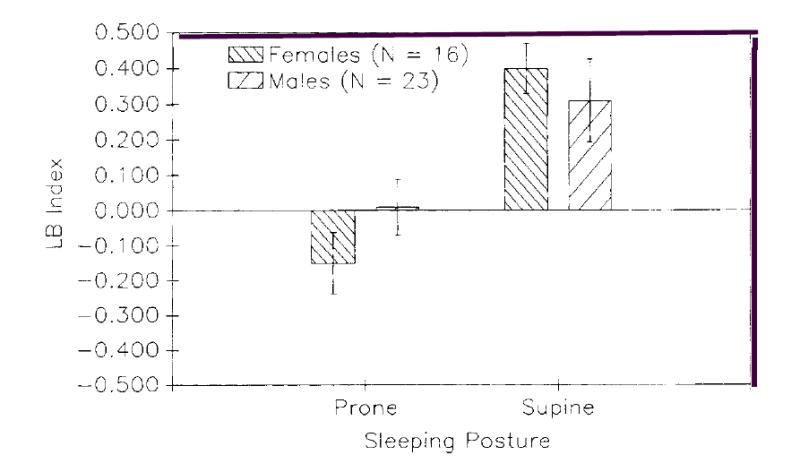
Average lateral bias (LB) score and standard errors for males and females as a function of sleeping posture.
Discussion
The data indicating a right-side bias in head orientation serve as further evidence that lateral biases are present in young nursery-reared chimpanzees. For example, 3-month-old chimpanzees show a right-hand bias in hand-to-mouth self-calming behavior (Bard et al., 1990; Hopkins & Bard. 1993a). There is also evidence that neonatal chimpanzees exhibit stronger and longer lasting gripping responses with the right hand compared with the left hand (Fagot & Bard, in press). Taken together with the result from this study, these data indicate that chimpanzees manifest population-level lateral bias early in life and that these biases are not restricted to a single behavioral measure.
Our results are similar to those from studies of humans in two important ways. First, the relative proportion of subjects exhibiting a right-side bias is comparable between the two species (see Barnes et al., 1985). Second, direction of lateral bias in head orientation was evident only in the supine posture. There is an equal distribution in lateral bias when subjects of both species are in a prone posture (Vles et al., 1991). Whether homologous or analogous neurobehavioral mechanisms account for this observation requires further experimentation.
Although not significant, our results are clearly suggestive of a sex difference in lateral bias. The stronger and more pronounced right-side bias for females is consistent with our previous findings on lateral bias in hand-to-mouth behaviors in chimpanzees (Bard et al., 1990; Hopkins & Bard, 1993a). Thus, although the behavioral measures of lateral bias differed between studies, we are repeatedly finding a sex difference in behavioral laterality.
In humans, attempts have been made to correlate asymmetries in head orientation with hand preference as well as hemispheric specialization in linguistic processing (Witelson, 1987). It is not clear whether asymmetries in head orientation are correlated with lateral bias in other behaviors of chimpanzees, and, in particular, hand preference. Chimpanzees do exhibit population-level hand preferences, and in most cases, the population biases are to the right side (Hopkins, 1993, 1994; Hopkins & Morris, 1993). However, we speculate that head-orientation asymmetries may correlate with lateral bias in leading limb in locomotion because both behaviors involve some degree of postural adjustment. Several recent studies have shown a right-side bias in the leading limb for locomotion in chimpanzees, orangutans, gorillas, gibbons (Heestand, 1986), and bonobos (Hopkins, Bennett, Bales, Lee, & Ward, 1993). The relative proportion of the population exhibiting a right leading limb asymmetry is comparable to the head-orientation asymmetry observed in the infant chimpanzees of this study.
Alternatively, it is possible that head-orientation asymmetries may account for the observed right hand-to-mouth asymmetries we have repeatedly observed in nursery-raised chimpanzees (Bard et al., 1990; Hopkins & Bard, 1993b). Simply stated, the chimpanzees may calm themselves by sucking on their right digits because the head is already turned right and therefore more proximal to the right hand. To date, about 75% of the chimpanzees exhibit a right hand-to-mouth bias, a value comparable to the right-head orientation bias when subjects were supine as reported in this study (79%). Preliminary analyses, however, did not find a correlation in the lateral bias of head orientation and hand-to-mouth behavior (Bard, 1994b). Moreover, the results of these analyses suggest rather that different neurobehavioral mechanisms underlie laterality: High arousal at 2 days of age is associated with nonright hand-to-mouth behavior, and a high level of orientation to environmental stimuli is associated with head orientation.
In conclusion, the results of this study serve as further evidence that behavioral asymmetries are present in chimpanzees very early in life. The possibility that these asymmetries are due to environmental factors such as learning or inadvertent shaping is unlikely; however, we would not rule out in utero factors such as fetal position or the possible role of intrauterine hormones (see Geschwind & Galaburda, 1985). These findings underscore the need for continued developmental studies that emphasize the role of early experiences on the outcome of adult competencies as they pertain to brain and behavior processes.
Acknowledgments
This investigation was supported in part by National Institutes of Health Grants RR-00165 (from the National Center for Research Resources), and RR-03591; RR-06158, NICHD Intramural Research Program funds; NICHD-NRSA Grant HD-07105; and NINDS Grant NS-29574, The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. We appreciate the supportive services provided by the Veterinary Department of the Yerkes Center. Data collection was facilitated with the assistance of Carolyn Fort and Kelly McDonald.
References
- Bard KA. Evolutionary foundations of intuitive parenting: A special case of maternal competence in chimpanzees. Early Development and Parenting. 1994a;3:19–28. [Google Scholar]
- Bard KA. Predicting laterality in young chimpanzees on the basis of neonatal neurobehavioral mechanisms; Paper presented at the International Conference on Infant Studies; Paris, France. 1994b. Jun, [Google Scholar]
- Bard KA. Similarities and differences in the neonatal behavior of chimpanzee and human infants. In: Eder G, Kaiser E, King FA, editors. The role of the chimpanzee in research. Basel, Switzerland: Karger; 1994c. pp. 43–55. [Google Scholar]
- Bard KA. Parenting in primates. In: Bornstein M, editor. Handbook of parenting: Volume 2: Biology and ecology of parenting. Hillsdale, NJ: Erlbaum; 1995. pp. 27–58. [Google Scholar]
- Bard KA, Gardner K. Influences on development in infant chimpanzees enculturation, temperament, and cognition. In: Russon A, Bard KA, Parker ST, editors. Reaching into thought: The minds of great apes. Cambridge, UK: Cambridge University Press; in press. [Google Scholar]
- Bard KA, Hopkins WD, Fort CL. Lateral bias in infant chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1990;104:309–321. doi: 10.1037/0735-7036.104.4.309. [DOI] [PubMed] [Google Scholar]
- Bard KA, Platzman KA, Lester BM, Suomi SJ. Orientation to social and nonsocial stimuli in neonatal chimpanzees and humans. Infant Behavior and Development. 1992;15:43–56. [Google Scholar]
- Barnes CL, Cornwell KS, Fitzgerald HE, Harris LJ. Spontaneous head positions in infants during the first 9 postnatal months. Infant Mental Health Journal. 1985;6:117–125. [Google Scholar]
- Brazelton TB. Neonatal behavioral assessment scale. Philadelphia: Spastics International Medical Publications; 1984. [Google Scholar]
- Brésard B, Bresson F. Handedness in Pongo pygmaeus and Pan troglodytes. Journal of Human Evolution. 1983;12:659–666. [Google Scholar]
- Chorazyna H. Shifts in laterality in a baby chimpanzee. Neuropscyhologia. 1976;14:381–384. doi: 10.1016/0028-3932(76)90033-6. [DOI] [PubMed] [Google Scholar]
- Cunningham D, Forsythe C, Ward J. A report of behavioral lateralization in an infant orangutan (Pongo pygmaeus) Primates. 1989;30:249–253. [Google Scholar]
- Fagot J. Ontogeny of object manipulation in the Guinea baboon. Preliminary observations. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. New York: Springer-Verlag; 1993. pp. 235–250. [Google Scholar]
- Fagot J, Bard KA. Lateralization of the grasping reflex in neonatal chimpanzees. Infant Behavior and Development in press. [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psychological Bulletin. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateraization Biological mechanisms, associations, and pathology: I A hypothesis and program for research. Archives of Neurology. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Harris LJ, Fitzgerald HE. Postural orientation in human infants: Changes from birth to three months. In: Young G, Segalowitz SJ, Corter CM, Trehub SE, editors. Manual specialization and the developing brain. New York: Academic Press; 1983. pp. 285–305. [Google Scholar]
- Hahn WK. Cerebral lateralization of function: From infancy through childhood. Psychological Bulletin. 1987;101:376–392. [PubMed] [Google Scholar]
- Heestand JE. Behavioral lateralization in four species of apes. University of Washington; Seattle: 1986. Unpublished doctoral dissertation. [Google Scholar]
- Hopkins WD. Posture and reaching in chimpanzees (Pan troglodytes) and orangutans (Pongo pygmaeus) Journal of Comparative Psychology. 1993;107:162–168. doi: 10.1037/0735-7036.107.2.162. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for bimanual feeding in 140 captive chimpanzees: Rearing and ontogenetic determinants. Developmental Psychobiology. 1994;27:395–408. doi: 10.1002/dev.420270607. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA. Hemispheric specialization in infant chimpanzees (Pan troglodytes): Evidence for a relation with gender and arousal. Developmental Psychobiology. 1993a;26:219–235. doi: 10.1002/dev.420260405. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA. The ontogeny of lateralized behavior in nonhuman primates with special reference to chimpanzees (Pan troglodytes) In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. New York: Springer-Verlag; 1993b. pp. 251–265. [Google Scholar]
- Hopkins WD, Bennett AJ, Bales S, Lee J, Ward JP. Behavioral laterality in captive bonobos (Pan paniscus) Journal of Comparative Psychology. 1993;107:403–410. doi: 10.1037/0735-7036.107.4.403. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Morris RD. Handedness in great apes: A review of findings. International Journal of Primatology. 1993;14:1–15. [Google Scholar]
- Lehman RAW. Manual preference in prosimians, monkeys, and apes. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. New York: Springer-Verlag; 1993. pp. 107–124. [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behavioral and Brain Sciences. 1987;10:247–303. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of function in apes: A meta-analysis of methods. Journal of Human Evolution. 1991;21:425–438. [Google Scholar]
- Michel GF. Right-handedness: A consequence of infant supine head-orientation preference. Science. 1981;212:685–687. doi: 10.1126/science.7221558. [DOI] [PubMed] [Google Scholar]
- Michel GF. Self-generated experience and the development of lateralized neurobehavioral organization in infants. Advances in the study of behavior. 1987;117:61–83. [Google Scholar]
- Molfese DL, Segalowitz SJ. Brain lateralization in children: Development implications. New York: Guilford Press; 1988. [Google Scholar]
- Previc FH. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychological Review. 1991;98:299–334. doi: 10.1037/0033-295x.98.3.299. [DOI] [PubMed] [Google Scholar]
- Provin KA. Early infant motor asymmetries and handedness: A critical evaluation of the evidence. Developmental Neuropsychology. 1992;8:325–365. [Google Scholar]
- Trehub SE, Corter CM, Shosenberg N. Neonatal reflexes: A search for lateral asymmetries. In: Young G, Segalowitz SJ, Corter CM, Trehub SE, editors. Manual specialization and the developing brain. New York: Academic Press; 1983. pp. 257–272. [Google Scholar]
- Turkewitz G. A prenatal source for the development of hemispheric specialization. In: Molfese DL, Segalowitz SJ, editors. Brain lateralization in children: Development implications. New York: Guilford Press; 1988. pp. 73–81. [Google Scholar]
- Veira Y, Bard KA. Developmental milestones for chimpanzees raised in a responsive care nursery. American Journal of Primatology. 1994;33:246. [Google Scholar]
- Viviani J, Turkewitz G, Karp E. A relationship between laterality of functioning at 2 days and 7 years of age. Bulletin of the Psychonomic Society. 1978;12:189–192. [Google Scholar]
- Vles J, Zutphen S, van Hasaart T, Dassen W, Lodder J. Supine and prone head orientation preference in term infants. Brain and Development. 1991;13:87–90. doi: 10.1016/s0387-7604(12)80112-4. [DOI] [PubMed] [Google Scholar]
- Ward JP, Hopkins WD. Primate laterality: Current behavioral evidence of primate asymmetries. New York: Springer-Verlag; 1993. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]
- Witelson SF. Neurobiological aspects of language in children. Child Development. 1987;58:653–688. [PubMed] [Google Scholar]


