Abstract
Background/Objective
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) is characterized by ventricular arrhythmias, sudden death, and fatty or fibrofatty replacement of right ventricular myocytes. Recent studies have noted an association between human ARVD/C and molecular remodeling of intercalated disc structures. However, progress has been constrained by limitations inherent to human studies. Here, we studied the molecular composition of the intercalated disc structure in a naturally occurring animal model of ARVD/C (boxer dogs).
Methods
We studied hearts from 12 boxers with confirmed ARVD/C and two controls. Ventricular sections from four animals were examined by immunofluorescent microscopy. Frozen tissue samples were used for western blot analysis. Proteins investigated were N-cadherin, plakophilin 2, desmoplakin, plakoglobin, desmin, and connexin43 (Cx43).
Results
In control dogs, all proteins tested by immunofluorescence analysis yielded intense localized signals at sites of end-to-end cell apposition. In contrast, myocardial tissues from ARVD/C-afflicted boxers displayed preservation of N-Cadherin staining but loss of detectable signal for Cx43 at the intercalated disc location. Western blots indicated that the Cx43 protein was still present in the samples. Gene sequencing analysis revealed no mutations in desmoplakin, plakoglobin, Cx43, or plakophilin 2.
Conclusions
Mutation(s) responsible for ARVD/C in boxers lead, directly or indirectly, to severe modifications of mechanical and electrical cell-cell interactions. Furthermore, significant reduction in gap junction formation may promote a substrate for malignant ventricular arrhythmias. This model may help advance our understanding of the molecular basis, pathophysiology and potential therapeutic approach to patients with ARVD/C.
Introduction
Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy (ARVD/C) is an inherited myocardial disease of unresolved pathogenesis that is associated with sustained monomorphic ventricular tachycardia and sudden cardiac death (SCD). Pathological lesions are distinctive and include progressive cardiac myocyte atrophy with replacement by fatty or fibro-fatty tissue most prominent in (but not limited to) the right ventricle.1 The arrhythmias are most commonly induced by exercise and, in humans, it is estimated that ARVD/C is responsible for as many as 10% of unexplained sudden cardiac deaths that occur under the age of 65.2-4
Recent studies have linked ARVD/C in humans with mutations in proteins of the cardiac desmosome5, a component of the intercalated disc essential for the mechanical coupling between cardiac cells.6 Though the loss of mechanical coupling may explain some of the phenotypical characteristics of the disease, it does not account for its highly arrhythmogenic nature, particularly in those cases where severe arrhythmias occur in the absence of substantial replacement of myocardium with fatty or fibrous tissue.5,7 In 2004, the group of Saffitz reported a significant disruption of gap junction integrity in hearts of patients afflicted with Naxos disease (caused by plakoglobin mutation).8 Further evidence of a link between desmosomes and gap junctions in the heart came recently from the analysis of cardiac tissue obtained from patients with Carvajal syndrome (a truncation of desmoplakin).7 Immunofluorescence staining of intercalated disc proteins showed the preservation of N-cadherin (and other proteins) at the sites of cell-cell apposition. However, gap junction plaques were absent or drastically reduced.7,8 Interestingly, Cx43 was still detectable by Western blot.8 These studies showed a link between desmosomal and gap junction integrity and were the first to postulate failure of this interaction as a potential pathophysiologic mechanism in ARVD/C.
The studies of Kaplan et al opened a fundamental venue for the understanding of the cellular and molecular mechanisms underlying the arrhythmic behavior prevalent in patients with ARVD/C.7,8 Yet, both Naxos and Carvajal diseases are rare, and some of the analysis on the mechanisms responsible for these syndromes is severely limited by the strict constraints inherent to human research. Recently, Basso et al reported inherited ARVD/C in boxer dogs. 9 The clinical manifestations of the disease in these animals included a high incidence of ventricular tachycardia and SCD. Here, we have applied immunochemical techniques to characterize the molecular phenotype of the cardiac intercalated disc in boxer dogs afflicted with ARVD/C. Our results are consistent with those reported by Kaplan et al indicating a significant loss of immunodetectable intercalated disc structures.7,8 In particular, afflicted boxers showed a drastic loss of gap junction plaques at the sites of cell apposition. The results strongly suggest that, as in the case of inherited ARVD/C in humans, the disease in boxers is associated with a significant remodeling of the structures involved in cell-cell communication. These experiments further validate the use of the boxer dogs as a model of the disease and support the notion that loss of gap junctions may represent a substrate in the development of ARVD/C-related ventricular arrhythmias.
Materials and Methods
Pathology
Hearts were fixed in 10% phosphate-buffered formalin and weighed. Wall thickness and chamber sizes were routinely assessed.10 Transmural tissue blocks were removed from the anterior, lateral, and posterior right ventricle (RV) and RV outflow tract; anterior, lateral, and posterior left ventricle (LV); anterior, medial, and posterior ventricular septum; and left and right atrial walls. Tissue sections were cut 5 μm thick and stained with hematoxylin and eosin and Masson's trichrome stains for routine histopathologic evaluation.
Immunolocalization experiments
Transmural blocks were taken from the LV free wall. The blocks were approximately 1.5 cm in length and 1 cm in width. The blocks were fixed in 10% buffered formalin, embedded in paraffin, and sectioned into 4 μm-thick slices. Protocols for immunolocalization of the relevant proteins followed those previously published.8 Briefly, sections were blocked in 3% BSA, 0.01% Triton buffer for 2 hours, and then incubated overnight at 4°C with the appropriate primary antibodies. After incubation, samples were rinsed in PBS and then incubated with the appropriate secondary antibodies for an additional period of 45 minutes, mounted and examined with a Zeiss Axiovision 2a microscope equipped with a 63X lens and Axioplan 2E imaging with structural illumination (Apotome). Primary antibodies used were the following: polyclonal rabbit anti-Cx43 (Chemicon), monoclonal mouse anti-PKP2a and 2b (BioDesign), polyclonal rabbit anti-DP (Serotec), polyclonal rabbit anti-pan cadherin (Sigma), monoclonal mouse anti-plakoglobin (Sigma), and polyclonal rabbit anti- desmin (Scy Tek). Secondary antibodies used were Alexafluor 598 goat anti-mouse, and Alexafluor 484 goat anti-rabbit (Molecular Probes).
Immunochemical detection of intercalated disc proteins in canine cardiac tissue lysates
Frozen tissue samples from one boxer (#6) and one unafflicted mongrel were homogenized in Laemmli buffer, and whole protein amounts measured by a Lowry protein assay. Proteins were separated by SDS-PAGE using an 8-16% tris-glycine gradient gel (Invitrogen), transferred onto nitrocellulose, blocked for 1 hour at room temperature with 5% non-fat milk in PBS-Tween (.5%), probed with the same antibodies specified for the immunofluorescence analysis. Cx43 was detected using an antibody that recognizes the amino terminal domain of the protein (Cx43NT, Fred Hutchinson Cancer Research Center). To ensure equal loading, blots were stripped with Restore Western Blot Stripping Buffer (Pierce), blocked again with 5% NFM in PBS-T for 1 hour at room temperature, and then incubated with primary monoclonal antibody to α cardiac actin (Fitzgerald).
Genetic Screening
RNA was isolated from the hearts of an afflicted boxer as well as from an un-afflicted mongrel dog. This RNA was reverse transcribed (Superscript III, Invitrogen) and subjected to PCR for amplification. As a first screening, we sequenced the cDNA coding for desmoplakin (DP), plakophilin 2 (PKP2), plakoglobin (PG), and connexin43 (Cx43). A series of primers were designed to amplify successive, overlapping fragments of each cDNA. These products were purified and sequenced by the DNA core sequencing facility at SUNY Upstate Medical University (Syracuse NY). Sequences derived from the afflicted boxer, from the control dog, and those predicted from the NCBI database were compared using ChromasPro Version 1.33 software (Technelysium Pty Ltd).
Results
Clinical Profile
Hearts were obtained from a total of 12 ARVD/C-afflicted boxers (9 studied by the Section of Cardiology, College of Veterinary Medicine, Cornell University and 3 studied by the Animal Medical Center in New York, NY). Animals were clinically diagnosed with ARVD/C by means of echocardiography, electrocardiography and 24-hour ambulatory electrocardiographic (Holter) monitoring. The clinical diagnosis was later confirmed by gross and histopathological examinations. Table 1 shows the general characteristics of the population. Ventricular tachycardia was present in 10 of the 12 dogs while atrial arrhythmias including atrial fibrillation were less common. Although polymorphic ventricular arrhythmias were present in dogs with clinical signs of left myocardial failure, monomorphic ventricular arrhythmias with a wide positive R wave in the anterior limb leads was the dominant pattern of arrhythmia (see Figure 1). These results were consistent with those previously described.11 Episodes of ventricular arrhythmias were often correlated with exercise or emotional stress, as it has been described both in the boxer and humans with ARVD/C.5,9 Eight dogs had evidence of myocardial failure based on clinical and echocardiographic examinations. Two of the twelve dogs also had mild subaortic stenosis (gradient < 40 mmHg). Six of the twelve dogs presented with syncope. The circumstances of death were sudden and unexpected in six of the twelve, while the remaining six were euthanized for medical reasons.
Table 1.
Summary of clinical findings in 12 ARVD/C afflicted boxer dogs. M = male, F = female, VT = ventricular tachycardia, AF = atrial fibrillation, APC = atrial premature complexes, N = no clinical signs, L = lethargy, S = syncope, A = ascites, PE = pulmonary edema, PLE = pleural effusion, LVDD = left ventricular diameter during diastole, LVDS = left ventricular diameter during systole, FS = % fractional shortening, LA/Ao = left atrial/aortic root ratio
| Dog ID | Age at Death (mos) | Sex | Rhythm | Clinical Signs | Echocardiography** | |||
|---|---|---|---|---|---|---|---|---|
| LVDD (mm) | LVSD (mm) | FS (%) | LA/Ao | |||||
| 1 | 52 | M | VT | L | 49.3 | 42.4 | 14 | 1.9 |
| 2 | 49 | F | VT | S | 35.9 | 24.6 | 31.5 | 1.85 |
| 3 | 136* | F | VT | S | 42.6 | 27.7 | 35 | 2.14 |
| 4 | 99* | M | VT | N | 55 | 42.7 | 22 | 2.07 |
| 5 | 93 | F | VT/AF | A | 45.2 | 44.3 | 2.2 | 2.23 |
| 6 | 153* | F | VT | N | 40 | 28.9 | 27.7 | 2.02 |
| 7 | 15* | F | AF | PE | 66.7 | 47.2 | 29 | 5.6 |
| 8 | 101 | F | VT | S/A/PLE | 62.7 | 44.7 | 28.7 | 2.44 |
| 9 | 94* | F | VT | L/PE | 52.3 | 41 | 21.1 | 1.74 |
| 10 | 118 | F | VT | L, S | 53.2 | 42.1 | 20.1 | 2.3 |
| 11 | 96 | M | VT | N | - | - | - | - |
| 12 | 58* | M | APC | PLE | 66 | 56.3 | 14.7 | 3.6 |
Indicates dog died suddenly rather than euthanized for medical reasons.
For unaffected boxer LVDD < 46 mm, LVDS < 29, FS > 27%, LA/Ao < 1.8
Figure 1.

Electrocardiographic trace (leads X, Y and Z) obtained during 24-hour Holter recording from a boxer dog presented with clinical signs of syncope. In this case, a run of monomorphic ventricular tachycardia degenerated into polymorphic ventricular tachycardia, eventually reverting, spontaneously, to sinus rhythm.
Pathological findings
Moderate right ventricular chamber dilation was judged on inspection to be present in 5 boxer dogs (41.7.3%) (Figure 2A). Hearts from all 12 boxer dogs displayed histopathological lesions that closely resembled those of human patients with ARVD/C,12,13 as well as lesions reported in an established spontaneous animal model of ARVD/C in the boxer dog.9 Most characteristic was substantial replacement of RV myocytes with adipocytes, or with adipose and fibrous tissue. This occurred in two morphologic patterns. The fatty form was characterized by diffusely distributed, multifocal regions of adipose cell replacement within the RV wall and trabeculae, extending from epicardium towards endocardium, and often associated with mild interstitial fibrosis (Figure 2B and 2C). The fibro-fatty form consisted of regions of focal or diffuse RV myocyte atrophy with adipose tissue and replacement-type fibrosis (Figure 2D and 2E). Both the fatty and fibro-fatty forms contained surviving myocytes embedded within areas of fat, and fibro-fatty tissue, respectively. Myocarditis, characterized by focal or multifocal inflammatory lymphocytic infiltrates associated with myocyte death, was identified in the RV of 5 of the 12 ARVD/C boxer dogs (41.7%). Left ventricular sections contained relatively minor lesions consisting largely of mild interstitial fibrosis or focal, fibrous tissue replacement, or scant, focal lymphocytic infiltrates. Overall, histopathological features of the 12 boxer dogs in the present study were consistent with the recent description of ARVD/C in the boxer dog.9 We therefore moved to assess the subcellular localization of intercalated disc proteins.
Figure 2.
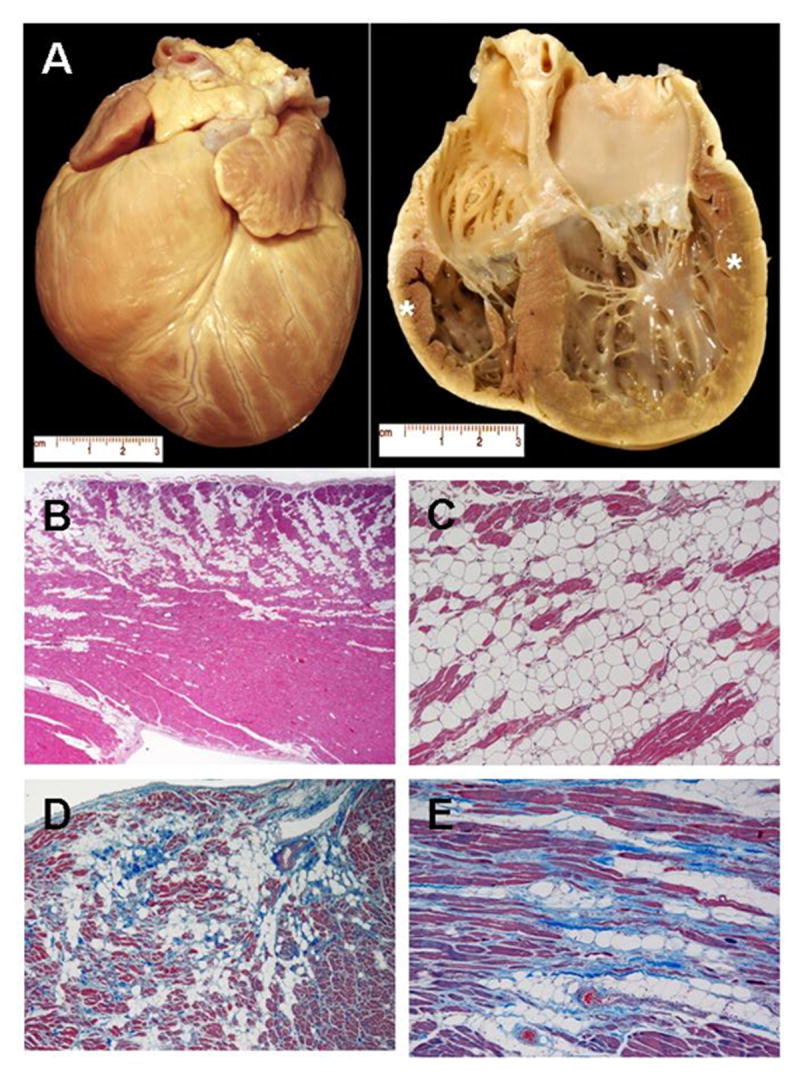
Pathological findings in boxer dogs afflicted with ARVD/C. Panel A: Heart from a dog with syncope and ventricular tachycardia. Gross specimen (left) and longitudinal four-chamber cut (right) illustrate cardiomegaly. Tissue sections examined by immunohistochemistry were taken from the anterolateral right ventricular wall (cranial star) and the left ventricular caudal wall (posterior star). Panels B and C. Histopathology of right ventricular (RV) wall from a boxer with ventricular tachycardia and fatty form of ARVD/C (Hematoxylin-eosin stain). B. There is diffuse myocyte loss associated with adipose tissue replacement extending predominantly from the epicardium to the mid-mural region (Magnification x2). C. Marked fatty replacement of atrophic myocardium is evident with small islands of surviving myocytes surrounded by fat (Magnification x10). Panels D and E. Histopathology of RV wall from a boxer with ventricular tachycardia (Masson’s trichrome stain). D. Diffuse subepicardial myocyte loss associated with adipose tissue and replacement-type fibrosis representative of the fibro-fatty form of ARVD/C (Magnification x4). E. Fibro-fatty tissue repair is evident (Magnification x10)
Immunolocalization of intercalated disc proteins in ARVD/C-afflicted boxers
Subcellular localization of relevant intercalated disc proteins was determined only in those hearts that were retrieved and fixed within two hours after time of death. The latter criteria avoided artifacts caused by postmortem proteolysis. Moreover, these experiments focused on tissue samples obtained from the left ventricular free wall since myocardial architecture was better preserved in these regions, compared with the RV which was characterized by substantial myocyte atrophy and fibro or fibro-fatty replacement.
Immunofluorescence analysis was conducted for the following proteins (the structure in which they integrate is indicated in parentheses): Cadherin (adherens junctions; see Figure 3), Plakophilin 2, Desmoplakin and Plakoglobin (desmosomes; Figure 4), Connexin43 (gap junctions; Figure 5), and Desmin (intermediate filaments; Figure 6). For the sake of consistency, tissue sections depicted in Figures 3-5 originate from the same hearts (either control mongrel, or case number 7 in Tables 1 and 2). A summary of the data is presented in Table 2. Samples obtained from control canines revealed that all proteins yielded intense localized signals at sites of end-end cell apposition, corresponding to the location of the intercalated disc. As shown in Figure 3, the distribution of cadherin in an ARVD/C afflicted heart was not different from that of the control animals. Also, plakophilin 2 and desmoplakin were present in the ARVD/C afflicted hearts. In one case (see Figure 4, panel B), the signal for plakophilin 2 was comparable to that observed in control; in three other cases, the signals were more sporadic and of weaker intensity than those obtained from the control animal (table 2). Similarly, desmoplakin was localized at the sites of cell apposition in all four dogs, though the intensity of the fluorescent signal was decreased when compared to that in the control (Figure 4D), whereas plakoglobin staining was not observed (Figure 4F). Perhaps most relevant from the perspective of arrhythmogenesis was the finding that tissue samples from all 4 ARVD/C boxer hearts, examined under the same conditions (i.e., stained in parallel and photographed using identical exposures), revealed no detectable signal originating at the end-end sites for Cx43. Indeed, the image shown in Figure 5B was obtained from the same heart stained for cadherin (Figure 3B), plakophilin 2 (Figure 4B) and desmoplakin (figure 4D). While signal for those proteins was clearly detected, gap junction plaques were not recognizable. Finally, tissue obtained from a control animal and treated with antibody to the intermediate filament protein desmin revealed an intense line of staining corresponding to the site of end-end contact, as previously described (see Figure 6A).5,6 A similar result was observed in the heart of one afflicted dog (case #7; see Figure 6B and table 2). However, in the remaining 3 boxer hearts, desmin could be detected throughout the cell, but a complete lack of signal was detected at the intercalated disc (Figure 6C). Overall, these results indicate a loss of gap junction plaques in ARVD/C afflicted hearts, even in cases where other structures involved in intercellular coupling remained identifiable at the sites of end-end contact.
Figure 3.

Immunohistochemical analysis of the adherens junctions protein cadherin in canine cardiac samples obtained either from a control animal (panel A) or from the heart of a boxer afflicted with ARVD/C (panel B; case # 7 in Table 1 and 2). Notice the preservation of the cadherin staining in the afflicted animal.
Figure 4.
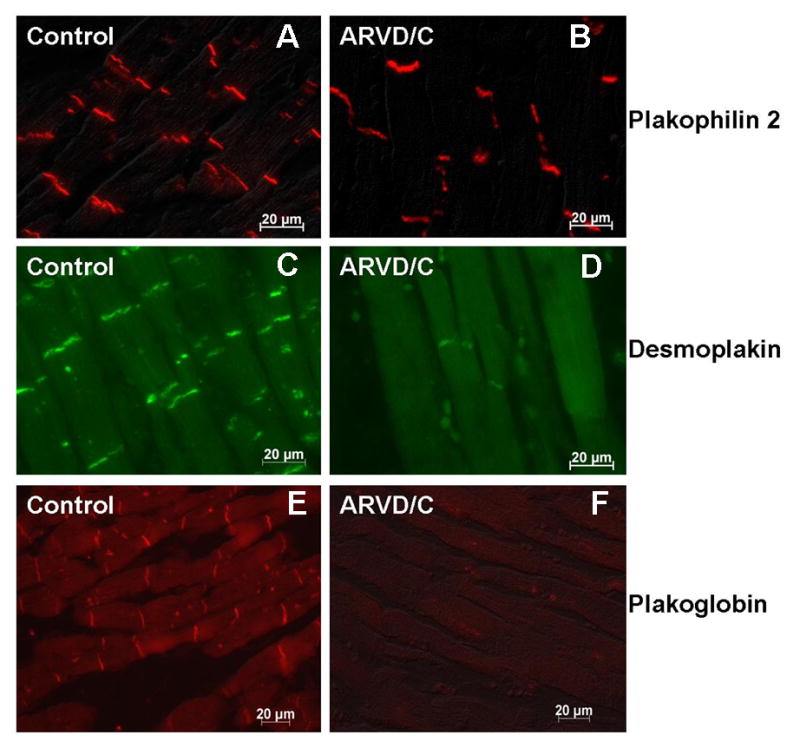
Immunohistochemical analysis of the desmosomal proteins plakophilin 2 (panels A and B), desmoplakin (panels C and D) and plakoglobin (panels E and F) in canine cardiac samples obtained either from a control animal (panels A, C and E) or from the heart of a boxer afflicted with ARVD/C (panels B, D and F; case # 7 in Table 1 and 2). Notice the preservation of the staining in the afflicted animal, though detectable desmoplakin signal was more sporadic and of lesser intensity.
Figure 5.

Cx43 expression and localization in control and afflicted canine cardiac tissues. Immunofluorescence images obtained from a control animal showed intense, regularly spaced signals located at sites of end-end apposition (panel A). In contrast, no selective signal was detected in tissue from an ARVD/C afflicted dog (panel B; case # 7 in table 1 and 2).
Figure 6.

Desmin expression and localization in control and afflicted canine cardiac tissue. Immunofluorescence images were obtained from control (panel A) and two different afflicted animals (panels B and C corresponding to samples from case # 7 and case # 6, respectively). For control, desmin staining was found throughout the cell, most prevalent at the site of the intercalated disc. A similar pattern was found for one afflicted boxer (panel B) whereas a loss of signal at the site of end-end contact was observed in the three other afflicted dogs studied (see, e.g., panel C).
Table 2.
Summary of proteins found at the intercalated disc of 4 ARVD/C afflicted boxers. Proteins examined were Cadherin (Cad), Plakoglobin (Pg), Plakophilin2 (PKP2), Desmoplakin (DP), Desmin (DSM) localization to the intercalated disc, and Connexin43 (Cx43).
| Dog ID# | Cad | Pg | PKP2 | DP | DSM at ID | Cx43 |
|---|---|---|---|---|---|---|
| 1 | Yes | No | faint | faint | No | No |
| 6 | Yes | No | faint | faint | No | No |
| 7 | Yes | No | bright | faint | bright | No |
| 9 | Yes | No | faint | faint | No | No |
Heart tissues from ARVD/C-afflicted dogs were collected within the first 2 hours after time of death; additional control experiments assessed whether this time frame could have interfered with the detection of gap junction plaques. Tissue from control canines was collected and placed into formalin either immediately after euthanasia (Figure 7, T0) or 2 hours post mortem (Figure 7, T2). Images taken with identical exposure times revealed that Cx43 signals were not affected by harvesting the tissue 2 hours post mortem. These results strongly suggest that the loss of Cx43 observed in ARVC/D –afflicted dogs occurred not because of the timing of tissue harvesting, but because of the pathological condition per se.
Figure 7.
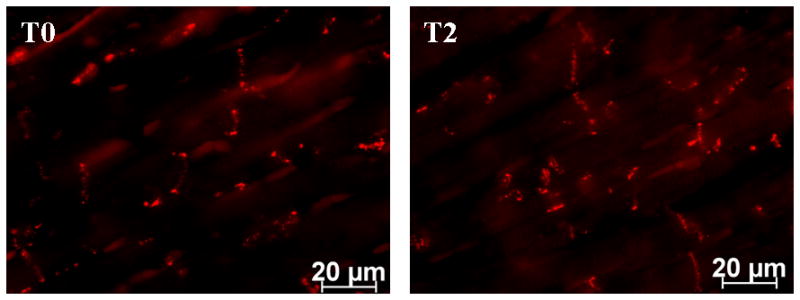
Cx43 expression and localization in control canine cardiac tissue harvested at time of death (T0), or 2 hours postmortem (T2). Data show preservation of Cx43-immunoreactive protein within the time frame used in this study.
Western blots for intercalated disc proteins in samples obtained from ARVD/C-afflicted boxers
Fluorescence microscopy allowed us to determine whether the molecular integrity of the intercalated disc was disrupted in ARVD/C-afflicted animals. As a next step, we explored whether the intercalated disc proteins were still present in tissue extracts obtained from an afflicted animal. Results are shown in Figure 8. All Western blots were obtained from the heart of one control animal, and one boxer dog (case # 6). Protein extracts were separated by conventional SDS-PAGE, transferred to nitrocellulose membranes and probed with antibodies specific for the proteins indicated on the right side of each panel. Separate samples were obtained from the right or left ventricles of either control or ARVD/C afflicted boxer dogs (location of the samples are noted by asterisks in Figure 1). Each lane was loaded with the same amount of total protein. As loading control, membranes were stripped and re-probed for either actin or heat shock protein 90 (hsp90). The results show that all proteins ran at the mobility predicted from their estimated molecular weight. For most of the proteins, signals of similar density were obtained from samples collected from the right or left ventricles, once loading was compensated by protein content. These results indicate that loss of detectable signal from the intercalated disc was not due to a loss of total protein content. Rather, our results suggest an inability of the pertinent proteins to localize and form a discrete functional unit. Yet, it is interesting to note that Western blots for PKP2 consistently showed a higher mobility band that was very weak in the control animal and much more intense in the sample obtained from the afflicted dog (see arrow in Figure 8). Whether this band relates to the possible genetic modification causative of ARVD/C in these animals remains to be determined. Finally, immunoblots from Cx43 revealed that the density of the high mobility band (relative to the band with lower mobility) was higher in the samples taken from the afflicted boxers. The latter would correlate with the apparent loss of gap junction plaques at the membrane, and is consistent with observations obtained in the hearts of patients afflicted with other variants of ARVD/C.7,8 We also noticed that, for equivalent loading, the intensity of the low mobility band in the right ventricle of the control dog was significantly higher than in the left ventricle of the same animal. This observation, though consistent in Western blots from the same heart, awaits confirmation in samples obtained from other control animals.
Figure 8.

Western blots obtained from ventricular tissue collected from either control or an afflicted boxer. LV or RV indicate that samples were collected from the left or the right ventricle, respectively. Proteins analyzed were cadherin (CAD), plakophilin 2 (PKP2), desmoplakin (DP), plakoglobin (PG), connexin43 (Cx43) and desmin (Dsm). Hsp90: Heat shock protein.
Sequencing of cDNA coding for junctional proteins in ARVD/C-afflicted boxers
Most of the mutations linked to ARVD/C in humans have been found in genes coding for desmosomal proteins.5 In addition, our data demonstrated significant Cx43 remodeling in the afflicted hearts. We therefore tested for modifications in the sequence of the cDNA coding for DP, PKP2, PG and the gap junction protein Cx43. The ARVD/C afflicted boxer sequence was compared to that obtained from an unafflicted mongrel, and also compared to the sequence reported in the dog genome (extrapolated from a boxer dog, NCBI). Consistent with the results obtained from Western blot, no signs of early termination, frame-shifts or missense mutations were encountered. Whether other proteins that associate to the intercalated disc are mutated remains to be determined.
Discussion
Previous studies have documented strong similarities between the clinical and pathological manifestations of ARVD/C in humans and those observed in purebred boxer dogs.9 Studies in humans have linked a number of cases of ARVD/C to mutations in desmosomal proteins5 and the studies of Kaplan et al, carried out in patients with Naxos disease8 and with Carvajal syndrome,7 have shown that mutations in desmosomal proteins can lead to a loss of gap junction plaques. Here, we have utilized a spontaneous canine model of ARVD/C to demonstrate that this condition is associated with a substantial loss of gap junction plaques as well as with remodeling of other intercalated disc structures. Overall, these data support the notion that ARVD/C may be a disease involving the molecular integrity of the intercalated disc as a whole.
We found significant loss of gap junction plaques in tissues from ARVD/C-afflicted animals. Yet, Western blot analysis showed that the Cx43 protein was indeed present in the tissue lysates. This apparent discrepancy may be due to our technical limitations in detecting a diffuse fluorescent signal in the immunolocalization experiments. Indeed, under normal conditions, the high concentration of connexin protein within a small area of the cell (at the plasma membrane) allows for proper visualization of the gap junction plaques by fluorescence microscopy. However, if the gap junction plaques are disorganized, with a diffuse protein pattern in the intracellular space, the immunofluorescent signal can fall below the level of detection even though the connexin protein is still identifiable in the cell lysate by Western blot.7,8 This observation has been reported in a variety of other circumstances, both in cardiac7,8 and in non-cardiac tissues14 and our results conform to the previous reports. It is also possible that part of the Cx43 signal detectable by Western blot originates from cells other than cardiac myocytes; indeed, cardiac fibroblasts are known to express Cx43; whether adipocytes in the area of the infiltrates would express Cx43 remains to be determined. Finally, it should be noted that altered phosphorylation may be an additional factor of functional relevance; previous studies have noted that phosphorylation of a variety of proteins is altered in the context of heart failure (with some proteins, such as RyR2, showing hyperphosphorylation15-16).
We found loss of desmin signal at the intercalated disc in three of the four dogs studied (see Figure 6C). Yet, in those three cases, immunoreactive desmin was still detected in the intracellular space, thus suggesting that the lack of signal at the site of end-end contact was a feature of the molecular phenotype of these animals, rather than an artifact of tissue preservation. The latter bears some similarity with observations made in the heart of a patient afflicted with Carvajal syndrome and may be suggestive of a disarray of the intermediate filaments at the intercalated disc due to the lack of organization of the desmosome.7 Our results show that desmoplakin signals were, though present, more sporadic and of weaker intensity. It is tempting to speculate that the loss of desmin signal may be related to changes in the integrity of the desmin-desmoplakin interactions. Additional experiments, exploring the ultra-structure of desmosomes and intermediate filaments in afflicted boxers, seem necessary to properly address this possibility.
Overall, these and other data support the notion that ARVD/C is associated with the loss of the spatial organization of the molecular components that normally maintain mechanical and electrical coupling between cardiac myocytes. It is important to emphasize that the samples utilized for immunochemistry were obtained from the left ventricles, and showed minimal fibro-fatty infiltration and a reasonable preservation of the myocardial architecture (see Figures 3-7). The latter suggests that, as pointed out by the results of Kaplan et al 7-8, the disruption of molecular coupling between the cells may precede the proliferation of fibrous and fatty tissue. Overall, the data point to a cross-talk between structures involved in mechanical coupling with those that maintain electrical communication between cells. As previously pointed out by Saffitz et al 17, at least two possible mechanisms can explain this cross-talk: a) There is a series of inter-molecular interactions between the components of both structures. Disruption of one molecule can interfere with the function of another, hence triggering a domino effect with the final disruption of the gap junctions. b) The two structures (desmosomes and gap junctions) may be molecularly independent. Yet, the physical strain imposed by the regular contraction of the heart will, in the absence of a functional mechanical coupling, lead to the disruption of the gap junction plaque.
With regards to the first mechanism, it is worth noting that previous studies have shown intermolecular interactions between Cx43 and other proteins that localize, at least under specific conditions, at the intercalated disc. Most relevant are the findings that Cx43 associates, directly or indirectly, with ZO-1,18-20 β-catenin,21 p120-catenin22 and with components of the sodium channel complex that localize at the intercalated disc.23 While the functional role of these protein-protein interactions has not been clearly established, they likely modulate intercellular communication and perhaps, play a role in coupling between inter- and intracellular signaling in Cx43-expressing tissues such as heart. Whether they represent a molecular network that could be disrupted by ARVD/C-relevant mutations is an interesting hypothesis that remains to be assessed.
With regards to the second mechanism, cell adhesion is generally recognized as a preamble to gap junction formation.24-29 Weak mechanical coupling due to desmosomal disruption could exert physical stress and eventually the physical separation of the neighboring myocytes, thus preventing the docking of the connexons and the disruption of the gap junction plaque. These hypotheses remain to be tested and also await further assessment for the specific mutation or mutations that are primarily responsible for ARVD/C in the boxer population.
Our experiments failed to identify a mutation in the three desmosomal proteins screened: DP, PG, and PKP2. We chose to sequence these genes based on previous data indicating their role in several cases of inherited human ARVD/C.5, 30 Furthermore, no mutation was found in the cDNA coding for Cx43. Yet, it is important to note that we could not obtain information as to the family history of the afflicted animal from whom the samples were taken. Moreover, we do not know whether this group of dogs suffered from a variety of diseases, where ARVD/C is an end point. Yet, the familial nature of the disease in this breed.31 supports the notion that the disease may be caused by a yet unknown genetic defect. In humans, investigators have reported a high incidence of PKP2 mutations in familial cases, but a very low incidence in the sporadic cases of the disease.32 Interestingly, our Western blot data for PKP2 revealed an intense band at ∼99 kD, directly beneath the signal corresponding to PKP2. A band of much lower intensity was observed in the same location in the control lanes (Figure 8). It is important to note that this lower band has previously been detected in Western blots for PKP233, and may be the result of post-translational modification of the protein. We speculate that the mutation causative of ARVD/C in this animal may be in a molecule functionally or structurally related to PKP2, which could secondarily disrupt the integrity of the intercalated disc structure.
Our results are consistent with the hypothesis that loss of gap junction-mediated coupling may be one of the substrates for arrhythmogenesis in ARVD/C. Yet, it is important to note that actual analysis of electrical communication has not been conducted as of yet. Studies in heterozygous Cx43-deficient mice have suggested that propagation can be maintained following a reduction in the abundance of Cx43 protein34, though the animals are more susceptible to ischemia-induced arrhythmias35. Furthermore, Danik et al (2004) used a conditional Cx43 knockout model to demonstrate that a reduction in cardiac Cx43 to about 18% from control correlated with a 50% decrease in conduction velocity and a significant increase in the inducibility of lethal tachyarrhythmias.36 Their results supported the notion that preservation of Cx43 expression is necessary for normal electrical synchrony in the heart. In our case, we have observed a loss of gap junction plaques, yet functional measurements have not been conducted as of yet. Thus, while the loss of gap junction plaques is suggestive of a loss of cell-cell communication, further experiments are necessary to assess whether electrical coupling has also been affected.
In conclusion, we have provided evidence demonstrating that ARVD/C in the boxer canine breed correlates with a substantial loss of myocyte gap junction plaques and other intercalated disc structures. These changes are observed in the presence of well preserved left ventricular myocardial structure. Our data support the hypothesis that intercalated disc remodeling originates at the cellular/molecular level and that this may represent a fundamental substrate that contributes to the clinical and pathologic characteristics of ARVD/C. Additional studies will be necessary to identify the responsible mutation in these animals and to elucidate the molecular cascades and/or the cellular events that lead to the loss of structures essential for intercellular communication in the heart.
Acknowledgments
This work was supported by grants HL39707, HL080602 and GM057691 from the National Institutes of Health, the Morris Animal Foundation, the Caspary Institute of the Animal Medical Center, the March of Dimes (ME, JS) and by an American Heart Association Fellowship (EO). The technical assistance of Shari Renaud-Farrell is greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, Nava A, Silvestri F, Blomstrom-Lundqvist C, Wlodarska EK, Fontaine G, Camerini F. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: A multicenter study. J Am Coll Cardiol. 1997;30:1512–20. doi: 10.1016/s0735-1097(97)00332-x. [DOI] [PubMed] [Google Scholar]
- 2.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318(3):129–33. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. clinical, demographic, and pathological profiles. JAMA. 1996;276:199–204. [PubMed] [Google Scholar]
- 4.Tabib A, Loire R, Chalabreysse L, Meyronnet D, Miras A, Malicier D, Thivolet F, Chevalier P, Bouvagnet P. Circumstances of death and gross and microscopic observations in a series of 200 cases of sudden death associated with arrhythmogenic right ventricular cardiomyopathy and/or dysplasia. Circulation. 2003;108:3000–5. doi: 10.1161/01.CIR.0000108396.65446.21. [DOI] [PubMed] [Google Scholar]
- 5.Sen-Chowdhry S, Syrris P, McKenna WJ. Genetics of right ventricular cardiomyopathy. J Cardiovasc Electrophysiol. 2005;16:927–35. doi: 10.1111/j.1540-8167.2005.40842.x. [DOI] [PubMed] [Google Scholar]
- 6.Hatsell S, Cowin P. Deconstructing desmoplakin. Nat Cell Biol. 2001;3(12):E270–2. doi: 10.1038/ncb1201-e270. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan SR, Gard JJ, Carvajal-Huerta L, Ruiz-Cabezas JC, Thiene G, Saffitz JE. Structural and molecular pathology of the heart in carvajal syndrome. Cardiovasc Pathol. 2004;13:26–32. doi: 10.1016/S1054-8807(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan SR, Gard JJ, Protonotarios N, Tsatsopoulou A, Spiliopoulou C, Anastasakis A, Squarcioni CP, McKenna WJ, Thiene G, Basso C, Brousse N, Fontaine G, Saffitz JE. Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (naxos disease) Heart Rhythm. 2004;1:3–11. doi: 10.1016/j.hrthm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Basso C, Fox PR, Meurs KM, Towbin JA, Spier AW, Calabrese F, Maron BJ, Thiene G. Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in boxer dogs: A new animal model of human disease. Circulation. 2004;109:1180–5. doi: 10.1161/01.CIR.0000118494.07530.65. [DOI] [PubMed] [Google Scholar]
- 10.Fox PR, Maron BJ, Basso C, Liu SK, Thiene G. Spontaneously occurring arrhythmogenic right ventricular cardiomyopathy in the domestic cat: A new animal model similar to the human disease. Circulation. 2000;102:1863–70. doi: 10.1161/01.cir.102.15.1863. [DOI] [PubMed] [Google Scholar]
- 11.Kraus MS, Moise NS, Rishniw M, Dykes N, Erb HN. Morphology of ventricular arrhythmias in the boxer as measured by 12-lead electrocardiography with pace-mapping comparison. J Vet Intern Med. 2002;16:153–8. doi: 10.1892/0891-6640(2002)016<0153:movait>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Fontaine G, Fontaliran F, Frank R. Arrhythmogenic right ventricular cardiomyopathies: Clinical forms and main differential diagnoses. Circulation. 1998;97:1532–5. doi: 10.1161/01.cir.97.16.1532. [DOI] [PubMed] [Google Scholar]
- 13.Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M. Arrhythmogenic right ventricular cardiomyopathy. dysplasia, dystrophy, or myocarditis? Circulation. 1996;94:983–91. doi: 10.1161/01.cir.94.5.983. [DOI] [PubMed] [Google Scholar]
- 14.Li WE, Nagy JI. Connexin43 phosphorylation state and intercellular communication in cultured astrocytes following hypoxia and protein phosphatase inhibition. Eur J Neurosci. 2000;12:2644–50. doi: 10.1046/j.1460-9568.2000.00162.x. [DOI] [PubMed] [Google Scholar]
- 15.Lehnart SE, Wehrens XH, Kushnir A, Marks AR. Cardiac ryanodine receptor function and regulation in heart disease. Ann N Y Acad Sci. 2004;1015:144–59. doi: 10.1196/annals.1302.012. [DOI] [PubMed] [Google Scholar]
- 16.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 17.Saffitz JE. Dependence of electrical coupling on mechanical coupling in cardiac myocytes. In: Thiene G, Pessina AC, editors. Advances in Cardiovascular Medicine. Padova, Italy: Universita degli Studi di Padova; 2003. pp. 15–28. [Google Scholar]
- 18.Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol. 1998;8:931–4. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 19.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem. 1998;273:12725–31. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- 20.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Molecular Biology of the Cell. 2005;16:5686–98. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. 2000;105:161–71. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, Thomas MP, Radice GL, Lo CW. Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. J Cell Biol. 2001;154:217–30. doi: 10.1083/jcb.200105047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malhotra JD, Thyagarajan V, Chen C, Isom LL. Tyrosine-phosphorylated and nonphosphorylated sodium channel beta1 subunits are differentially localized in cardiac myocytes. J Biol Chem. 2004;279:40748–54. doi: 10.1074/jbc.M407243200. [DOI] [PubMed] [Google Scholar]
- 24.Kostin S, Hein S, Bauer EP, Schaper J. Spatiotemporal development and distribution of intercellular junctions in adult rat cardiomyocytes in culture. Circ Res. 1999;85:154–67. doi: 10.1161/01.res.85.2.154. [DOI] [PubMed] [Google Scholar]
- 25.Zuppinger C, Schaub MC, Eppenberger HM. Dynamics of early contact formation in cultured adult rat cardiomyocytes studied by N-cadherin fused to green fluorescent protein. J Mol Cell Cardiol. 2000;32:539–55. doi: 10.1006/jmcc.1999.1086. [DOI] [PubMed] [Google Scholar]
- 26.Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol. 2004:811–38. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- 27.Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc Res. 2004;62:233–45. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Wei CJ, Francis R, Xu X, Lo CW. Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J Biol Chem. 2005;280:19925–36. doi: 10.1074/jbc.M412921200. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Patel VV, Kostetskii I, Xiong Y, Chu AF, Jacobson JT, Yu C, Morley GE, Molkentin JD, Radice GL. Cardiac-specific loss of N-cadherin leads to alteration in connexins with conduction slowing and arrhythmogenesis. Circ Res. 2005;97:474–81. doi: 10.1161/01.RES.0000181132.11393.18. [DOI] [PubMed] [Google Scholar]
- 30.Pilichou K, Nava A, Basso C, Beffagna G, Bauce B, Lorenzon A, Frigo G, Vettori A, Valente M, Towbin J, Thiene G, Danieli GA, Rampazzo A. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:1171–9. doi: 10.1161/CIRCULATIONAHA.105.583674. [DOI] [PubMed] [Google Scholar]
- 31.Meurs KM, Spier AW, Miller MW, Lehmkuhl L, Towbin JA. Familial ventricular arrhythmias in boxers. J Vet Intern Med. 1999;13:437–9. doi: 10.1892/0891-6640(1999)013<0437:fvaib>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 32.van Tintelen JP, Entius MM, Bhuiyan ZA, Jongbloed R, Wiesfeld AC, Wilde AA, van der Smagt J, Boven LG, Mannens MM, van Langen IM, Hofstra RM, Otterspoor LC, Doevendans PA, Rodriguez LM, van Gelder IC, Hauer RN. Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2006;113:1650–8. doi: 10.1161/CIRCULATIONAHA.105.609719. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Bonne S, Hatzfeld M, van Roy F, Green KJ. Protein binding and functional characterization of plakophilin 2. evidence for its diverse roles in desmosomes and beta - catenin signaling. J Biol Chem. 2002;277:10512–22. doi: 10.1074/jbc.M108765200. [DOI] [PubMed] [Google Scholar]
- 34.Morley GE, Vaidya D, Samie FH, Lo C, Delmar M, Jalife J. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J Cardiovasc Electrophysiol. 1999;10:1361–75. doi: 10.1111/j.1540-8167.1999.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 35.Lerner DL, Yamada KA, Schuessler RB, Saffitz JE. Accelerated onset and increased incidence of ventricular arrhythmias induced by ischemia in Cx43-deficient mice. Circulation. 2000;101:547–52. doi: 10.1161/01.cir.101.5.547. [DOI] [PubMed] [Google Scholar]
- 36.Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res. 2004;95:1035–41. doi: 10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]


