Abstract
The future of rapid point-of-care diagnostics depends on the development of cheap, noncomplex, and easily integrated systems to analyze biological samples directly from the patient (eg. blood, urine, saliva). A key concern in diagnostic biosensing is signal differentiation between non-specifically bound material and the specific capture of target molecules. This is a particular challenge for optical detection devices in analyzing complex biological samples. Here we demonstrate a porous silicon (PSi) label-free optical biosensor that has intrinsic size-exclusion filtering capabilities which enhances signal differentiation. We present the first demonstration of highly repeatable, specific detection of immunoglobulin G (IgG) in serum and whole blood samples over a typical physiological range using the PSi material as both a biosensor substrate and filter.
Keywords: porous silicon, optical microcavity, biosensor, whole blood, blood serum, IgG, biotion/streptavidin
1.0 Introduction
Whole blood tests are desirable as they enable fast turnaround and a reduction in pre-analytical error arising from centrifugation, dilution, and transportation of the sample. Biosensor analyses of complex biological solutions remain problematic due to high background levels, baseline drift and deviations in sensitivity due to cross-reactivity with interferents that are present in the sample (Byrne et al., 2006). One strategy to reduce these spurious effects on target detection is to filter the sample; however this often adds complexity and cost to the process. In this paper we demonstrate that the inherent filtering capabilities and unique signal generation properties of porous silicon (PSi) devices can be exploited in optical biosensing to size exclude cells and proteins larger than the pores from interacting with the transducer surface. The integrated filter/sensor device is inexpensive to fabricate and noncomplex to operate. It can be used to rapidly (<1 hr) and reliably detect IgG target (95% confidence compared to ELISA) using a small volume (15 μl) of whole blood or blood serum.
Electrochemically etched PSi exhibits many features that are leveraged in the design of biosensors such as its tunable morphology, large internal surface area, intrinsic optical properties and compatibility with silicon microelectronics processing (Vinegoni et al., 2001; Ouyang et al., 2005; Dancil et al., 2002; DeLouise and Miller, 2004a; Lehmann et al., 2002). Exploitation of the porous morphology for filtering has been considered in size-exclusion-based separation techniques (Létant et al., 2003; Collins et al., 2002) and in the design of extremely low refractive index optical layers (Rabus et al., 2007), but the intrinsic filtering capabilities of the material have not previously been emphasized in a biosensor application. Because the optical response from a PSi sensor can be specifically monitored to report binding events that occur only within the 3D porous matrix, the ability to filter a complex biological sample such as blood provides an advantage over planar biosensing techniques. In the latter case, false positives and/or a high baseline drift during reference measuring commonly arise from interference of blood constituents (erythrocytes, leukocytes, platelets) that contaminate the transducer surface (Schneider et al., 2000; Lim et al., 2004; Shih et al., 2005). Specific detection of target binding to receptors immobilized within the 3D porous matrix is monitored as an optical shift in the white light reflectance spectrum. The shift indicates a change in the effective refractive index of the device caused by a change in porosity. The Bruggeman effective medium approximation relates the refractive index to porosity of the sensor matrix (Vinegoni et al., 2001; Bruggeman et al., 1935). It is important to note that the optical wavelength shift is linear with pore filling (change in dielectric environment) which simplifies quantification of target binding (DeLouise et al., 2005).
2.0 Materials and Methods
2.1 PSi Biosensor Fabrication
The PSi photonic microcavity sensors used in this study were electrochemically etched into highly doped n-type silicon using procedures detailed in previously (Vinegoni et al., 2001; Ouyang et al., 2005; Dancil et al., 2002; DeLouise and Miller, 2004a; Létant et al., 2003). The pore diameter, porosity and thickness of each layer are controlled by the magnitude and duration of the applied current density cycle and the constituents of the electrolyte solution. PSi sensors were made by anodic etching of n-type, Sb-doped, <100> oriented silicon, with resistivity range of 0.007-0.02 ohm-cm (SHE America, Inc.) in an aqueous electrolyte solution of 5% Hydrofluoric acid and 0.1% Pluronic L31 (BASF) surfactant. The sensor fabrication process begins with forming a sacrificial layer (current density, J=60 mA cm-2 for 30 sec) that was etched off with two short duration current pulses of J=300 mA cm-2 for 1.5 s each. The sacrificial layer creates defects on the n-type silicon surface, in which holes are the minority carrier, which help to produce a more consistent distribution of pore formation and inter-pore distances in etching subsequent layers (Ouyang et al., 2005). The λ/2 microcavity structure consisted of alternating layers of J=30 and 60 mA cm-2 for 3.23 s and 2.87 s respectively with 10 periods per mirror. After etching the electrolyte was rinsed off using ethanol then water and dried under nitrogen gas. The pore diameters of our microcavity sensors range from 88.2±39.3 to 106.9±40.8 nm (for low and high porosity layers, respectively). The etching process produced a porous silicon device with planar diameter of ∼1.4 cm, which was diced evenly into six individual porous silicon sensors. Dry thermal oxidation was conducted using a three-zone Lindberg tube furnace at 900 °C for 3 min. to impart greater stability of the PSi structure and to increase hydrophilicity of the material.
2.2 PSi Biosensor Functionalization
Unless otherwise stated, all materials were purchased from Sigma-Aldrich and used without further purification. After oxidation the porous silicon sensor was silanized with 2 % aqueous 3-aminopropyltrimethoxysilane (APTMS, Gelest) mixed in 50 % ethanol for 15 min. The sensors were rinsed with ethanol then water and dried with nitrogen gas. The sensors were then kept at 100 °C for 10 min. to crosslink the silane layer and evaporate any remaining solvents. Amine reactive sulfo-NHS-LC-LC-biotin (sulfosuccinimidyl-6-(biotinamido)-6-hexanamido hexanoate, 669.75 Da, Pierce), was diluted in phosphate buffered saline (PBS) buffer (pH=7.4) and immobilized to the amine-terminated porous silicon surface by adding 15 μl of 0.5 mg ml-1 sulfo-NHS-LC-LC-biotin on the sensor surface by pipette and leaving for 30 min in a humidified enclosure to prevent evaporation. The solution was rinsed off with PBS buffer and dried under nitrogen gas. Immunopure streptavidin (53 kDa, Pierce) was diluted to 0.07 mg ml-1 in 20 mM potassium phosphate buffer (pH 6.5), and 15 μl was incubated on PSi for 45 min. in a humidified enclosure. The streptavidin solution was rinsed off with PBS buffer and dried with nitrogen gas.
The receptor molecule (biotinylated whole anti-rabbit IgG (H&L) from goat) and control receptor molecule (biotinylated anti-chicken IgG (H&L) from goat) were diluted in PBS buffer and 15 μl of a 0.07 mg ml-1 solution was soaked on the sensor within a humidified enclosure for 1 hour. Samples were rinsed and soaked for 5 min in 5 ml of PBS buffer prior to rinsing with 0.1% Tween 20, 1% trehalose aqueous solution and drying with nitrogen. Blocking was performed by addition of 15 μl of 1% BSA to the sensor within a humidified enclosure for 30 min. Then the sensor was rinsed with PBS buffer and 0.1% Tween 20, 1% trehalose aqueous solution before drying with nitrogen.
Blood samples were collected from male Dutch belted rabbits and combined with an anti-coagulant (4% sodium citrate in PBS) to prevent pore clogging. One part sodium citrate was added to nine parts whole blood directly after retrieval from the rabbit. This concentration of anti-coagulant was found to keep blood samples fluid over a 60 min. period (data not shown) within a humidified enclosure, therefore allowing sufficient time for pore infiltration and binding of the target antibodies to the functionalized PSi sensor without fouling or clogging. Serum was obtained from whole blood samples by centrifugation.
All target solutions were incubated on the sensor in a humidity chamber for 1 hour at room temperature, which is after the saturation point of 45 min. previously determined by kinetic studies (Bonanno and DeLouise, 2007). The sensor was rinsed with PBS and then soaked in 5 ml of PBS buffer for 30 min on a rocker plate at room temperature and then was rinsed with 0.1% Tween 20 detergent to reduce non-specific binding.
2.3 SEM Imaging of Filtered Erythrocytes
SEM micrographs were obtained by incubation of whole blood (0.4% sodium citrate) on the sensor for 1 hour within a humidified enclosure. The sensor was gently rinsed with PBS buffer (pH=7.4) and fixed with 1% glutaraldehyde; then serially dehydrated in ethanol (25 %, 50 %, 75 %, 100 %) prior to imaging.
2.4 Optical Detection
The sensor surface was exposed to a normal incident beam of white light (spot size of ∼13 mm2). The optical reflectance spectrum was measured using an Ocean Optics Spectrophotometer HR2000 with an optical resolution of 0.33 nm pixel-1. All measurements were taken on dry samples following exposure and wash procedures as described above. Each experiment was repeated at least twice on two separate PSi sensors with 3 measurement positions on each sensor. All plots within this paper contain error bars that represent the standard deviation of each data point. Nonlinear least squares curve fitting was performed with Origin 7.0.
2.5 Direct ELISA
Serum or IgG was immobilized to an ELISA plate. A secondary reporter antibody (whole anti-rabbit IgG (H&L) from goat) with a conjugated alkaline phosphatase enzyme was used to quantify the amount of IgG present in the wells. A negative human serum control was used for all experiments and blocking was completed with 1% BSA.
3.0 Results
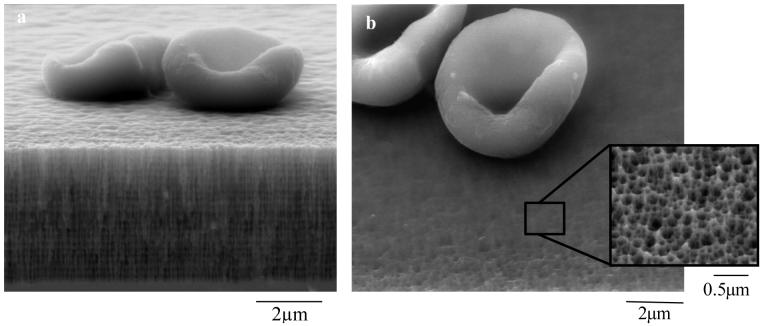
Typical device morphology of a PSi biosensor used to filter and analyze whole blood is illustrated in SEM micrographs (Fig. 1). The images were taken after glutaraldehyde fixation and dehydration of a blood sample on the PSi sensor and effectively show how erythrocytes (diameter ∼4 μm) are excluded from diffusing into the porous matrix of the sensor. The pore diameters (88/114 nm) of this device are sized to allow infiltration of antibodies, target IgG radius ∼5.6 nm (Taylor and Parker, 2003), while excluding large proteins and cells present in blood. Our studies show that if the sensor is designed with pore diameters <40 nm, large proteins (>50 kDa) including antibodies will be excluded from pore infiltration (DeLouise and Miller, 2004b). It is important to note that the methodology used to fix and dehydrate the blood sample for SEM analysis, as shown in Fig. 1, differs from the procedure used to conduct the biosensor measurement. As described in the methods section, the blood sample for biosensing is mixed with an anticoagulant and then applied to the sensor housed in a humidity chamber. This keeps the blood a fluid state, preventing thrombus formation on top of the porous layer which would otherwise block inward diffusion of target molecules into transducer.
Figure 1. Demonstration of the size -exclusion capability of the porous sensor.
a-b; SEM images of PSi microcavity structure (biosensor substrate) showing erythrocytes filtered out of porous matrix and crosslinked to the surface via glutaraldehyde fixation. a, cross-sectional view. Note the high and low porosity layers in microcavity structure; b, top view with inset showing magnified pores (∼88 nm pore diameter).
Recent literature has highlighted a fundamental challenge in developing biosensors is the homogeneous attachment of protein receptors to the transducer surface in a site-directed manner with control over immobilization density, distance from the surface, and affinity to the surface (Medinitz et al., 2006). The versatile biotin-streptavidin linking chemistry employed in these experiments satisfy the ‘universal’ conditions outlined (Medinitz et al., 2006) where any biotinylated receptor antibody may be immobilized to the PSi sensor surface with a known affinity (Kd = 10-15 M) (Gonzales et al., 1997) and surface density. Here the amount of bound biotinylated receptor antibody is controlled by the surface concentration of immobilized streptavidin (Ouyang et al., 2005; Bonanno and DeLouise, 2007).
Sensors fabricated for this study were designed to detect rabbit IgG (target) using immobilized biotinylated anti-rabbit IgG antibody (α-Rab receptor). To maintain stability and preserve activity of the functionalized sensor (attached receptor antibody) during optical read out and storage, the sensor was rinsed with a buffer solution containing 1% trehalose prior to drying the device (Nan et al., 2004; Jairo and Michel, 2004). Non-specific binding of various proteins and blood serum constituents that are small enough to diffuse into the porous matrix was minimized using bovine serum albumin (1% BSA) as a blocking agent and stringent rinsing with a wash buffer containing detergent (0.1% Tween 20).
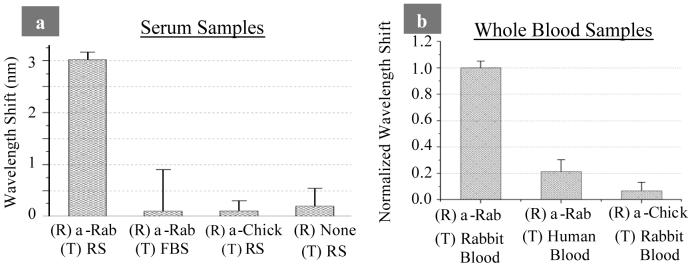
Target binding specificity was tested by varying the composition of the receptor antibody (α-rabbit and α-chicken), as well as testing the binding of these receptors to various targets including rabbit serum (RS), fetal bovine serum (FBS), rabbit blood, and human blood. The amount of bound rabbit IgG (target) is quantified by measuring the magnitude of the wavelength shift of the optical reflectance spectrum. Results from these measurements are displayed in Fig. 2a for serum samples and Fig. 2b for whole blood samples. A large wavelength shift (target binding) was observed only when the correct receptor and target pair (α-rabbit IgG and rabbit serum/blood) were tested. Minimal non-specific binding of FBS (Fig. 2a) and human blood (Fig. 2b) to the α-Rab receptor was observed. A control receptor antibody (α-chicken IgG) was tested against rabbit serum (Fig. 2a) and rabbit blood (Fig. 2b) and resulted in minimal cross-reactivity. This was confirmed with results from enzyme-linked immunosorbent assay (ELISA) which indicated ∼10 % cross-reactivity between the α-chicken IgG antibody receptor with both purified rabbit IgG in solution and rabbit serum samples. When no receptor was attached to the sensor little non-specific binding of the rabbit serum was observed (Fig. 2a), illustrating the effectiveness of the BSA blocking agent.
Figure 2. Specificity of biosensor to detect rabbit IgG in rabbit serum (RS) and whole blood samples.
Receptor antibody (R) and target solution (T) were varied to determine specificity of linking chemistry. a, IgG detection in serum quantified with biotin/streptavidin linking chemistry, [IgG]=2.8 mg ml-1 per ELISA. (Standard deviations shown in plot were taken from 9 measurements) b, IgG detection in whole blood samples with biotin/streptavidin linking chemistry. Data normalized with blood samples from 2 different rabbits (tests on each rabbit were performed on 2 different sensors, each having 3 measurement positions) with IgG concentrations of 6.8 and 8.7 mg ml-1 per ELISA.
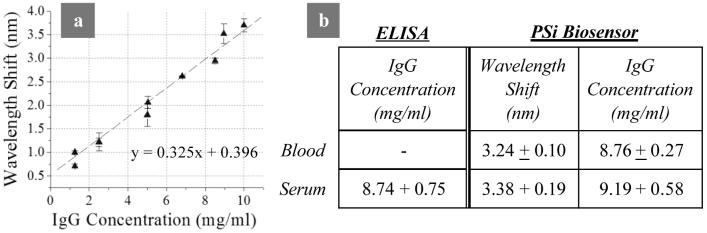
To test the analytical capability of the whole blood sensor an IgG calibration curve was made with whole rabbit IgG antibody serially diluted in 1% BSA. The immobilized concentration of each layer in the linking chemistry (biotin, SA, receptor α-Rab IgG) was optimized, following procedures documented elsewhere (Bonanno and DeLouise, 2007), to achieve a linear detection range of rabbit IgG (target) between the values of 2-10 mg ml-1 (Fig. 3a). This detection range includes normal physiologic IgG levels expressed in rabbits (∼6.34-8.91 mg ml-1) (Weisbroth et al., 1974). To determine the concentration of IgG in serum and blood samples, we correlated the PSi sensor wavelength shift response (Fig. 3b) to the IgG concentration calibration curve (Fig. 3a). Similar results were achieved in experiments on 2 different rabbits (expressing IgG serum levels of 7.85 and 8.74 mg ml-1). Figure 3b tabulates results obtained from 2 separate experiments on 1 of the rabbits (each experiment contained at least 3 measurements). Results show that the PSi biosensors produce repeatable measurements of IgG (coefficient of variation <15 %) in whole blood and serum samples.
Figure 3. Biosensor calibration curve for IgG and tabulated comparison between biosensor and ELISA quantification of rabbit IgG in rabbit blood.
a; IgG calibration curve depicts rabbit IgG binding (wavelength shift) per known concentrations of rabbit IgG applied to sensor surface. (Standard deviations taken from 9 measurements) b; PSi biosensor quantification of IgG compared to standard immunoassay (ELISA plate) results on same sample. Rabbit blood samples were collected from male Dutch Belted rabbit. Both immunoassay and PSi biosensor test were conducted in 2 separate experiments with at least 3 measurements per each experiment. A t-test showed ELISA and Biosensor results for serum to not be statistically different (95% confidence).
To validate the accuracy of the PSi sensor, results were compared to ELISA measurements of the rabbit IgG concentration present in the serum sample. The ELISA and PSi biosensor results gave statistically equivalent values of the IgG concentration (Fig. 3b). These results suggest that PSi optical biosensor technology may offer several advantages over the benchmark ELISA bioanalytical technique. First, a serum sample can be analyzed directly using PSi sensors whereas for ELISA it must be diluted ∼10,000 fold to conduct analysis. This adds process steps and cost to the technique which also requires use of a secondary reporter antibody. Second, despite the fact that the benchmark ELISA and surface plasmon resonance (SPR) bioanalytical techniques exhibit significantly better detection sensitivity, neither is particularly well suited for conducting protein analysis on whole blood samples. These techniques perform best in analysis of transparent test fluids (Suenaga and Abdou, 1995; Matveeva et al., 2005) however, SPR has proven useful in studying blood cell - antibody interactions (Quinn et al., 2005), plasma and whole blood coagulation (Hansson et al., 1999; Hansson et al., 2002) and in numerous other affinity-based interactions in biomedicine (Ramanavièius et al., 2005).
4.0 Conclusions
This work addresses specific material issues in the design of optical biosensors and demonstrates a proof of concept that the filtering capabilities of PSi devices can be leveraged to enable specific detection of immunoglobulin target in whole blood. The ability to tune the morphology of porous silicon over a broad range of pore diameters (< 2 nm in microporous structures up to tens of microns in macroporous structures) allows customization of the filtered biosensing technique for a specific target size. Our sensor utilizes the versatile biotin-streptavidin linking chemistry which can be employed to immobilize any biotinylated receptor antibody to detect its corresponding target. Here, we have demonstrated specific IgG target detection by varying both the receptor and complexity of the target solution. We have developed simple protocols employing solutions containing additives (surfactants, trehalose, BSA and citrate) to prevent pore clogging from blood coagulation and protein precipitation, to minimize spurious signal from generation nonspecific binding on the transducer surface and we have addressed issue of stability and storage. We demonstrate for the first time specific and repeatable biodetection of IgG in whole blood and serum samples with 95% confidence and values are comparable to an ELISA standard. Our progress points to the feasibility for translating this technology for development of diagnostic devices for a broad range of immunological diseases and conditions pending availability of whole or antibody fragments. On-going work is focused on increasing the sensitivity of the PSi filter/biosensor for applications where a lower limit of detection is needed. For example, to diagnose hyper IgE syndrome on the basis of elevated IgE levels (∼0.005 - 0.132 mg ml-1), which is important for diagnosis of pathologic skin disorders in clinical research (Grimbacher et al., 1999).
Acknowledgments
The authors would like to acknowledge support from the NIH / NIAID 5K25AI060884-02 and helpful discussions with Prof. Benjamin L. Miller.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bonanno LM, DeLouise LA. Langmuir. 2007;23:5817–5823. doi: 10.1021/la063659c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggeman DAG. Ann. Phys. 1935;24:636. [Google Scholar]
- Byrne R, Diamond D. Nature Mater. 2006;5:421–424. doi: 10.1038/nmat1661. [DOI] [PubMed] [Google Scholar]
- Collins BE, Dancil KS, Abbi G, Sailor MJ. Adv. Funct. Mater. 2002;12:187–191. [Google Scholar]
- Dancil KS, Greiner DP, Sailor MJ. J. Am. Chem. Soc. 1999;121:7925–7930. [Google Scholar]
- DeLouise LA, Miller BL. Mater. Res. Soc. Symp. Proc. 2004a;782:A5.3.1. [Google Scholar]
- DeLouise LA, Miller BL. Anal Chem. 2004b;76:6915–6920. doi: 10.1021/ac0488208. [DOI] [PubMed] [Google Scholar]
- DeLouise LA, Kou PM, Miller BL. Anal. Chem. 2005;77:3222–3230. doi: 10.1021/ac048144+. [DOI] [PubMed] [Google Scholar]
- González M, Gagatolli LA, Echabe I, Arrondo JLR, Argarańa CE, Cantor CR, Fidelio GD. J. of Biol. Chem. 1997;272:11288–11294. doi: 10.1074/jbc.272.17.11288. [DOI] [PubMed] [Google Scholar]
- Grimbacher B, Holland SM, Gallin JI, Greenberg F. The New England J. of Med. 1999;340:692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- Hansson KM, Vikinge TP, Rånby M, Tengvall P, Lundström I, Johansen K, Lindahl TL. Biosens. Bioelectron. 1999;14:671–682. doi: 10.1016/s0956-5663(99)00050-0. [DOI] [PubMed] [Google Scholar]
- Hansson KM, Tengvall P, Lundström I, Rånby M, Lindahl TL. Biosens.Bioelectron. 2002;17(9):747–759. doi: 10.1016/s0956-5663(02)00048-9. [DOI] [PubMed] [Google Scholar]
- Jairo J, Michel P. Current Medicinal Chem. 2004;11:439–446. [Google Scholar]
- Lehmann V. Nature Mater. 2002;1:12–13. doi: 10.1038/nmat707. [DOI] [PubMed] [Google Scholar]
- Lètant SE, Hart BR, Van Buuren AW, Terminello LJ. Nature Mater. 2003;2:391–395. doi: 10.1038/nmat888. [DOI] [PubMed] [Google Scholar]
- Lim C, Slack S, Ufer, Stefan, Lindner E. Pure Appl. Chem. 2004;76:753–764. [Google Scholar]
- Matveeva EG, Gryczynski Z, Malicka J, Lukomska J, Makowiec S, Berndt KW, Lakowicz JR, Gryczynski I. Anal. Biochem. 2005;344:161–167. doi: 10.1016/j.ab.2005.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medintz I. Nature Mater. 2006;5:842–842. doi: 10.1038/nmat1776. [DOI] [PubMed] [Google Scholar]
- Nan X, Shumaker-Parry JS, Zareie MH, Campbell CT, Castner DG. Langmuir. 2004;20:3710–3716. doi: 10.1021/la035864n. [DOI] [PubMed] [Google Scholar]
- Ouyang H, Christophersen M, Viard R, Miller BL, Fauchet PM. Adv. Funct. Mater. 2005;15:1851–1859. [Google Scholar]
- Quinn JG, O’Neill S, Doyle A, McAtamney C, Diamond D, MacCraith BD, d O‘Kennedy R. Anal. Biochem. 2000;281:135–143. doi: 10.1006/abio.2000.4564. [DOI] [PubMed] [Google Scholar]
- Rabus DG, DeLouise LA, Ichihashi, Yauhisa submitted..... [DOI] [PubMed]
- Ramanavièius A, Herberg FW, Hutschenreiter S, Zimmermann B, Lapënaitë I, Kaušaitë A, Finkelšteinas A, Ramanavièienë A. Acta Medica Lituanica. 2005;12:1–9. [Google Scholar]
- Schneider BH, Dickinson EL, Vach MD, Hoijer JV, Howard LV. Biosens. Bioelectron. 2000;15:597–604. doi: 10.1016/s0956-5663(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Shih C, Shih C, Su Y, Gerhardt R, Lin S. J. of Biomed. Mater. Res.Part. 2005;74A(3):325–337. doi: 10.1002/jbm.a.30255. [DOI] [PubMed] [Google Scholar]
- Suenaga R, Abdou NI. Lupus. 1995;4:57–62. doi: 10.1177/096120339500400112. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Parker JC. J. of Physiology. 2003;553(2):333. doi: 10.1113/jphysiol.2003.053595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinegoni C, Cazzanelli M, Pavesi L. Silicon-Based Materials and Devices. Vol. 2. Academic Press; New York: 2001. pp. 124–188. [Google Scholar]
- Weisbroth SH, Flatt RE, Kraus AL. The Biology of the Laboratory Rabbit. Academic Press; New York: 1974. pp. 62–64. [Google Scholar]