Abstract
This study evaluates the structural and biochemical alterations of the elbow capsule following trauma through microscopy and immunohistochemistry. A total of thirty-seven capsules from patients undergoing surgery for elbow contracture were compared to normal capsules of seven donors. Contracture capsules were significantly thicker than that of the controls (p<0.05) and exhibited extensive disorganization of collagen fiber bundle arrangement. Levels of specific cytokines involved in connective tissue turnover were measured. The results demonstrate that levels of the cytokines MMP-1, MMP-2, and MMP-3 were greater as compared to controls (p<0.05). This was associated with collagen disorganization, fibroblast infiltration, and in some specimens, lymphocytic infiltration in the capsular tissue. In contracture specimens, there was a localization of TIMP-2 staining only in the vicinity of the synovial membrane and in blood vessels. Immunohistochemistry for collagen type III showed greater presence in the control capsules compared to contracture capsules. This study demonstrates pathologic thickening, disorganization of the collagen fiber arrangement as well as involvement of cytokines in the pathology of posttraumatic contracture of the elbow. However, the mechanism of contracture tissue formation probably differs from that observed in wound healing due to the association of decreased collagen type III with contracture.
Keywords: elbow, elbow contracture, matrix metalloproteinase, collagen type III
INTRODUCTION
Post-traumatic stiffness of the elbow is common.29 While this may be multifactorial, the capsule clearly plays a role.4,5,15,25 Release or excision of the elbow capsule is necessary when treating arthrofibrosis surgically, and diminished compliance of the capsular tissue itself has been documented following trauma.10
To our knowledge, only one study has examined the physiologic profile of elbow contracture.11 These investigators demonstrated relative increases in the mRNA levels, as assessed through reverse transcription-polymerase chain reaction) for collagens Types I, III, and V, and various matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases (MMPs) known to be involved in connective tissue turnover. The MMPs, as matrix degrading enzymes, would be expected to be integral players in this turnover and, therefore, may play a role in capsular alterations following trauma.6,28
This is the first study to evaluate both the morphological characteristics and the profiles of specific MMPs in the capsule of contracted elbows. Specifically, capsular thickness, collagen fiber organization, immunohistochemical profiles of cytokines MMP-1, MMP-2, and MMP-3, tissue inhibitor of matrix metalloproteinase (TIMP)-2 and collagen type III were studied in an effort to identify the structural and biochemical alterations of the elbow capsule that lead to pathologic limitation of joint motion following trauma.
MATERIALS AND METHODS
Specimens
Thirty-seven anterior elbow capsules were collected, with institutional IRB approval at the time of joint release for post-traumatic contracture. Patients ranged in age from 13 to 60 with a mean of 39 years. There were 13 females and 24 males. Preoperatively, elbow extension averaged 35° (range 40–80°), and flexion averaged 94° (range 80 to 115°).
All joints were exposed through an open lateral approach. The interval between the extensor carpi radialis longus and brevis was identified and the brachialis muscle elevated to expose the anterior joint capsule.8,9 Pericapsular adhesions were released, and the anterior capsule was completely resected for evaluation. Capsules from seven cadaveric elbows with no history of trauma or pathology were harvested as controls. The mean age of these cadaveric donors at death was 63 years.
Histological Preparation and Polarizing and Ordinary Light Microscopy
Specimens were placed in 10% neutral buffered formalin, paraffin embedded, and sectioned to 5-µm thickness. For polarizing microscopy, three sections from each specimen were treated with 2.0 mg bovine testicular hyaluronidase (Sigma-Aldrich, St. Louis, MO) in 1.0 ml 0.1M phosphate buffer at pH 6.0 to remove chondroitin sulfate molecules of the matrix. Sections were subsequently stained with a 0.1% sirius red F3B (Polysciences, Warrington, PA), dissolved in saturated picric acid, washed and dehydrated.31
The sections were evaluated with polarizing microscopy for comparison of collagen fiber orientation as obtained through small-angle X-ray diffraction. Birefringence was determined by rotating the slide in two opposite directions. The presence or absence of birefringence suggests the orientation of the collagen fibers.14 Three sections from each tissue block were analyzed. For ordinary light microscopy, the polarizer was removed from the light path.
In addition, for ordinary light microscopy, three sections from each tissue block were stained with hematoxylin and eosin for cellular features. Capsular and synovial cells were identified by their light microscopic appearance. Capsular thickness was measured using a micrometer setting.
Immunohistochemistry
Immunohistochemistry was performed on paraffin sections from specimens using standard immunoperoxidase techniques. Endogenous peroxidase activity was blocked with 0.3 H2O2 in methanol for 10 minutes. Non-specific binding was blocked with 10% normal goat serum. The sections were then incubated overnight with the appropriate dilution of primary antibody at 4°C. For negative controls, the primary antibodies were replaced with buffered saline. The ImmunoPure ABC Alkaline Phosphatase Staining Kit (Pierce, Rockford, IL) was utilized with a biotinylated secondary antibody. The color reaction product was developed alkaline phosphatase-based one-step NBT/BCIP substrate (ImmunoPure ABC kit; Pierce). To inhibit endogenous alkaline phosphatase activity, ImmunoPure Phosphatase Suppressor (levamisole, Pierce) was used. Antibodies to MMP-1, MMP-2, MMP-3, and TIMP-2, were used.
The distribution of collagen type III was investigated with the use of purifed rabbit polyclonal anti-human collagen type III (Cedarlane Laboratories Limited, Ontario, Canada) at a dilution of 1:100.
Negative control immunoglobulins were used with each specimen to evaluate nonspecific staining.
Grading of the Immunostained Sections
Each of the immunostained sections was graded by an examiner who was blinded to the clinical diagnosis or control status of the specimens. Scores for cellular staining are as follows: 0 = less than 30% specific staining for cells or matrix and 1 = staining in 30% or more of the cells or matrix within the sections. Specific staining in blood vessels was given a score of 0 if fewer than 30% of the blood vessels within the sections were stained and 1 if 30% or more were stained. The 30% level was chosen since anything below 30% was considered to be minimally stained. The Mann-Whitney test was used to determine significance between control and contracture specimens.
The anti-collagen type III stained sections were graded in their entirety according to the following scale: grade 0 = no staining, grade 1 = weak staining, and grade 2 = strong staining.
Data examination
The data was examined for relationships between the presence/level of MMP, TIMP, and collagen type III staining and the following parameters: patient age, gender, previous steroid injection, collagen disorganization, lymphocytic infiltration, and level of pre-operative motion.
RESULTS
Gross Morphological Characteristics
The thickness of the elbow capsules ranged from 0.5 mm for a control capsule to 8 mm for a contracture capsule. The mean thickness of the control capsules was 0.6 mm ± 0.2 mm and, for the contracture specimens, was 4.0 mm ± 2.1. Grossly, the control capsules appeared thin and of uniform thickness throughout, whereas the contracture specimens were thick and displayed an inconsistency in thickness within the length of the sample.
Light Microscopy
Hematoxylin and Eosin-stained sections and polarizing light microscopy of all controls capsules revealed a well organized, parallel arrangement of collagen fibers with intervening fibroblasts (Fig. 1). No lymphocytes were present in any of the sections from control specimens. All contracture capsules revealed thickening of the tissue with extensive disorganization of the collagen fiber bundle arrangement as assessed with polarized light microscopy (Fig. 1). More fibroblasts, particularly in large groups, were also found in these specimens compared to the controls. Additionally, five of these specimens displayed moderate to severe lymphocyte infiltration (Fig. 2). No correlation was identified between these cases and those without lymphocytes in any clinical parameter (age, gender, time from injury, preoperative motion, etc.). No difference in the level of collagen disorganization between males and females was observed.
Figure 1.
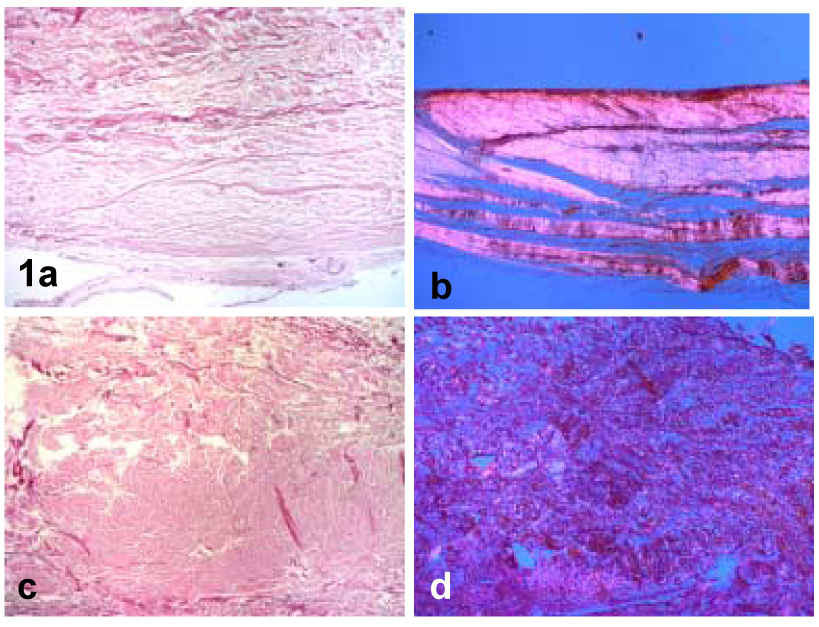
(a) Control specimen viewed by ordinary light and (b) polarizing light. (c) Contracture specimen viewed by ordinary light and (d) polarizing light. Note the well-organized bundles of collagen fibers with intervening fibroblasts in the control specimens versus the collagen disorganization in the contracture specimens (40x).
Figure 2.
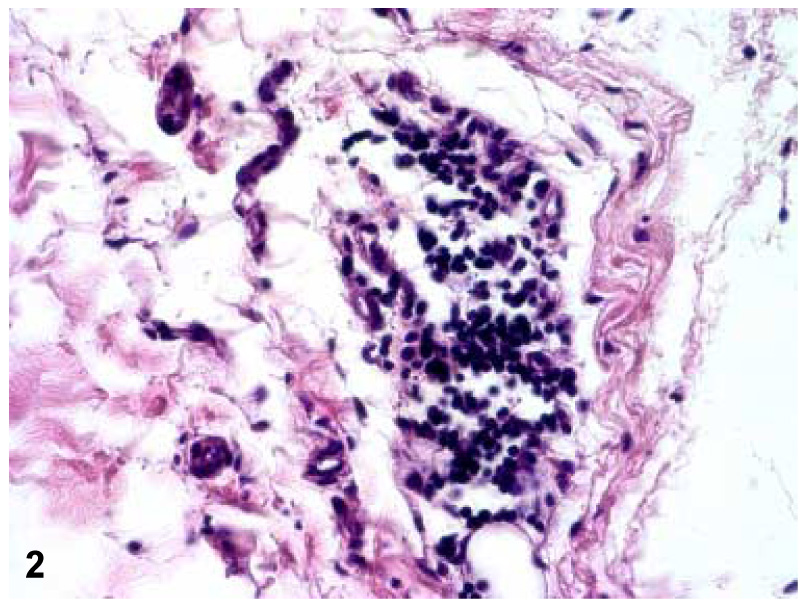
A 100x magnification of one of the five contracture specimens displaying lymphocyte infiltration as can be seen in the center of the field.
Immunohistochemical Staining
The results of the percentage of control and patient elbow capsules with positive staining (more than 30% of the cells and/or matrix within all sections) for each of the cytokines are shown graphically in Figure 3. MMP-1, MMP-2, and MMP-3 staining were significantly increased in the contracture group compared to the controls (p<0.05).
Figure 3.
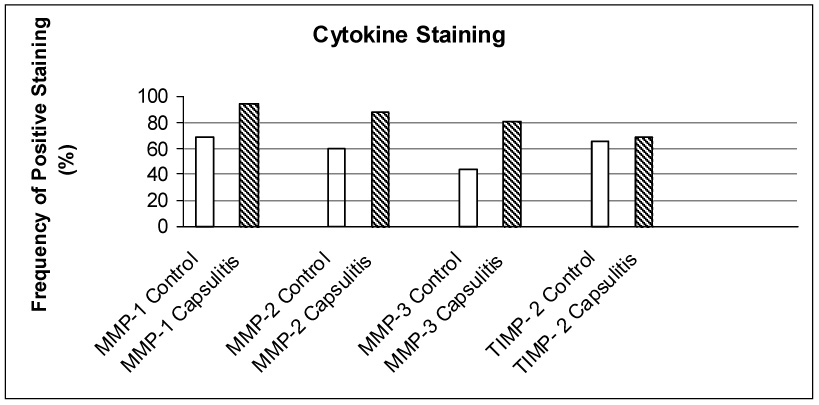
Percentage of control and contracture specimens displaying more than 30% of the cells and/or matrix within all sections for MMP-1, MMP-2, MMP-3 and TIMP-2. For each of the MMPs, there was significantly greater staining in the contracture specimens compared to the controls (p<0.05). Although the TIMP-2 specimens showed no difference in the overall level of staining between the two groups, the capsules specimens showed TIMP-2 staining primarily in the blood vessels and synovium.
Examples of typical specimens with each of the MMP cytokine stains are shown in Figure 4. MMP-1 staining was found in the fibroblast cell membrane and in the matrix within the control and contracture specimens, as well as in the synovial cells of four and in the blood vessels of two contracture specimens. The frequency of antibody staining for MMP-1 was significantly higher in the contracture specimens.
Figure 4.
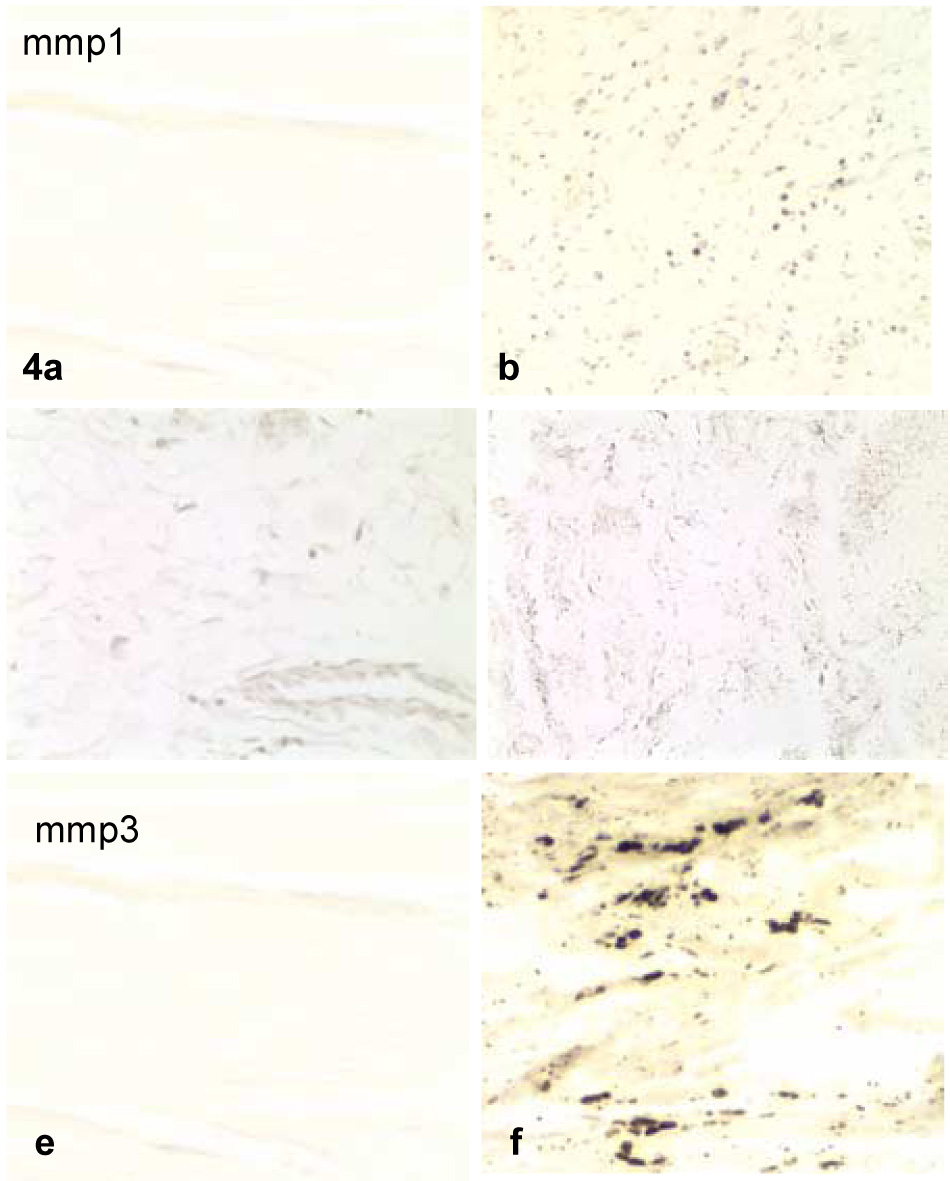
Examples of control (a,c,e) and contracture (b,d,f) specimens displaying more than 30% of the cells and/or matrix within all sections for MMP-1 (a,b), MMP-2 (c,d), and MMP-3 (e,f). The contracture specimens displayed distinctively more staining than the controls for each cytokine.
MMP-2 staining was found in the fibroblast cell membrane and in the matrix within the control and contracture specimens. In addition, MMP-2 staining was found in and near the synovial cells of two specimens. These specimens were not the same as those whose synovial cells stained for MMP-1 as noted above. The frequency of antibody staining for MMP-2 was significantly higher in the contracture specimens.
Positive staining for MMP-3 was significantly greater in the contracture specimens where it was found primarily in and near the synovial membrane and blood vessels. A lesser amount of staining could be found among the cells of the capsule. Each of the five contracture capsules having the presence of lymphocytes also showed MMP staining. No differences were identified when these were compared to the other contracted capsules.
Staining for TIMP-2 was fairly evenly dispersed in the control specimens, while the contracture specimens displayed concentrated staining only in and near the synovial membrane and in the blood vessels (Figure 5). No correlation was identified between MMP and TIMP-2 staining and any clinical measure (age, time from injury, preoperative motion, etc.)
Figure 5.
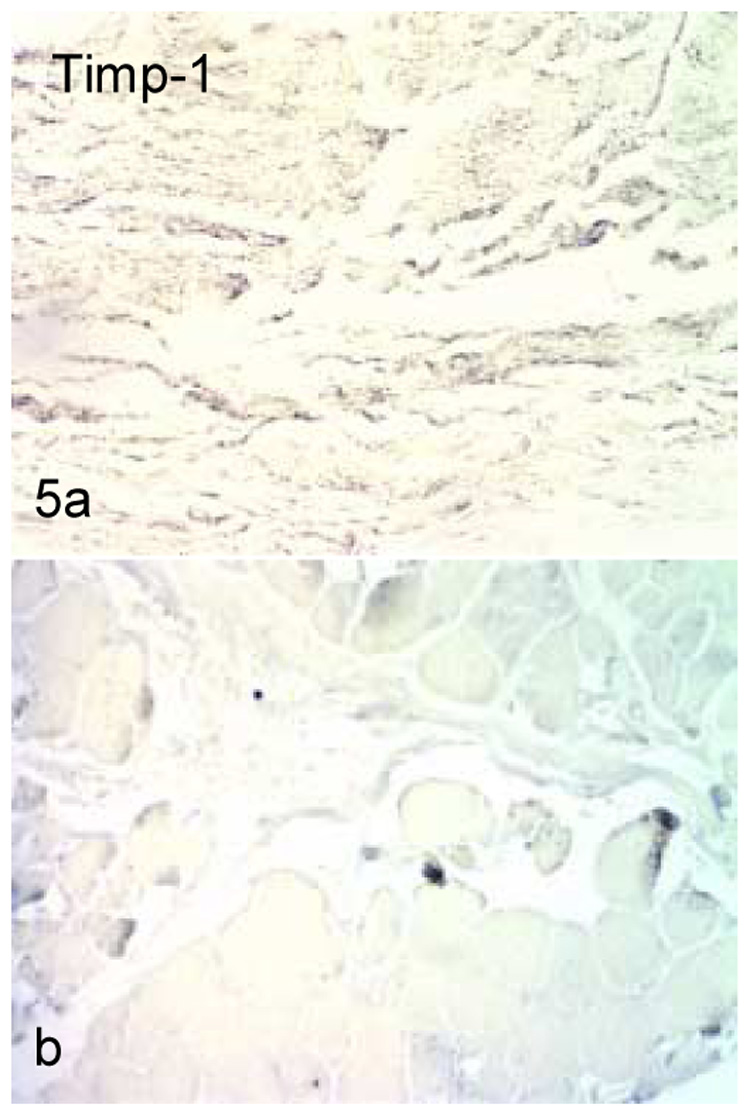
TIMP-2 staining in control (a) and contracture (b) specimens. Staining was dispersed throughout the tissue in the control tissues, but was limited to the blood vessels and synovial membrane in the contracted capsules.
Staining for collagen type III was much more prominent in the control capsules than in the contracture capsules, with the mean score for the control capsule at 2 + 0 and the mean score for the contracture capsules at 1 + 0 (p<0.05). It was quite apparent that every control capsule displayed stronger staining throughout the tissue (intima and subintima) compared to that found in every contracture sample (Figure 6a,b). This distinction could be observed at both low (20x) and high (400x) magnification levels. Since the level of detectable immunohistochemical staining was approximately the same for all contracture specimens, no correlation could be made between this parameter and levels of MMP staining or pre-operative motion,
Figure 6.
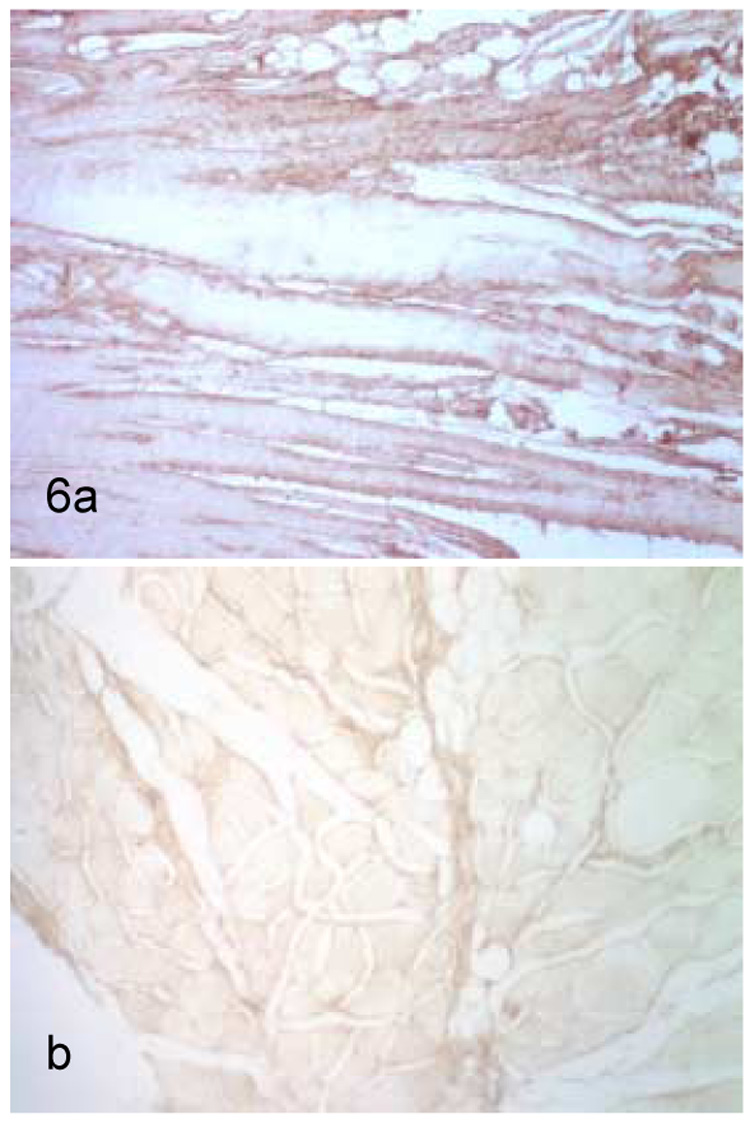
Staining for collagen type III in control (a) and contracture (b) specimens. Staining was greater in every control specimens as compared to every contracture specimen. Time of surgical excision of contracture from identifiable related trauma had no relationship to level of collagen type III staining.
No differences in any MMP staining between males and females were observed.
MMP Staining Related to Collagen Disorganization
Because the gradations used to determine MMP staining were general and the use of polarized light microscopy is not quantitative, it was impossible to make specimen determinations as to a relationship between the level of staining and the degree of collagen organization. Furthermore, since all contracture specimens showed a great deal of collagen disorganization, quantitative distinctions were not possible.
Previous steroid injection
Ten (27% of total sample) contracture patients had been administered a previous steroid injection as follows: cortisone (N = 7) and prednisone (N = 3). However, no correlation was found between the level of MMP or TIMP staining or the extent of the collagen disorganization and whether or not the patient was previously treated with steroid. Furthermore, there were capsules from patients receiving previous treatment and from patients not receiving previous treatment that stained for MMP and/or TIMP.
The time that elapsed between steroid injection and surgery (at which time the capsular tissue was taken) ranged from 1.5 to 36 months with a mean of 7.6 months. There was no correlation between whether or not the patient had a previous steroid injection nor with the time that elapsed with the degrees of motion measured just prior to surgery, with MMP or TIMP levels, or presence/absence of collagen type III.
DISCUSSION
The elbow is particularly prone to stiffness following traumatic injury. This propensity has been attributed to several factors, including the congruous nature of the joint, the presence of three articulations within a synovial lined cavity, and the close relationship of the joint capsule to the intracapsular ligaments and surrounding muscles.1,2,3,7,15,17,19,20,22,25,30 In addition, the elbow capsule itself plays a role in arthrofibrosis. Gallay et. al10 found the stiff elbow capsule to be one sixth as compliant as that of the normal elbow, concluding that it is altered structurally and biomechanically. They hypothesized that these changes involved hypertrophy of the capsule or a change in its collagen content.
The joint capsule of the elbow is composed primarily of collagen fibers and the fibroblasts responsible for the production and maintenance of these fibers. The normal anterior capsule is quite thin and compliant, with well organized collagen fibers. Every contracture specimen was characterized by a marked thickening, on the average of approximately seven times (and up to 13 times) that of the normal capsule. This thickening was accompanied by collagen disorganization and fibroblast infiltration (Figure 1). The presence of lymphocytic infiltration in some of the specimens suggests that an inflammatory process was active to some degree in these cases.
In contracture of the shoulder, it is clear that pathological molecular and structural changes occur within the capsule and synovial membrane of the joint. Rodeo et al. evaluated immunolocalization of cytokines and growth factors and concluded that contracture of the shoulder is both an inflammatory and a fibrotic condition.24 Matrix metalloproteinases are cytokines known to be involved in connective tissue turnover. The MMPs, as a group of zinc endopeptidases, degrade different components of the extracellular matrix. MMP-1 (interstitial collagenase) is known to cleave collagens I, II, II, VII, and X. MMP-2 (gelatinase A), cleaves collagens I, IV, V, VII, and X, while MMP-3, (stromelysin 1) cleaves collagens III, IV, IX, and X among other substrates. TIMP 2 binds to MMP2, therefore, preventing its proteolytic action.5,13,21,26,27,32
The presence of frozen shoulder in individuals given an inhibitor of MMP’s (TIMP) for malignancy suggests a role for MMP and its relationship to TIMP in the pathology of contracture of the shoulder.12 Although a direct comparison of post-traumatic contracture of the elbow and contracture of the shoulder cannot be made, we did find the cytokines, MMP-1, MMP-2, MMP-3, and TIMP, to be present in greater levels in contracture elbow capsules compared to control specimens. These increased levels were seen in association with collagen disorganization and a fibroblastic response. While a specific cause and effect cannot be concluded, these abnormal cytokines may be involved in the remodeling or repair process following trauma. A delicate balance between MMPs and their inhibitors (TIMPs) is an essential element in the homeostasis of the connective tissue matrix. Any connective tissue breakdown, remodeling, or repair must, therefore, involve the production or release of at least some MMPs and a decrease in their inhibitors.
Our findings of increased MMP in contracture elbow capsules is in agreement with the results of Hildebrand et al.11 They identified relatively higher mRNA levels of MMPs (as determined through reverse transcription-polymerase chain reaction) in elbow contracture specimens compared with controls. However, as opposed to these authors, we did not demonstrate an overall decrease in the relative levels of TIMP-2 in the contracture specimens. A difference was identified in the localization of TIMP-2, whereby the contracture specimens displayed concentrated staining only in and near the synovial membrane and in the blood vessels with little or no staining elsewhere. The control capsules, in turn, displayed a rather evenly distributed TIMP staining throughout the tissue. Thus, we thus did observe relatively less TIMP staining in the collagen tissue itself.
Because immunohistochemistry cannot be evaluated in a particularly quantitative manner, the results may not be exact. In fact, we were unable to determine an association between MMP staining and the degree of collagen disorganization which was seen in all contracture specimens. We found nearly all of the contracture capsules to have more than 30% of the cells stained for antibodies against the MMPs studied here and without large differences between them. Additionally, because the data are not truly quantitative, correlations between the level of staining and the presence of inflammatory cells could only be made to the extent that the five contracture capsules (of a total of 37) that displayed the presence of lymphocytes also showed anti-MMP staining. However, not all anti-MMP stained tissues displayed lymphocytes.
Interestingly, the five contracture capsules with the presence of lymphocytes were also among the 6 thickest capsules. This, however, had no apparent relationship to the degree of preoperative motion or the time since the initial injury. In fact, one capsule displaying heavy lymphocytic infiltration was surgically removed from the patient ten years after the related trauma. Thus, although we were not able to study the capsules over time, we found no specific evidence for an inflammatory based process associated with the observed structural changes in the elbow capsule matrix.
It has previously been demonstrated that collagen types I, III, and VI are present in each part of the capsule of the human finger joint16, and thus, these molecules would be expected to be present in the normal elbow capsule. However, the determination of levels of collagen type III is of particular significance in the profiling of the pathologic changes associated with contracture in that an increase in the level of collagen Type III is associated with wound healing 23,26 This response involving tissue turnover and remodeling might be thought to be a possible mechanism for elbow contracture, particularly following injury. However, we found that immunohistochemical staining levels of collagen type III were consistently weaker in the contracture specimens than in the control capsules. This is in agreement with work carried out by Matsumoto et al.18 demonstrating that a lower level of collagen type III was associated with knee joint contracture in a rat model of knee joint immobilization. Taken together, these results suggest a mechanism of contracture tissue formation that differs from that observed in wound healing.
No gender differences were noted in any of the parameters studied. It is both possible that there truly are no differences, that our sample size was not large enough to determine gender differences, or that immunohistochemistry is not sensitive enough to determine slight differences.
Last, although the mean age of the contracture patients and controls was significantly different (36 vs. 63, respectively), we do not suspect this to contribute to any differences due to age, since all control capsules displayed the normal morphological appearance that is representative of this type of normal tissue. Additionally, no differences in any parameters were noted across age within the contracture group which ranged from 13 to 60 years.
In summary, the results of the present study are the first to characterize the histological profile in post-traumatic contracture of the elbow capsule and associate this to the presence of MMPs and their inhibitors. Collagen type III is present in the normal elbow capsule, but by comparison, its immunohistochemical staining is consistently diminished in the contracture capsule. Further characterization is essential to identify elbow-specific processes and to propose treatment strategies to decrease this pathological alteration of capsular tissue. Although difficult to obtain, the evaluation of elbow capsules from individuals suffering trauma without a subsequent joint contracture might be very helpful in understanding the normal and pathological processes involved.
ACKNOWLEDGEMENTS
This work was supported, in part, by NIH grant RO1-48292. We would like to thank Angela Babbo, M.D. for her role in the histological preparation of the specimens and Kristin R.L. Abboud, OTR,CHT for her review of the clinical material and data collection.
Supported, in part, by NIH grant RO1 AR48292.
REFERENCES
- 1.Akeson WH. An experimental study of joint stiffness. J Bone Joint Surg Am. 1961;43:1022–1034. [PubMed] [Google Scholar]
- 2.Akeson WH, Amiel D, Woo SL. Immobility effects on synovial joints: The pathomechanics of joint contracture. Biorheology. 1980;17:95–110. doi: 10.3233/bir-1980-171-212. [DOI] [PubMed] [Google Scholar]
- 3.Akeson WH, Amiel D, Mechanic GL, Woo SL, Harwood FL, Hamer ML. Collagen cross-linking alterations in joint contractures: Changes in the reducible cross-links in periarticular connective tissue collagen after nine weeks of immobilization. Connect Tissue Res. 1977;5:15–19. doi: 10.3109/03008207709152607. [DOI] [PubMed] [Google Scholar]
- 4.Aldridge JM, 3rd, Atkins TA, Gunneson EE, Urbaniak JR. Anterior release of the elbow for extension loss. J Bone Joint Surg Am. 2004;86:1955–1960. doi: 10.2106/00004623-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Billinghurst RC, Pidoux I, Ionescu M, Reiner A, Pidoux I, Webber C, et al. Membrane-type matrix metalloproteinast digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;292:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 6.Bunker TD, Reilly J, Baird KS, Hamblen DL. Expression of growth factors, cytokines and matrix metalloproteinases in frozen shoulder. J Bone Joint Surg. 2000;82B:768–773. doi: 10.1302/0301-620x.82b5.9888. [DOI] [PubMed] [Google Scholar]
- 7.Byrd JW. Elbow arthroscopy for arthrofibrosis after type I radial head fractures. Arthroscopy. 1994;10:162–165. doi: 10.1016/s0749-8063(05)80087-8. [DOI] [PubMed] [Google Scholar]
- 8.Diwan DB, Murrell GAC. An evaluation of the effects of the extent of capsular release and of postoperative therapy on the temporal outcomes of contracture. J Arthrosc Rel Surg. 2005;21:1105–1113. doi: 10.1016/j.arthro.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MS, Hastings H. Post-Traumatic Contracture of the Elbow: Operative Release using a Lateral Collateral Ligament Sparing Approach. J Bone Joint Surg. 1998;80B:805–812. doi: 10.1302/0301-620x.80b5.8528. [DOI] [PubMed] [Google Scholar]
- 10.Gallay SH, Richards RR, O’Driscoll SW. Intraarticular capacity and compliance of stiff and normal elbows. Arthroscopy. 1993;9(1):9–13. doi: 10.1016/s0749-8063(05)80336-6. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrand KA, Zhang M, Hart DA. High rate of joint capsule matrix turnover in chronic human elbow contractures. Clin Orthop Rel Res. 2005;439:228–234. doi: 10.1097/01.blo.0000177718.78028.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchinson JW, Tierney GM, Parsons SL, Davis RC. Dupuytren’s disease and frozen shoulder induced by treatment with a matrix metalloproteinase inhibitor. The Journal of Bone and Joint Surgery. 1998;80B:907–908. doi: 10.1302/0301-620x.80b5.8464. [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey JJ. Interstitial Collagenases. In: Parks WC, Mecham RP, editors. Matrix Metalloproteinases. New York: Academic Press; 1998. pp. 15–42. [Google Scholar]
- 14.Junquiera LCU, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 15.Jupiter JB, O’Driscoll SW, Cohen MS. The Assessment and Management of the Stiff Elbow. In: Ferlic DC, editor. Instructional Course Lectures. Vol. 52. Rosemont: American Academy of Orthopaedic Surgeons; 2003. pp. 93–112. [PubMed] [Google Scholar]
- 16.Lewis AR, Ralphs JR, Kneafsey B, Benjamin M. Distribution of collagens and glycosaminoglycans I the joint capsule of the proximal interphalangeal joint of the human finger. Anat Rec. 1998;250:281–291. doi: 10.1002/(SICI)1097-0185(199803)250:3<281::AID-AR3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Mansat P, Morrey BF. The column procedure: a limited lateral approach for extrinsic contracture of the elbow. J Bone Joint Surg Am. 1988;80(11):1603–1615. [PubMed] [Google Scholar]
- 18.Matsumoto F, Trudel G, Uhthoff HK. High collagen type I and low collagen type III levels in knee joint contracture. Acta Orthop Scan. 2002;73:335–343. doi: 10.1080/000164702320155365. [DOI] [PubMed] [Google Scholar]
- 19.Morrey BF. Surgical Treatment of extraarticular albow contracture. Clin Orthop Relat Res. 2000;370:57–64. doi: 10.1097/00003086-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Morry BF. The posttraumatic stiff elbow. Clin Orthop Relat Res. 2005;431:26–35. [PubMed] [Google Scholar]
- 21.Nagase H. Stromelysins 1 and 2. In: Parks WC, Mecham RP, editors. Matrix Metalloproteinases. New York: Academic Press; 1998. pp. 43–84. [Google Scholar]
- 22.Nickolson GP. Arthroscopic capsular release for stiff shoulders: effect of etiology on outcomes. Arthroscopy. 2003;19:40–49. doi: 10.1053/jars.2003.50010. [DOI] [PubMed] [Google Scholar]
- 23.Robins SP, Milne G, Duncan A, Davies C, Butt R, Greiling D, James IT. Increased skin collagen extractability and proportion of collagen type III are not normalized after 6 months healing of human excisional wounds. J Invest Dermatol. 2003;121:267–272. doi: 10.1046/j.1523-1747.2003.12373.x. [DOI] [PubMed] [Google Scholar]
- 24.Rodeo SA, Hannafin JA, Tom J, Warren RF, Wickeiwicz TL. Immunolocalization of cytokines and their receptors in contracture of the shoulder. J Orthop Res. 1997;15:427–436. doi: 10.1002/jor.1100150316. [DOI] [PubMed] [Google Scholar]
- 25.Safran MR, Baillargeon D. Soft-tissue stabilizers of the elbow. J Shoulder Elbow Surg. 2004;14:179S–185S. doi: 10.1016/j.jse.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 26.Sculean A, Junker R, Donos N, Berakdar M, Brecx M, Dunker N. Immunohistochemical evaluation of matrix molecules associated with wound healing following regenerative periodontal treatment in monkeys. Clin Oral Investig. 2002;6:175–182. doi: 10.1007/s00784-002-0161-8. [DOI] [PubMed] [Google Scholar]
- 27.Seibel MJ. In: Dynamics of Bone and Cartilage Metabolism. Seibel MJ, Robins SP, Bilezikian JP, editors. Academic Press; 1999. pp. 141–147. [Google Scholar]
- 28.Suzuki K, Attia ET, Hannafin JA, Rodeo SA, Warren RF, Bhargava MM. The effect of cytokines on the migration of fibroblasts derived from different regions of the canine shoulder capsule. J Shoulder Elbow Surg. 2001;10:62–67. doi: 10.1067/mse.2001.109559. [DOI] [PubMed] [Google Scholar]
- 29.Walker-Bone K, Palmer KT, Reading I, Coggon D, Cooper C. Prevalence and impact of musculoskeletal disorders of the upper limb in the general population. Arthritis Rheum. 2004;51:642–651. doi: 10.1002/art.20535. [DOI] [PubMed] [Google Scholar]
- 30.Weiss AP, Sachar K. Soft tissue contractures about the elbow. Hand Clin. 1994;10:439–451. [PubMed] [Google Scholar]
- 31.Williams JM, Uebelhart D, Thonar EJ, Kocsis K, Modis L. Alteration and recovery of the spatial orientation of the collagen network of articular cartilage in adolescent rabbits following intra-articular chymopapain injection. Connect. Tissue Res. 1996;34:105–117. doi: 10.3109/03008209609021496. [DOI] [PubMed] [Google Scholar]
- 32.Woessner JF, Nagase H. Matrix Metalloproteinases and TIMPS. In: Woessner JF, Nagase H, editors. Matrix Metalloproteinases and TIMPS. Oxford: Oxford University Press; 2000. pp. 1–70. [Google Scholar]


