Abstract
In developing male embryos, the female reproductive tract primordia (Müllerian ducts) regress due to the production of testicular anti-Müllerian hormone (AMH). Because of the association between secreted frizzled-related proteins (SFRPs) and apoptosis, their reported developmental expression patterns and the role of WNT signaling in female reproductive tract development, we examined expression of Sfrp2 and Sfrp5 during development of the Müllerian duct in male (XY) and female (XX) mouse embryos. We show that expression of both Sfrp2 and Sfrp5 is dynamic and sexually dimorphic. In addition, the male-specific expression observed for both genes prior to the onset of regression is absent in mutant male embryos that fail to undergo Müllerian duct regression. We identified ENU-induced point mutations in Sfrp5 and Sfrp2 that are predicted to severely disrupt the function of these genes. Male embryos and adults homozygous for these mutations, both individually and in combination, are viable and apparently fertile with no overt abnormalities of reproductive tract development.
Keywords: SFRP, Müllerian duct regression, mesonephros, mouse sexual development, ENU mutagenesis
INTRODUCTION
WNT signaling influences a variety of developmental processes, ranging from specification of cell fate and cell polarity to apoptosis. In the canonical pathway, WNT ligand binds to its target Frizzled (Fz) receptor and initiates an intracellular signal resulting in the nuclear accumulation of nonphosphorylated β-catenin and consequent activation of TCF/LEF-dependent target genes (Wodarz and Nusse, 1998). Secreted frizzled-related proteins (SFRPs) are a family of secreted glycoproteins that contain a cysteine-rich domain (CRD) at their N-terminus that is homologous to the Fz CRD (Jones and Jomary, 2002; Kawano and Kypta, 2003). Several lines of evidence suggest that SFRPs can antagonize WNT-mediated signaling by direct competitive interaction with WNT ligands via the CRD (Leyns et al., 1997; Wang et al., 1997; Dann et al., 2001) or by formation of nonsignaling complexes with Frizzled proteins (Bafico et al., 1999). A possible role for SFRPs in regulating the impact of the WNT signaling pathway in a variety of tissues was postulated soon after their discovery (Moon et al., 1997), though direct evidence supporting a developmental role in vivo is still scant.
In the mouse, five members of the Sfrp gene family have been identified using a variety of approaches including database searches (Rattner et al., 1997), a screen for genes containing signal sequences (Shirozu et al., 1996), co-purification with HGF/SF (Finch et al., 1997), and a screen for genes regulating apoptosis in cell culture (Melkonyan et al., 1997). The latter screen identified SFRP1, SFRP2, and SFRP5, and implicated these in the modulation of cellular responses to cytotoxic signals. Phylogenetic analysis using protein sequence comparisons also indicates that SFRP1, SFRP2, and SFRP5 are the most closely related members of the family. An analysis of Sfrp1 and Sfrp2 during mouse embryogenesis revealed widespread and overlapping expression patterns (Leimeister et al., 1998). Sites of Sfrp2 expression include the comma- and S-shaped bodies of the kidney, the retina of the eye and the forebrain, hindbrain, and neural tube. Sfrp1 was detected in the kidney, eye, brain, teeth, salivary gland, and small intestine. Sfrp5 is expressed in the anterior visceral endoderm and foregut endoderm from 5.5 dpc of mouse development and later in the dorsal neural tube and forebrain (Finley et al., 2003). Sfrp5 is also expressed at high levels in the retinal pigment epithelium of the eye, in a manner complementary to that of Sfrp2, which is restricted to cells of the inner nuclear layer (Chang et al., 1999). It has been suggested that the expression of Sfrp2 and Sfrp5 in this fashion reflects a role in the establishment of photoreceptor cell polarity or apoptosis during retinal development. A role for Sfrp2 in modulation of apoptosis has also been described in the avian hindbrain (Ellies et al., 2000).
Programmed cell death is a common event during mammalian development, where it results in the formation and deletion of structures, the control of cell numbers, and the elimination of abnormal cells (Baehrecke, 2002). An example of developmentally regulated deletion of a structure through apoptosis is the regression of the Müllerian duct in male embryos (Dyche, 1979; Roberts et al., 1999; Jamin et al., 2002; Josso and Clemente, 2003; Kobayashi and Behringer, 2003; MacLaughlin and Donahoe, 2004). The Müllerian ducts form adjacent to the Wolffian ducts from approximately 11.5 dpc in the mouse embryonic mesonephros through invagination of the coelomic epithelium. In females, these ducts differentiate into the oviducts, uterus, cervix, and upper vagina of the reproductive tract. However, in male embryos the Müllerian duct regresses due to the action of testicular anti-Müllerian hormone (AMH; also known as Müllerian inhibiting substance, MIS), which signals through its type II receptor (AMHR2) on the periductal mesenchymal cells to result in epithelial cell regression (Behringer et al., 1994; Mishina et al., 1996). Disruption of the basement membrane surrounding ductal epithelial cells is an important part of this process and is associated with the appearance of apoptotic cells and the transformation of healthy neighbors into migratory mesenchymal cells (Allard et al., 2000). One candidate for a molecule activated by AMH signaling and responsible for basement membrane disruption is the matrix metalloproteinase MMP2 (Roberts et al., 1999). However, it still remains to be determined precisely what are the paracrine signals produced by mesenchymal cells and what changes these induce in the underlying epithelium that result in regression of the Müllerian duct.
WNT signaling is required for Müllerian duct regression and female reproductive tract development more generally. Female mice homozygous for a null allele of Wnt4 lack a reproductive tract due to the failure of the Müllerian duct to form (Vainio et al., 1999). Similarly, embryonic Müllerian duct extension is blocked in the absence of WNT9B (Carroll et al., 2005), resulting in mutant females that lack a uterus and upper vagina. Male mice lacking WNT7A fail to undergo Müllerian duct regression due to the absence of Amhr2 expression in ductal mesenchyme (Parr and McMahon, 1998; Parr et al., 1998). Females lacking WNT7A also exhibit a variety of abnormalities suggesting that WNT7A signaling maintains the molecular and morphological boundaries of distinct cellular populations along the anteroposterior and radial axes of the female reproductive tract (Miller and Sassoon, 1998).
Due to the association between SFRPs and apoptosis, their reported developmental expression patterns and the broader role of WNT signaling in female reproductive tract development, we examined expression of Sfrp2 and Sfrp5 during development of the Müllerian duct in male (XY) and female (XX) mouse embryos. Here, we describe the expression of these genes during Müllerian duct development from the stage immediately prior to the onset of male-specific regression and show that Sfrp2 and Sfrp5 are expressed in a sexually dimorphic manner. In addition, we show that the male-specific expression patterns observed for these two genes prior to the onset of regression is lost in mutant male embryos that fail to undergo regression. We have previously identified ENU-induced point mutations in both Sfrp2 and Sfrp5 that are predicted to disrupt function (Quwailid et al., 2004). Here, we report that male embryos and adults homozygous for these mutations, both individually and in combination, are viable and fertile with no overt abnormalities of reproductive tract development.
MATERIALS AND METHODS
Expression Analysis
Wild-type embryos were generated by timed matings of C3H/HeH females and 101 males. Pairs were set up at approximately 3 pm and females were checked for vaginal plugs the following morning. Noon on the day of the plug was counted as 0.5 dpc. Embryos were harvested at different times of the day and staged accurately based on the number of tail somites or limb and gonad morphology. Wholemount in situ hybridization (WMISH) analysis of explanted gonad-mesonephros pairs and sample sectioning were performed as previously described (Grimmond et al., 2000). A cDNA clone corresponding to Sfrp2 (Accession no. AI047549) was identified in the embryonic urogenital ridge library NMUR (Grimmond et al., 2000). An Sfrp5 probe was obtained as an EST (Accession no. AA797570). The Amhr2 (Mishina et al., 1996) and Lim1 (Kobayashi et al., 2004) probes utilized have been described.
Mouse Mutants Utilized and Genotyping
The postaxial hemimelia (px) mutation is caused by a 515 base pair (bp) deletion of the Wnt7a gene (Parr et al., 1998). Homozygotes were identified by polymerase chain reaction (PCR) analysis utilizing primers 5′-GGACGAGGGCTGGAAGTG-3′ and 5′-GGTAGATCCTTAGGTTTGCCAGAA-3′. Amplification with these primers gives a 654-bp product for the wild-type allele and a 139 bp-product for the mutant allele.
The identification of the Sfrp2HC50F and Sfrp5HQ27stop mutations has been described previously (Quwailid et al., 2004). Mice heterozygous for these mutations were maintained on a C3H/HeH background for at least three generations before homozygotes were generated by heterozygote intercrosses. Genotyping of mice harboring these mutations was achieved using an allelic discrimination PCR (AD-PCR) assay. This genotyping was performed using an ABI Prism 7000 Sequence Detection System according to manufacturer's guidelines. Briefly, for each mutation two allele-specific Taqman probes (wild-type and mutant) were designed using the Primer Express program (ABI). These probes each have a distinct fluorophor conjugated to their 5′ end: FAM for the wild-type probe and VIC for the mutant probe. The 3′ end of each probe is modified with a nonfluorescent quencher (NFQ) and a minor groove binder (MGB) (Applied Biosystems, Foster City, CA). PCR was performed using primers that span the site of the mutation and probe binding, in combination with the probes, in qPCR MasterMix Plus (Eurogentec, Southampton, UK). The comparative fluorescence of free FAM and VIC was measured during and after PCR to determine the genotype of the sample.
Mice doubly homozygous for the Sfrp2HC50F and Sfrp5HQ27stop mutations were generated by crossing individual homozygotes (−/−, +/+) to generate doubly heterozygous mice (−/+, −/+). Males and females of this genotype were crossed to Sfrp5HQ27stop homozygotes to generate mice of the genotype −/−, −/+ (where the genotype of Sfrp5 is denoted first and that of Sfrp2 second). Mice of this genotypic class were then inter-crossed and generated doubly homozygous individuals at the predicted ratio. These mice were identified using the AD-PCR assay described above.
Histological Analysis of Adult Male Reproductive Organs and Pathology Phenotyping
A number of Sfrp2HC50F and Sfrp5HQ27stop homozygotes (and littermate controls) generated to analyze reproductive tract morphology were subsequently investigated in a wider organ (approximately 30) and tissue pathology screen according to a necropsy standard operating procedure (see http://www.eumorphia.org/EMPReSS/). Reproductive organs examined histologically included the testis and male accessory glands and the ovary, oviduct, uterus, and vagina.
To assess fertility, double homozygotes of both sexes were mated with wild-type mice of the C3H/HeH strain. Three doubly homozygous males were each mated to two C3H/HeH females. All of these females became pregnant and produced offspring; the average litter size was 6.5. In addition, three doubly homozygous females were each mated with a C3H/HeH male. All three females became pregnant and produced offspring; the average litter size was 9.3.
RESULTS
Expression of Sfrp5 and Sfrp2 During Mouse Mesonephros Development
Because morphological changes associated with the onset of Müllerian duct regression are first observed at approximately 13.5 dpc in the mouse (Dyche, 1979), we examined Sfrp gene expression in the developing male and female gonad and mesonephros from 12.5 dpc.
The onset of expression of Sfrp5 in the female mesonephros appears to be delayed with respect to that in the male (Fig. 1). Sfrp5 expression is first observed at approximately 13.0 dpc in the male mesonephros (Fig. 1A). All male samples harvested at 13.0 dpc show expression along the cranial end of the mesonephros, but the caudal limit of this expression varies between samples (see Fig. 3C and data not shown). This variation is likely to reflect differences in the precise developmental stage of the samples. No expression is observed in female mesonephroi of the same stage. The initial expression in males, which is somewhat diffuse and associated with the ductal epithelium and surrounding mesenchyme, becomes restricted to the epithelium by 13.5 dpc (Fig. 1B,D). Low levels of expression are first observed at approximately 13.5 dpc in female mesonephroi (Fig. 1B) and by 14.0 dpc strong Sfrp5 expression is visible in the female Müllerian duct (Fig. 1E). This expression is restricted to the ductal epithelium (data not shown). The spatial distribution of the earliest expression along the cranial-caudal axis of the female duct is variable, and there appears to be no consistent association with the cranial end. Expression in the male ductal epithelium is still observed at 14.0 dpc (Fig. 1E). This expression domain in the male duct is greatly reduced by 14.5 dpc, due to physical narrowing of the duct (Fig. 1F). This narrowing is a morphological feature of the onset of regression in males (Dyche, 1979). Strong expression is still observed in female ductal epithelium cells at 14.5 dpc (Fig. 1F). No gonadal expression was observed in any samples.
Fig. 1.
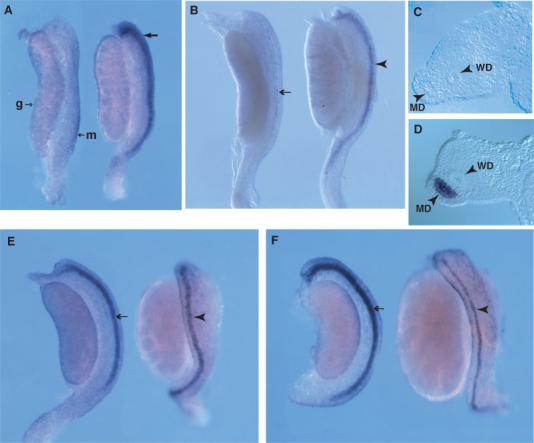
Sexually dimorphic expression of Sfrp5 in the developing mesonephros. Wholemount in situ hybridization analysis of Sfrp5 expression in male and female mesonephros and gonad samples at 13.0 (A), 13.5 (B, C, D), 14.0 dpc (E), and 14.5 dpc (F). In A, B, E, and F, the female sample is on the left, the male on the right. The mesonephros (m) and gonad (g) are labeled in the female sample in A. A: At 13 dpc, Sfrp5 demonstrates diffuse expression at the lateral edge of the male mesonephros (arrow), but is not expressed in the female mesonephros. Examination of wholemount samples indicates that Sfrp5 expression in males at this stage is detectable in the Müllerian duct (MD) epithelium at highest levels, but also in the surrounding mesenchyme and the coelomic epithelium. This localization was confirmed by transverse sections (data not shown). B: By 13.5 dpc, male-specific Sfrp5 expression is restricted to the MD epithelium (arrowhead), whilst expression in females is detected only at low levels (arrow). Transverse sections of the mesonephric region in 13.5 dpc samples confirms this epithelial expression in males (MD arrowhead in D). Expression in females is often undetectable at this stage after sectioning (MD arrowhead in C). By 14.0 dpc (E), prominent expression is detectable in the MD epithelium of females (arrow). Expression in the MD epithelium of males (arrowhead) is reduced at this stage, due to the onset of MD regression. By 14.5 dpc (F), expression has increased in the female MD epithelium (arrow) whilst the narrowing and fragmentation of expression in the male duct (arrowhead) is even more pronounced. WD, Wolffian duct.
Fig. 3.
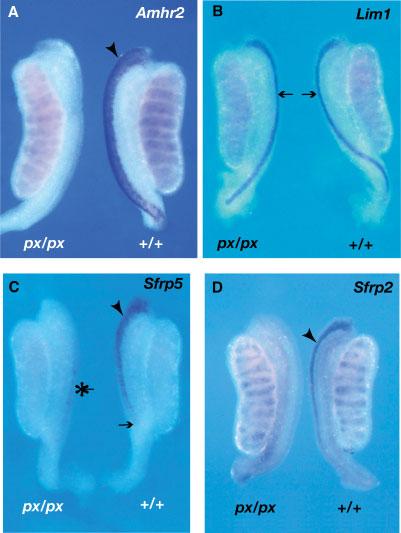
Expression of Sfrp5 and Sfrp2 in developing mesonephroi from px/px homozygotes. Expression of Amhr2 (A), Lim1 (B), Sfrp5 (C), and Sfrp2 (D) was examined by WMISH analysis of mesonephroi dissected from male embryos (13.0–13.5 dpc) homozygous for the postaxial hemimelia mutation (px/px). Mutant homozygous males fail to undergo MD regression due to the absence of AMH type 2 receptor (Amhr2) expression in the MD periductal mesenchyme. A: Amhr2 is a marker of the developing MD mesenchyme at approximately 13.5 dpc and exhibits a broad domain of expression at the lateral edge of the mesonephros in wild-type male embryos (arrowhead). In contrast, no expression is seen in mesonephroi from embryonic px/px homozygotes at the same stage. Amhr2 is also expressed in the testis cords of both wild-type and mutant samples. B: Lim1 marks the MD epithelium at approximately 13.0 dpc. This expression is clearly visible as narrow regions of expression (arrows) in both mutant and wild-type mesonephroi. This indicates that the MD epithelium is still present in px/px homozygotes at 13.0 dpc. C: At approximately 13.0 dpc, Sfrp5 expression is observed in the wild-type embryonic mesonephros as described in Figure 1. Note the relatively broad domain of expression at the cranial end of the mesonephros (arrowhead) and the absence of expression in the most caudal regions of the duct (arrow). In male mesonephroi from px/px homozygotes Sfrp5 expression is negligible at the same stage. Expression is apparent at low levels (asterisk), but does not exhibit the cranial to caudal progression apparent in wild-type males. D: At approximately 13.5 dpc, Sfrp2 is expressed in the MD epithelium of wild-type males (arrowhead) as described in Figure 2. In contrast, this expression is absent in the px/px male mesonephros. Testis cord expression is undiminished in the mutant sample.
Sfrp2 expression in the Wolffian duct and mesonephric tubules of the mouse embryo at 9.5 dpc and the metanephric kidney from 12.5 dpc has been described previously (Leimeister et al., 1998; Lescher et al., 1998). As with Sfrp5, we observe sexually dimorphic expression of Sfrp2 in the mesonephros from 13.0 dpc onwards. At 13.0 and 13.5 dpc, a narrow band of expression, corresponding to the ductal epithelium, is seen in males (Fig. 2A,B,D). Variation between embryos in the extent of the caudal progression of this expression is also observed (data not shown). In contrast, Sfrp2 in the female Müllerian duct is observed in a broader domain of expression at these stages (Fig. 2A,B). Sectioning of female samples at 13.5 dpc reveals this domain to correspond to elevated expression in the periductal mesenchyme (Fig. 2C). By 14.0 dpc, Sfrp2 is expressed in both the ductal mesenchyme and epithelium of females (Fig. 2E,F), and by 14.5 dpc, the epithelium is the most prominent site of expression (Fig. 2H). Expression in males, whilst prominent in the ductal epithelium and coelomic epithelium at 14.0 dpc (Fig. 2E,G) is almost lost by 14.5 dpc due to onset of regression of the duct (Fig. 2H). Expression in the testis cords is also prominent at 13.5 and 14.0 dpc (Fig. 2B,E).
Fig. 2.
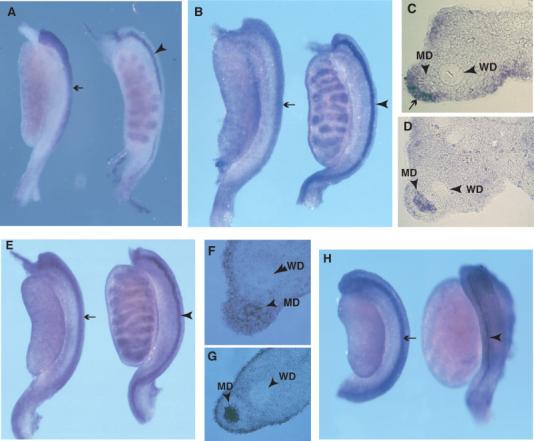
Sexually dimorphic expression of Sfrp2 in the developing mesonephros. Wholemount in situ hybridization analysis of Sfrp2 expression in male and female mesonephros and gonad samples at 13.0 (A), 13.5 (B–D), 14.0 (E–G), and 14.5 dpc (H). In A, B, E, and H, the female sample is on the left. A: A narrow band of expression in the male mesonephros (arrowhead) indicates expression in the MD epithelium at 13.0 dpc. In contrast, a broader domain of expression at the lateral edge of the mesonephros is observed in females (arrow). Low levels of expression are apparent in the testis cords. B: By 13.5 dpc, expression in the MD epithelium of males is more prominent (arrowhead), as is expression in the testis cords. This epithelial expression is confirmed by analysis of transverse sections (MD arrowhead in D). The broader domain of expression in females (arrow in B) is confirmed to be predominantly mesenchymal in origin (arrow in C). The female MD epithelium does not express Sfrp2 at high levels at this stage (MD arrowhead in C), although comparison with the adjacent Wolffian duct (WD arrowhead in C) suggests low-level expression. (D) By 14.0 dpc, the MD epithelium expression is still strong in males (arrowhead) (confirmed by section analysis in G (arrowhead)). At this stage, expression is still found in the MD periductal mesenchyme of females, but a similar level of expression in the MD epithelium is now apparent (MD arrowhead in F). H: By 14.5 dpc, strong expression is now detectable in the MD epithelium of females (arrow) and surrounding mesenchyme, whereas expression in the male MD epithelium is greatly diminished (arrowhead) due to the onset of regression. In all samples examined there appears to be low-level expression in most regions of the mesonephros and gonad, including the surrounding coelomic epithelium.
Examination of Sfrp gene Expression in a Mouse Model of Persistent Müllerian Duct Syndrome
We have shown that the Müllerian duct-associated expression profiles of both Sfrp5 and Sfrp2 are sexually dimorphic prior to the onset of overt regression in males, data that suggest a role for these genes in this process. In order to examine further the relationship between these expression patterns and Müllerian duct regression, we next examined expression in mesonephroi from embryos homozygous for the mutation postaxial hemimelia (px) (Searle, 1964). The px mutation arose spontaneously and male adult homozygotes are infertile due to the persistence of the Müllerian duct. Molecular analysis revealed that the px mutation results from a deletion of the Wnt7a gene (Parr et al., 1998), and identical persistence of the Müllerian duct is observed in male mice homozygous for a targeted Wnt7a deletion (Parr and McMahon, 1998). The failure of the Müllerian duct to regress in these mutants is due to the absence of expression of the Amhr2 gene in the periductal mesenchyme of developing mesonephroi (Parr and McMahon, 1998; Parr et al., 1998). We first confirmed the absence of Amhr2 expression in mesonephroi from 13.0 dpc px/px embryos (Fig. 3A) and the continued expression of the duct epithelium marker, Lim1 (Fig. 3B), indicating the structural integrity of the duct in the mutant embryos. We then examined the expression of Sfrp5 in the mutants. In contrast to wild-type embryos at 13.0 dpc, which show prominent Sfrp5 expression, there is virtually no expression in the px/px male embryonic mesonephros (Fig. 3C). In similar fashion, Sfrp2 expression in male px/px embryonic mesonephroi is almost undetectable (Fig. 3D). Thus, the male-specific expression of these genes in the Müllerian duct epithelium at approximately 13.0 dpc is lost in male embryos that are not competent to undergo duct regression.
Identification of Mutant Alleles of Sfrp5 and Sfrp2
Given the expression profiles exhibited by the Sfrp genes described above, we were interested in determining the phenotypic consequences of eliminating their function by chemical mutagenesis. The use of mouse mutant archives derived from the F1 male progeny of N-ethyl–N-nitrosourea (ENU)-mutagenized male mice is now a viable alternative to gene targeting for functional gene studies in the mouse (Coghill et al., 2002). We have previously described the screening of a large (>5,000 individuals) archive to identify point mutations in Sfrp5 and Sfrp2 (Quwailid et al., 2004). Briefly, we screened exon 1 of each of these genes for mutations because this exon contains the whole of the CRD, the domain thought to be responsible for WNT and Fz domain interaction (Bafico et al., 1999; Dann et al., 2001). Our studies focused on two mutations detected: Sfrp5HQ27stop and Sfrp2HC50F. The Sfrp5HQ27stop allele is caused by a C to T transition that results in the conversion of a glutamine codon to a stop codon at amino acid position 27. Given this very early chain termination, the allele is predicted to be a null mutation for Sfrp5. The Sfrp2HC50F allele is caused by a G to T transversion that results in the replacement of 1 of the 10 canonical cysteine residues of the CRD by a phenylalanine. Given the critical role played by these cysteines in the formation of disulphide bridges required for the functional conformation of the CRD (Chong et al., 2002), it is likely that Sfrp2HC50F represents a loss-of-function allele of Sfrp2.
Analysis of the Reproductive Tracts of Sfrp5HQ27stop and Sfrp2HC50F Homozygotes
Frozen sperm carrying the Sfrp5HQ27stop and Sfrp2HC50F mutations were used for in vitro fertilization in order to generate mice heterozygous for each mutation. Carriers were distinguished from wild-type sibs by the use of an allelic discrimination assay (Materials and Methods). A further three rounds of backcrossing to the C3H/HeH strain were performed in order to remove unlinked mutations. Homozygotes were then generated by intercrossing of heterozygotes. In the case of both mutant alleles, homozygotes were born in the expected Mendelian ratios (data not shown). Male Sfrp5HQ27stop and Sfrp2HC50F homozygotes appeared overtly normal and were fertile. Examination of the reproductive tracts of adult male homozygotes at between 9 and 14 weeks of age revealed no gross abnormalities and no evidence of persistent Müllerian duct derivatives (Fig. 4A-C). Histological analysis of the testis in Sfrp5HQ27stop and Sfrp2HC50F homozygous males revealed apparently normal spermatogenesis associated with intraluminal spermatozoa in the epididymis and vas deferens, and unremarkable prostate, seminal vesiscle, and coagulating glands. Examination of Müllerian duct regression in homozygous male embryos of both genotypes at 16.5 dpc also revealed no abnormalities in the temporal progression of this process (data not shown). Female homozygotes of both genotypes also appeared normal and were fertile (data not shown). A general histological screen also revealed no significant abnormalities in male or female mice, homozygous or heterozygous, that could be attributed to the Sfrp mutations.
Fig. 4.
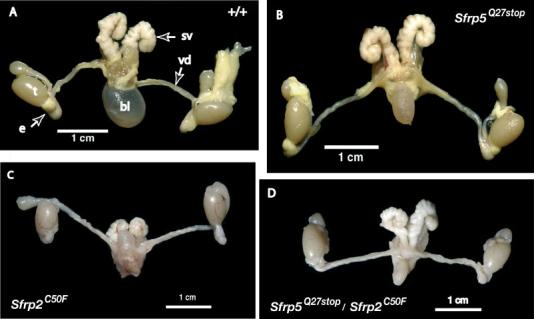
Morphological analysis of adult male reproductive organs in wild-type, Sfrp5HQ27stop homozygotes, Sfrp2HC50F homozygotes and doubly homozygous mutant mice. (A) Wholemount image of 9-week-old wild-type male reproductive organs indicating the testis (t), cauda epididymis (e), vas deferens (vd), seminal vesicle (sv), and bladder (bl). There are no observable abnormalities of the reproductive tracts in adult males of the following genotypes: Sfrp5HQ27stop/Sfrp5HQ27stop (B, 9-week-old male); Sfrp2HC50F/Sfrp2HC50F (C, 14-week-old male); Sfrp5HQ27stop/Sfrp5HQ27stop, Sfrp2HC50F/Sfrp2HC50F (double homozygote) (D, 13-week-old male).
The absence of any overt abnormalities in Sfrp5HQ27stop and Sfrp2HC50F homozygotes raises the possibility of functional redundancy between Sfrp5 and Sfrp2. To address this, we generated mice doubly homozygous for these mutant alleles, a possibility due to the fertility of both homozygotes (see Materials and Methods for breeding scheme). Double homozygotes were born in the expected Mendelian ratio. Males and females of this genotype were apparently fertile and males exhibited no gross morphological abnormalities of the adult reproductive tracts (Fig. 4D). A subtle abnormality in the histology of the adult testis was detected, with double homozygotes exhibiting elevated numbers of residual bodies in some tubules (data not shown). The histology of the ovary, oviduct, uterus, and vagina in two 11-week-old double homozygous females was unremarkable (data not shown). All male and female double homozygotes, however, exhibited tail kinks of varying severity (data not shown).
DISCUSSION AND CONCLUSIONS
Expression studies described here demonstrate that both Sfrp5 and Sfrp2 show male-specific expression in the Müllerian duct epithelium between 13.0 and 13.5 dpc, prior to the regression of this embryonic tract. Furthermore, this male-specific Sfrp expression is lost in mesonephroi lacking the competence to undergo Müllerian duct regression. Taken together, these data suggest a role for Sfrp5 and Sfrp2 in the regression of the primitive female reproductive tract in male embryos. Whilst the alterations in Sfrp2 and Sfrp5 expression reported here in px homozygotes place them strictly downstream of WNT7A signaling, their sexually dimorphic pattern at 13.0 dpc is most simply explained on the basis of male-specific AMH signaling, which is also absent in px mutants. Thus, Sfrp5 and Sfrp2 represent epithelial markers of the male Müllerian duct primed for regression. The only other molecule reported to be induced in the Müllerian duct in vivo in a male-specific fashion by AMH, the matrix metalloproteinase gene Mmp2, is expressed in periductal mesenchymal cells (Roberts et al., 2002). The cranial to caudal progression of the expression of these genes between 13.0 and 13.5 dpc may result from the similarly progressive expression of AMHR2, which is itself responsible for the cranial to caudal wave of apoptosis that results in Müllerian duct regression (Allard et al., 2000).
The male-specific expression of Sfrp5 and Sfrp2 at 13.0–13.5 dpc in the ductal epithelium is unlikely to be a result of direct regulation by AMH, because the AMHR2 is expressed on the surface of periductal mesenchymal cells. Some paracrine signal (Roberts et al., 1999) from the mesenchymal cells to the epithelial cells must, therefore, be responsible for upregulation of these genes. The later expression of Sfrp2 and Sfrp5 in the Müllerian duct of female embryos suggests a role for these genes in the subsequent development of this tract in females.
The absence of any gross abnormalities in mice harboring mutant copies of Sfrp5 and Sfrp2 can be explained in number of ways. It seems highly likely that the Sfrp5HQ27stop mutation is a null allele. The whole of the CRD of Sfrp5 is encoded by its first exon and so no possibility for alternative splicing exists that might allow the production of a protein with residual function. Given this, it is reasonable to conclude that SFRP5 is dispensable for normal development in the mouse, at least when these are housed in standard, pathogen-free conditions. Sequence comparison of members of the SFRP gene family suggests that two closely related sub-groups exist (Jones and Jomary, 2002). SFRP1, SFRP2, and SFRP5 form one sub-group, whilst SFRP3/FRZB and SFRP4 comprise the second. Thus, the search for molecules that act to compensate for the absence of functional SFRP5 might profitably start with a study of SFRP2 and SFRP1. For this reason, and due to its striking expression profile during Müllerian duct development, we identified and rederived a mutation in mouse Sfrp2. The Sfrp2HC50F allele lacks one of the canonical cysteine residues of the CRD of SFRP2, the domain thought to mediate interaction with WNT proteins (Lee et al., 2000). This mutation is therefore likely to disrupt the structure of the CRD of SFRP2. However, the severity of the mutation remains unclear, and consequently it is more difficult to interpret the absence of an abnormal phenotype in Sfrp2HC50F homozygotes. It is worth noting, however, that the presence of the tail-kink phenotype in mice doubly homozygous for Sfrp2HC50F and Sfrp5HQ27stop suggests that the Sfrp2HC50F mutation has some functional deficit. A recent report describes the generation of mice lacking SFRP1 and SFRP2 using gene targeting (Satoh et al., 2006). Consistent with data presented here, mice lacking SFRP2 are viable and fertile, as are those lacking SFRP1. Interestingly, mice lacking both SFRP1 and SFRP2 have defects in anteroposterior axis elongation and somite segmentation during embryogenesis. These observations suggest that tail-kinks in mice doubly homozygous for Sfrp2HC50F and Sfrp5HQ27stop may be due to subtle abnormalities in somitogenesis. They also reveal that SFRP2 and SFRP1 can act in a functionally redundant manner.
The possibility remains that the mutant alleles described here represent null or severely hypomorphic alleles. If this is the case, it will be important to investigate the role of Sfrp1 during Müllerian duct development in the context of disrupted Sfrp5 and Sfrp2. It has been reported that SFRP1-deficient mice exhibit reduced osteoblast and osteocyte apoptosis (Bodine et al., 2004). However, despite the variety of sites of expression of Sfrp1 in embryonic (Leimeister et al., 1998) and adult tissues (Rattner et al., 1997), no abnormalities associated with these were observed, consistent with the observations of Satoh et al. (2006). These data suggest that functional redundancy between Sfrp family members is common, although the possibility that other, structurally unrelated, molecules may compensate cannot be excluded.
SFRP genes have been associated with a variety of apoptotic events (Melkonyan et al., 1997; James et al., 2000; Jones et al., 2000; Schumann et al., 2000; Lacher et al., 2003; Bodine et al., 2004). Whilst these potential roles of SFRPs in regulating apoptosis in different contexts are likely to be mediated by their interaction with WNT proteins, in vitro data suggest that this role may extend beyond the inhibition of canonical WNT signaling (He et al., 2005). The identity of WNT molecules with which SFRPs interact during Müllerian duct development is unclear. Wnt7a is expressed in the ductal epithelium (Parr and McMahon, 1998), whilst Wnt4 is also expressed in the developing mesonephros (Vainio et al., 1999). Interestingly, it has been proposed that SFRP2 is able to disrupt the antagonistic action of SFRP1 on WNT4 during metanephric kidney development (Yoshino et al., 2001). Preliminary data indicates that Sfrp1 is expressed in the embryonic mesonephros (SC and AG, unpublished data). No role for WNT4 in Müllerian duct regression has yet been described, however, the role of WNT signaling in duct regression is suggested by the male-specific accumulation of cytoplasmic and nuclear β-catenin in cells of the periductal mesenchyme (Allard et al., 2000; Xavier and Allard, 2003). It is, therefore, a possibility that SFRP2 and WNT4 may act in concert to regulate Müllerian duct regression.
In summary, data described here suggest a role for Sfrp2 and Sfrp5 in male-specific Müllerian duct regression during mouse mesonephros development. Analysis of ENU-induced mutants for these two genes does not establish these roles, but suggests a requirement for analysis of individuals harboring multiple Sfrp mutant alleles in order to overcome the probable functional redundancy that exists between family members.
ACKNOWLEDGMENTS
We thank Akio Kobayashi and Richard Behringer for the kind gift of the Lim1 and Amhr2 probes. We also thank Martin Fray and staff of the FESA Core for assistance with in vitro fertilization, Jim Humphreys, Kate Vowell, David Shipston, and Terry Hacker for histology support, Steve Thomas for help with electronic imaging, Sara Wells, Elaine Whitehill, Michelle Hammett, and Lucie Vizor for animal husbandry and Ruth Arkell, Sean Grimmond, and Rachel Larder for earlier contributions to this work. Thanks also to Richard Sharpe for advice on testis histology. This research was supported by the Medical Research Council and animal experiments described were covered by UK Home Office Project License PPL 30/1879.
Grant sponsor: Medical Research Council.
REFERENCES
- Allard S, Adin P, Gouedard L, di Clemente N, Josso N, Orgebin-Crist MC, Picard JY, Xavier F. Molecular mechanisms of hormone-mediated Müllerian duct regression: Involvement of beta-catenin. Development. 2000;127:3349–3360. doi: 10.1242/dev.127.15.3349. [DOI] [PubMed] [Google Scholar]
- Baehrecke EH. How death shapes life during development. Nat Rev Mol Cell Biol. 2002;3:779–787. doi: 10.1038/nrm931. [DOI] [PubMed] [Google Scholar]
- Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem. 1999;274:16180–16187. doi: 10.1074/jbc.274.23.16180. [DOI] [PubMed] [Google Scholar]
- Behringer RR, Finegold MJ, Cate RL. Müllerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, Gaur T, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Chang JT, Esumi N, Moore K, Li Y, Zhang S, Chew C, Goodman B, Rattner A, Moody S, Stetten G, Campochiaro PA, Zack DJ. Cloning and characterization of a secreted frizzled-related protein that is expressed by the retinal pigment epithelium. Hum Mol Genet. 1999;8:575–583. doi: 10.1093/hmg/8.4.575. [DOI] [PubMed] [Google Scholar]
- Chong JM, Uren A, Rubin JS, Speicher DW. Disulfide bond assignments of secreted Frizzled-related protein-1 provide insights about Frizzled homology and netrin modules. J Biol Chem. 2002;277:5134–5144. doi: 10.1074/jbc.M108533200. [DOI] [PubMed] [Google Scholar]
- Coghill EL, Hugill A, Parkinson N, Davison C, Glenister P, Clements S, Hunter J, Cox RD, Brown SD. A gene-driven approach to the identification of ENU mutants in the mouse. Nat Genet. 2002;30:255–256. doi: 10.1038/ng847. [DOI] [PubMed] [Google Scholar]
- Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
- Dyche WJ. A comparative study of the differentiation and involution of the Mullerian duct and Wolffian duct in the male and female fetal mouse. J Morphol. 1979;162:175–209. doi: 10.1002/jmor.1051620203. [DOI] [PubMed] [Google Scholar]
- Ellies DL, Church V, Francis-West P, Lumsden A. The WNT antagonist cSFRP2 modulates programmed cell death in the developing hindbrain. Development. 2000;127:5285–5295. doi: 10.1242/dev.127.24.5285. [DOI] [PubMed] [Google Scholar]
- Finch PW, He X, Kelley MJ, Uren A, Schaudies RP, Popescu NC, Rudikoff S, Aaronson SA, Varmus HE, Rubin JS. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc Natl Acad Sci USA. 1997;94:6770–6775. doi: 10.1073/pnas.94.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley KR, Tennessen J, Shawlot W. The mouse secreted frizzled-related protein 5 gene is expressed in the anterior visceral endoderm and foregut endoderm during early post-implantation development. Gene Expr Patterns. 2003;3:681–684. doi: 10.1016/s1567-133x(03)00091-7. [DOI] [PubMed] [Google Scholar]
- Grimmond S, Van Hateren N, Siggers P, Arkell R, Larder R, Soares MB, de Fatima Bonaldo M, Smith L, Tymowska-Lalanne Z, Wells C, Greenfield A. Sexually dimorphic expression of protease nexin-1 and vanin-1 in the developing mouse gonad prior to overt differentiation suggests a role in mammalian sexual development. Hum Mol Genet. 2000;9:1553–1560. doi: 10.1093/hmg/9.10.1553. [DOI] [PubMed] [Google Scholar]
- He B, Lee AY, Dadfarmay S, You L, Xu Z, Reguart N, Mazieres J, Mikami I, McCormick F, Jablons DM. Secreted frizzled-related protein 4 is silenced by hypermethylation and induces apoptosis in beta-catenin-deficient human mesothelioma cells. Cancer Res. 2005;65:743–748. [PubMed] [Google Scholar]
- James IE, Kumar S, Barnes MR, Gress CJ, Hand AT, Dodds RA, Connor JR, Bradley BR, Campbell DA, Grabill SE, Williams K, Blake SM, Gowen M, Lark MW. FrzB-2: A human secreted frizzled-related protein with a potential role in chondrocyte apoptosis. Osteoarthritis Cartilage. 2000;8:452–463. doi: 10.1053/joca.1999.0321. [DOI] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Behringer RR. Genetic studies of MIS signalling in sexual development. Novartis Found Symp. 2002;244:157–164. discussion 164-158, 203-156, 253-157. [PubMed] [Google Scholar]
- Jones SE, Jomary C. Secreted Frizzled-related proteins: Searching for relationships and patterns. Bioessays. 2002;24:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- Jones SE, Jomary C, Grist J, Stewart HJ, Neal MJ. Modulated expression of secreted frizzled-related proteins in human retinal degeneration. Neuroreport. 2000;11:3963–3967. doi: 10.1097/00001756-200012180-00012. [DOI] [PubMed] [Google Scholar]
- Josso N, Clemente N. Transduction pathway of anti-Mullerian hormone, a sex-specific member of the TGF-beta family. Trends Endocrinol Metab. 2003;14:91–97. doi: 10.1016/s1043-2760(03)00005-5. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4:969–980. doi: 10.1038/nrg1225. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Shawlot W, Kania A, Behringer RR. Requirement of Lim1 for female reproductive tract development. Development. 2004;131:539–549. doi: 10.1242/dev.00951. [DOI] [PubMed] [Google Scholar]
- Lacher MD, Siegenthaler A, Jager R, Yan X, Hett S, Xuan L, Saurer S, Lareu RR, Dharmarajan AM, Friis R. Role of DDC-4/sFRP-4, a secreted frizzled-related protein, at the onset of apoptosis in mammary involution. Cell Death Differ. 2003;10:528–538. doi: 10.1038/sj.cdd.4401197. [DOI] [PubMed] [Google Scholar]
- Lee CS, Buttitta LA, May NR, Kispert A, Fan CM. SHH-N upregulates Sfrp2 to mediate its competitive interaction with WNT1 and WNT4 in the somitic mesoderm. Development. 2000;127:109–118. doi: 10.1242/dev.127.1.109. [DOI] [PubMed] [Google Scholar]
- Leimeister C, Bach A, Gessler M. Developmental expression patterns of mouse sFRP genes encoding members of the secreted frizzled related protein family. Mech Dev. 1998;75:29–42. doi: 10.1016/s0925-4773(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Lescher B, Haenig B, Kispert A. sFRP-2 is a target of the Wnt-4 signaling pathway in the developing metanephric kidney [In Process Citation] Dev Dyn. 1998;213:440–451. doi: 10.1002/(SICI)1097-0177(199812)213:4<440::AID-AJA9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaughlin DT, Donahoe PK. Sex determination and differentiation. N Engl J Med. 2004;350:367–378. doi: 10.1056/NEJMra022784. [DOI] [PubMed] [Google Scholar]
- Melkonyan HS, Chang WC, Shapiro JP, Mahadevappa M, Fitzpatrick PA, Kiefer MC, Tomei LD, Umansky SR. SARPs: A family of secreted apoptosis-related proteins. Proc Natl Acad Sci USA. 1997;94:13636–13641. doi: 10.1073/pnas.94.25.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development. 1998;125:3201–3211. doi: 10.1242/dev.125.16.3201. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, Behringer RR. Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996;10:2577–2587. doi: 10.1101/gad.10.20.2577. [DOI] [PubMed] [Google Scholar]
- Moon RT, Brown JD, Yang-Snyder JA, Miller JR. Structurally related receptors and antagonists compete for secreted Wnt ligands. Cell. 1997;88:725–728. doi: 10.1016/s0092-8674(00)81915-7. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395:707–710. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- Parr BA, Avery EJ, Cygan JA, McMahon AP. The classical mouse mutant postaxial hemimelia results from a mutation in the Wnt 7a gene. Dev Biol. 1998;202:228–234. doi: 10.1006/dbio.1998.9007. [DOI] [PubMed] [Google Scholar]
- Quwailid MM, Hugill A, Dear N, Vizor L, Wells S, Horner E, Fuller S, Weedon J, McMath H, Woodman P, Edwards D, Campbell D, Rodger S, Carey J, Roberts A, Glenister P, Lalanne Z, Parkinson N, Coghill EL, McKeone R, Cox S, Willan J, Greenfield A, Keays D, Brady S, Spurr N, Gray I, Hunter J, Brown SD, Cox RD. A gene-driven ENU-based approach to generating an allelic series in any gene. Mamm Genome. 2004;15:585–591. doi: 10.1007/s00335-004-2379-z. [DOI] [PubMed] [Google Scholar]
- Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci U S A. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LM, Hirokawa Y, Nachtigal MW, Ingraham HA. Paracrine-mediated apoptosis in reproductive tract development. Dev Biol. 1999;208:110–122. doi: 10.1006/dbio.1998.9190. [DOI] [PubMed] [Google Scholar]
- Roberts LM, Visser JA, Ingraham HA. Involvement of a matrix metalloproteinase in MIS-induced cell death during urogenital development. Development. 2002;129:1487–1496. doi: 10.1242/dev.129.6.1487. [DOI] [PubMed] [Google Scholar]
- Satoh W, Gotoh T, Tsunematsu Y, Aizawa S, Shimono A. Sfrp1 and Sfrp2 regulate anteroposterior axis elongation and somite segmentation during mouse embryogenesis. Development. 2006;133:989–999. doi: 10.1242/dev.02274. [DOI] [PubMed] [Google Scholar]
- Schumann H, Holtz J, Zerkowski HR, Hatzfeld M. Expression of secreted frizzled related proteins 3 and 4 in human ventricular myocardium correlates with apoptosis related gene expression. Cardiovasc Res. 2000;45:720–728. doi: 10.1016/s0008-6363(99)00376-4. [DOI] [PubMed] [Google Scholar]
- Searle AG. The genetics and morphology of two ‘luxoid’ mutants in the house mouse. Genet Res, Camb. 1964;5:171–197. [Google Scholar]
- Shirozu M, Tada H, Tashiro K, Nakamura T, Lopez ND, Nazarea M, Hamada T, Sato T, Nakano T, Honjo T. Characterization of novel secreted and membrane proteins isolated by the signal sequence trap method. Genomics. 1996;37:273–280. doi: 10.1006/geno.1996.0560. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling [In Process Citation] Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Wang S, Krinks M, Lin K, Luyten FP, Moos M., Jr. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–766. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Xavier F, Allard S. Anti-Mullerian hormone, beta-catenin and Mullerian duct regression. Mol Cell Endocrinol. 2003;211:115–121. doi: 10.1016/j.mce.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Yoshino K, Rubin JS, Higinbotham KG, Uren A, Anest V, Plisov SY, Perantoni AO. Secreted Frizzled-related proteins can regulate metanephric development. Mech Dev. 2001;102:45–55. doi: 10.1016/s0925-4773(01)00282-9. [DOI] [PubMed] [Google Scholar]


