Abstract
Three potential routes to generation of reactive oxygen species from α-tocopherolquinone have been identified. The quinone of the water-soluble vitamin E analogue Trolox C (Trol-Q) is reduced by hydrated electron and isopropanol α-hydroxyalkyl radical, and the resulting semiquinone reacts with molecular oxygen to form superoxide with a second order rate constant of 1.3 × 108 dm3 mol−1 s−1, illustrating the potential for redox cycling. Illumination (UV-A, 355 nm) of the quinone of 2,2,5,7,8-pentamethyl-6-hydroxychromanol (PMHC-Q) leads to a reactive short-lived (ca 10−6 s) triplet state, able to oxidise tryptophan with a second order rate constant greater than 109 dm3 mol−1 s−1. The triplet states of these quinones sensitize singlet oxygen formation with quantum yields of about 0.8. Such potentially damaging reactions of α-tocopherolquinone may in part account for the recent findings that high levels of dietary vitamin E supplementation lack any beneficial effect and may lead to slightly enhanced levels of overall mortality.
Keywords: Tocopherolquinone, radical, superoxide, singlet oxygen, redox cycling, triplet
1. INTRODUCTION
Recent meta-analysis of data from trials of high levels of dietary supplementation with vitamins, including vitamin E, surprisingly suggests an associated increased risk of cancer and overall morbidity [1-3]. Previously, little risk was attached to vitamin E supplementation and there are indications that it is beneficial in preventing some major diseases such as atherosclerosis and cancer [4-7]. Vitamin E exists in several forms of which α-tocopherol is the best antioxidant, although a specific role for γ-tocopherol has also been claimed [8,9]. It is now appreciated that in addition to their widely acknowledged antioxidant activities as chain-breaking inhibitors of lipid peroxidation [10,11], tocopherols also have some important non-antioxidant activities in vivo [12,13]. There are therefore many aspects of the biochemistry of vitamin E which might be explored in relation to its potentially harmful effects.
Oxidation of α-tocopherol (α-TOH) is known to give a range of products. In vitro, exposure to peroxyl radicals generated from the azoinitiator 2,2'-azobis(2,4-dimethylvaleronitrile) gave mainly the peroxyl adduct and a spirodimer with a smaller yield of α-tocopherolquinone (α-TQ) [14]. In microsomes the same radical source also generated α -TQ which to a variable extent was found to be converted back to the α -tocopherolhydroquinone (α-TQH2) [15]. Moore and Ingold have reported on conversion of α-TQ back to α-TOH in man and reviewed some of the earlier findings related to formation and metabolism of α-TQ in animal models [16]. More recently Yamauchi [17] has reviewed the formation of tocopherolquinones during lipid peroxidation in a range of systems. Unlike the non-arylating quinone of α-tocopherol, the arylating γ- and δ-tocopherolquinones are mutagenic [18], cytotoxic [19] and capable of stimulating apoptosis [20]. Maroz and Brede [21] have shown that β-hydroxyalkyl radical addition only occurs with unsubstituted quinones and that duroquinone (2,3,5,6-tetramethyl-1,4-benzoquinone, DQ) is unreactive and not able to act as a radical trap. By analogy with substituted benzoquinones, radical scavenging through adduct formation might be possible in other tocopherolquinones (i.e γ-TQ and δ-TQ) but less so for the fully substituted α-TQ. α-TQH2 is reported to be a good reductant, reacting more rapidly with peroxyl radicals than either α-TOH or reduced ubiquinone and hence is proposed to be an effective antioxidant [22-24]. The detoxification of α-TQ by DT-diaphorase (NAD(P)H: quinone oxidoreductase) therefore not only removes a reactive metabolite and prevents potential generation of reactive oxygen species (ROS) by redox cycling of the quinone, but also generates the antioxidant hydroquinone [25]. Other biochemical functions for α-TQ have also been suggested including acting as a cofactor for fatty acid dehydrogenases [26] and as an anti-clotting factor [27].
The reducing power of α-TOH (Eo'(α-TO•, H+/ α-TOH), 480 mV) endows it with antioxidant function, but similarly allows it to act as a prooxidant under particular conditions, especially in the presence of transition metal ions [28]. Such prooxidant activities might help explain the potentially harmful effects of vitamin E. We have shown that on photolysis at 308 nm α-TOH and a model compound, 2,2,5,7,8-pentamethyl-6-hydroxychroman (PMHC), generate singlet oxygen [29], a biologically damaging ROS. However the overall quantum yield for formation of singlet oxygen was rather small (ca 10%) and requires excitation of vitamin E within its absorption band in the UVB region. We have now explored the possibility that α-TQ may be more effective than α-TOH in generating ROS.
2. MATERIALS AND METHODS
Pulse radiolysis experiments were carried out using the 12 MeV linear accelerator at CCLRC Daresbury Laboratory [30]. Samples were irradiated in a 2.5 cm pathlength capillary cell using doses of either ca. 3 Gy/pulse (kinetics) or ca 7 Gy/pulse (transient spectra). Spectra are shown as the product of extinction coefficient and radiation chemical yield (G-value) based on dosimetry with thiocyanate. Time-resolved singlet oxygen luminescence measurements were performed with a pulsed Nd:YAG laser (Continuum Surelight II-10, 355 nm, 5 ns pulses, ∼ 1 mJ at the sample) and a fast germanium photodiode (Edinburgh Instruments El-P) with a 1275 nm interference filter (NDC Infrared Engineering, 25 nm bandpass). Laser flash photolysis used a system based on a YAG laser (355 nm, 10 ns pulses) as previously described [31]. Data was analysed using the Origin 7.5 software package.
Solutions were prepared either in Milli-Q grade water or in HPLC grade solvents and saturated with the gases described before use. The pH was adjusted with phosphate buffers and by the addition of perchloric acid when required. The three quinones investigated (see Scheme 1 for structures) were α-tocopherolquinone (α-TQ, 2-(3-hydroxy-3,7,11,15-tetramethyl-hexadecyl)-3,5,6-trimethyl-[1,4]-benzoquinone), the quinone from Trolox C (Trol-Q, 2-(3-hydroxy-3-methylbutanoic acid)-3,5,6-trimethyl-[1,4]-benzoquinone), and the quinone from 2,2,5,7,8-pentamethyl-6-hydroxychroman (PMHC-Q, 2-(3-hydroxy-3-methylbutyl)-3,5,6-trimethyl-[1,4]-benzoquinone). Both Trolox C and vitamin E were obtained from Sigma-Aldrich. PMHC was synthesised as described by Smith et al [32]. Conversion of the phenols to quinones was achieved by oxidation with FeCl3 in a single step [33], followed with purification by chromatography on a column of silica gel (Fluka, silica gel 60, 220-440 mesh). Identity and purify was established by thin layer chromatography and NMR.
Scheme 1.
Solutions were saturated with gases by bubbling. For varying the oxygen concentration, nitrogen was mixed with either oxygen or air in a twin flowmeter assembly. The solubilities of oxygen in common solvents were taken from Battino et al [34]. The oxygen concentration in oxygen-saturated water/ethanol (1:1 v/v) was calculated from the data of Cargill [35] to be 1.7 mmol dm−3. Methods for obtaining singlet oxygen quantum yields followed those described by Nonell and Braslavsky [36], using phenalenone (perinaphthenone) as a standard with a quantum yield of 0.95 ± 0.05 [37].
3. RESULTS AND DISCUSSION
3.1 Pulse radiolysis studies of one-electron reduction of Trol-Q
Pulse radiolysis was used to study the one-electron reduction of the water-soluble vitamin E quinone analogue Trol-Q. From radiolysis [38] of deaerated aqueous solutions (reaction (1)) containing 2-methylpropanol as a hydroxyl radical scavenger (reaction (2)), both hydrated electrons and hydrogen atoms are available at neutral and alkaline pH values as one-electron reductants with yields of 0.28 and 0.06 μmol dm−3 Gy−1 respectively. In acidic solutions below about pH 4, hydrated electrons are converted by reaction (3) to hydrogen atoms.
| ...(1) |
| ...(2) |
| ...(3) |
In addition, studies of one-electron reduction may also employ the isopropyl radical, formed with an overall yield of 0.72 μmol dm−3 Gy−1 in nitrous oxide saturated solutions (reactions (4) and (5)).
| ...(4) |
| ...(5) |
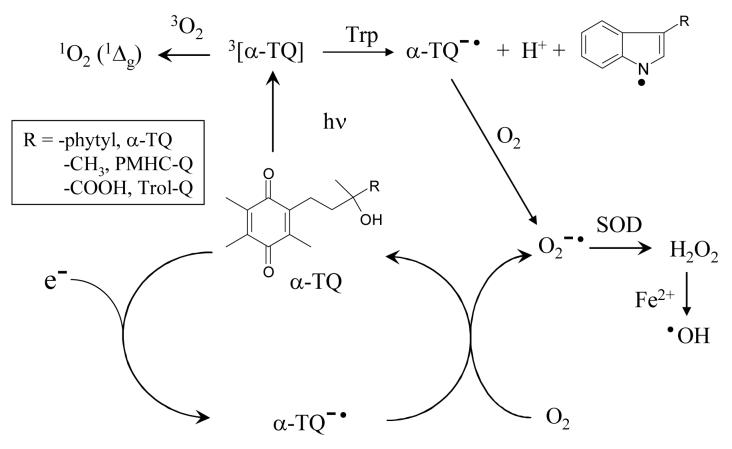
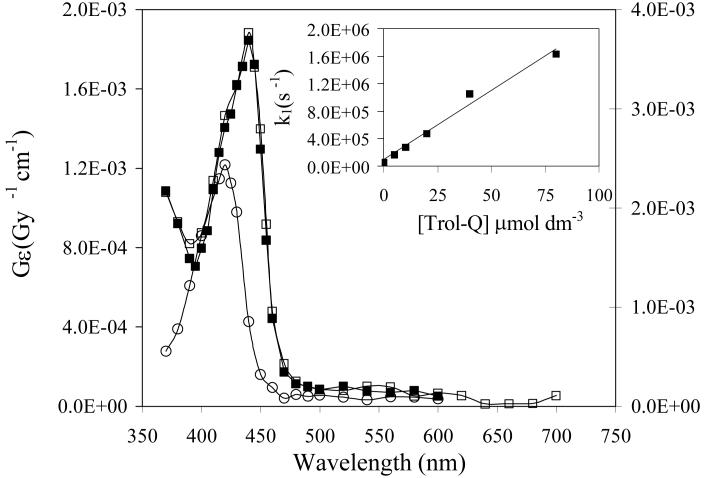
At neutral pH the second order rate constant for reaction of hydrated electrons with Trol-Q was measured from the first order rate of decay of the absorption of e−aq at 700 nm (inset to Figure 1) giving a value of (2.0 ± 0.1) × 1010 dm3 mol−1 s−1, at the diffusion controlled limit. Figure 1 shows the transient absorption spectra measured from reduction of Trol-Q in pulse radiolysis experiments. The spectra obtained by reduction with hydrated electrons at pH 9.2 and with propan-2-ol radicals at pH 7.3 are essentially identical with a peak at 440 nm, except for the two-fold increase in intensity of the latter due to the respective G-values of the reducing radicals as described above. The extinction coefficient at the peak absorbance (440 nm) of the spectrum at pH 9.2 is 6.7 × 103 dm3 mol−1 cm−1 based on a G-value for e−aq of 0.28 μmol dm−3 Gy−1. This is comparable with the value of 7.6 × 103 dm3 mol−1 cm−1 reported for the durosemiquinone radical anion [39]. At pH 1.8 a different spectrum from reaction of hydrogen atoms was observed with a peak at 420 nm and a lower extinction coefficient compared with that from reduction with hydrated electrons at pH 7.4. By analogy with other semiquinone radicals [39], it is clear that the different spectra represent the anionic (Q•−) and neutral (QH•) semiquinone radicals at pH >7 and pH 1.8 respectively. The intensity of the transient absorbance at 445 nm over a range of pH values is shown in Figure 2 and gives a pKa value for QH• of 4.9 ± 0.1. This compares with a value of 5.0 determined previously for DQH• [39].
Figure 1.
Transient absorption spectra, as product of radiation chemical yield (G-value) and extinction coeeficient, from pulse radiolysis of N2-saturated aqueous solutions containing Trol-Q (200 μmol dm−3), 2-methylpropanol (0.1 mol dm−3) and phosphate buffer (10 mmol dm−3) at pH 9.2 (□) and pH 1.8 (○) (left hand scale). Also shown is the transient spectrum from N2O-saturated solutions of Trol-Q (200 μmol dm−3) and propan-2-ol (20% v/v) in phosphate buffer (10 mmol dm−3) at pH 7.3 (■) (right hand scale). Inset:- First order rate constant for decay of the hydrated electron at 700 nm versus Trol-Q concentration in N2-saturated solutions containing 2-methylpropanol (0.1 mol dm−3) and phosphate buffer (10 mmol dm−3, pH 7.3).
Figure 2.
Effect of pH on the transient absorbance at 445 nm from pulse radiolysis of N2-saturated aqueous solutions containing Trol-Q (200 μmol dm−3), 2-methylpropanol (0.1 mol dm−3) and phosphate buffer (10 mmol dm−3). Inset:- First order rate constant for decay of the transient absorbance of the semiquinone radical at 440 nm versus oxygen concentration in solutions containing 2-methylpropanol (0.1 mol dm−3) and phosphate buffer (10 mmol dm−3, pH 7.3).
The rate of reaction of Q•− with dioxygen (reaction (6)) at neutral pH was measured from the increasing first order rate constant for disappearance of the absorption at 440 nm with increasing oxygen concentration as shown by the results in the inset to Figure 2, giving a second order rate constant of (1.2 ± 0.1) × 108 dm3 mol−1 s−1. This is slightly slower than the value for durosemiquinone radical anion (2 × 108 dm3 mol−1 s−1) determined by Patel and Willson [39]. Also in comparison with their results it is noticeable that using the lower concentration of quinone (2×10−4 mol dm−3) the reverse reaction (6) was not significant and the reaction proceeded almost entirely in the forward direction, as expected from the value for K6 of 43 determined by Patel and Willson for duroquinone. Overall the results illustrate the potential for tocopherolquinones to redox cycle in vivo with the concomittent generation of damaging superoxide radical as shown in Scheme 1.
| .............(6) |
3.2 Laser flash photolysis of tocopherolquinones
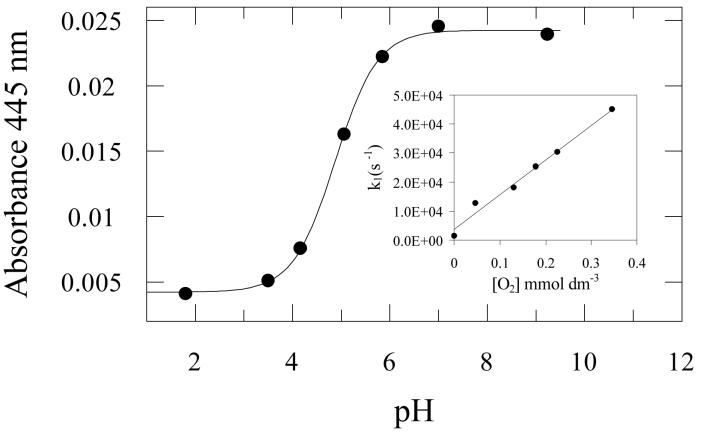
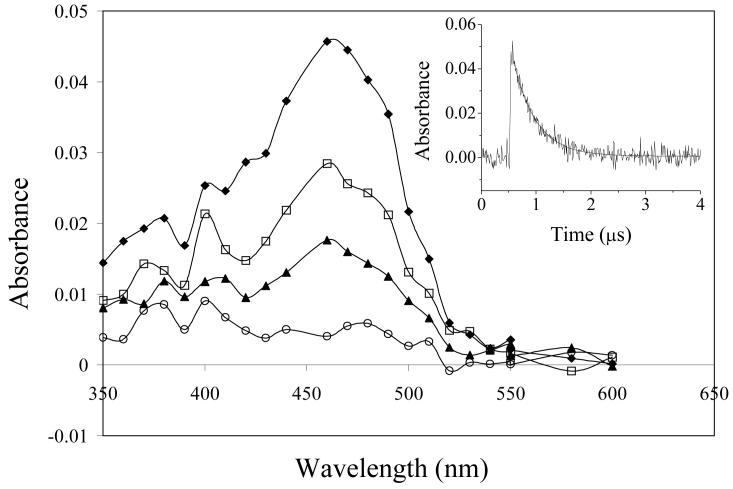
Laser flash photolysis at 355 nm of PMHC-Q in deaerated (N2-saturated) methanol revealed the transient absorption spectra shown in Figure 3. Immediately after the laser pulse there is a broad absorption with a peak at 450-460 nm which decays with bi-exponential kinetics with first order rates (lifetimes) of 0.91 × 106 s−1 (1.1 ± 0.1 μs) and 8 × 104 s−1 (13 ± 3 μs). Simultaneously new absorption bands were formed at in the regions of 430 nm and 500 nm which decay with a lifetime of about 10 microseconds. Finally, a more stable product with a lifetime of some hundreds of microseconds is revealed between 400 and 430 nm. The initial species observed at 450 nm is clearly the triplet state, based on its rapid quenching by oxygen (k2 (2.2 ± 0.1) × 109 dm3 mol−1 s−1 in methanol, inset to Figure 5), and is formed by rapid intersystem crossing from the initial singlet excited state. The spectrum is very similar to that of the triplet formed from duroquinone (3DQ) [40, 41] although the lifetime is shorter than for 3DQ in methanol (τ ∼ 5 μs, [41]). The absorption band at 480-520 nm has been assigned by Kemp and Porter [43] to the quinone methide anion formed following rapid proton loss (Scheme 1) and is not observed to any significant extent for 3DQ [41, 44]. The long-lived transient at 410-430 nm is ascribed to the semiquinone radical as already described in the pulse radiolysis experiments. In the photolysis experiment it is formed by electron abstraction and proton transfer from the solvent [41]. In ethanol:water (1:1 v/v buffered to pH 7) the decay of the triplet was slower (k = 5 × 105 s−1) and the reaction with oxygen was also somewhat slower (k2 1.33 ± 0.05 × 109 dm3 mol−1 s−1, see inset to Figure 5). Although Kemp and Porter [43] did not measure transient spectra, their results are consistent with those found here.
Figure 3.
Laser flash photolysis (355 nm, 10 ns) of PMHC-Q (2 mmol dm−3) in deaerated methanol. Transient spectra were measured 150 ns (■), 1.5 μs (○) and 9 μs (▲) after the laser flash. Inset: Absorption transients recorded at (a) 450 nm, and (b) 520 nm.
Figure 5.
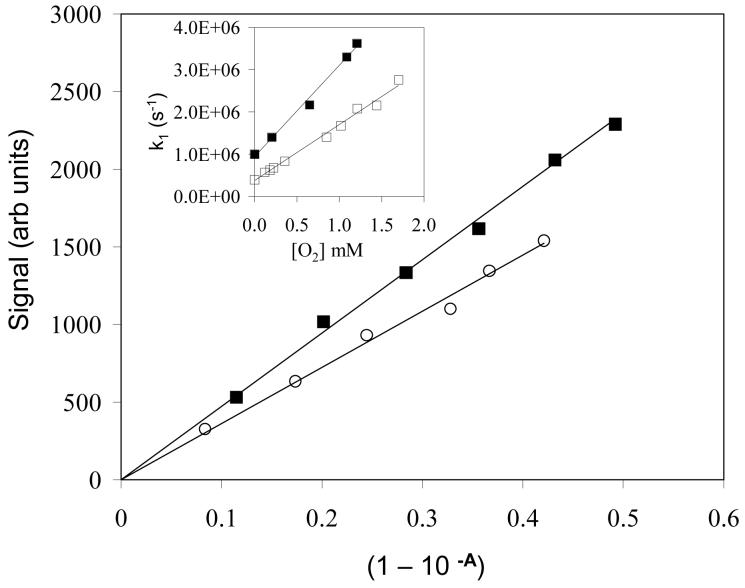
Intensities of 1270 nm singlet oxygen luminescence versus fraction of light absorbed (1-10−A) in solutions of perinaphthenone (■) and PMHC-Q (○) in oxygen-saturated acetonitrile. Inset: First order rates of decay of triplet PMHC-Q versus oxygen concentration in methanol (■) and ethanol/water (1:1 v/v buffered to pH 7 with 10 mmol dm−3 phosphate) (□).
In hexane solution the spectra shown in Figure 4 were obtained and indicate that only the triplet state is observed. The decay of the triplet is first order and more rapid (first order rate constant, k1 2.4 × 106 s−1 (lifetime 0.41 μs)) than in methanol. Again these observations are supported by those of Kemp and Porter [43] in a hydrocarbon solvent (liquid paraffin). This contrasts with the behaviour of 3DQ in hexane when a second order decay from triplet-triplet interactions is observed and the overall lifetime is longer than in methanol under typical conditions of the experiment [41]. This suggests that deactivation of the triplet state of PMHC-Q in hexane occurs through an intramolecularly hydrogen-bonded state involving the quinone carbonyls and the side-chain hydroxyl group, possibly through internal proton/charge transfer.
Figure 4.
Laser flash photolysis of PMHC-Q (2 mmol dm−3) in deaerated n-hexane. Spectra are shown 100 ns (◆), 300 ns (□), 500 ns (▲) and 1000 ns (○) after the laser flash. Inset: decay of the transient at 460 nm with the solid line indicating the fitted exponential decay with τ = 0.41 μs.
3.3 Singlet oxygen yields from photolysis of PMHC-Q
Singlet oxygen was detected by time-resolved near infrared luminescence at 1270 nm using a fast Ge photodiode detector. In oxygen-saturated solutions decays were first order and were extrapolated to back to the mid-point of the laser pulse in order to provide the relative intensity of emission [36]. At low oxygen concentrations both growth and decay of the signal was observed and was fitted using a double exponential function. Relative singlet oxygen intensities (at zero time) from photolysis of tocopherolquinones were plotted against the fraction of light absorbed (1-10−A) from a series of solutions of increasing absorbance (A) up to ∼ 0.3 [36]. The quantum yields of singlet oxygen formation from vitamin E quinones were determined by comparison of the slopes of such plots with that obtained with perinaphthenone, for which the quantum yield for singlet oxygen formation of 0.95 in oxygen saturated solutions is assumed for all solvents used [37]. Typical results are shown in Figure 5.
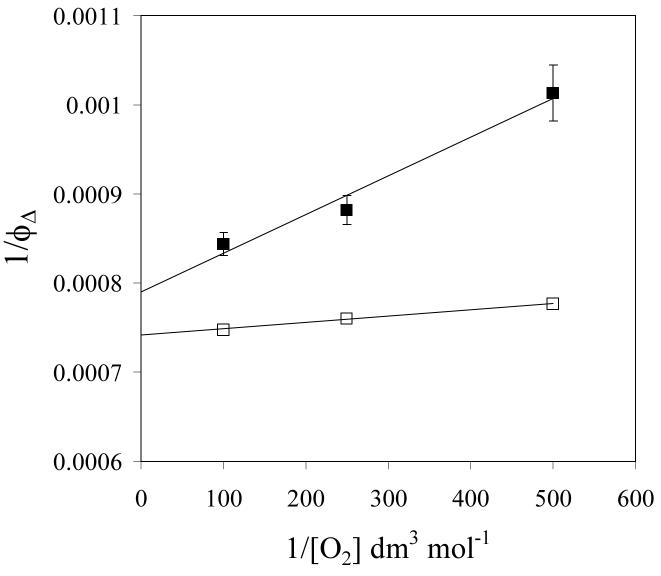
The singlet oxygen yields determined in this way are shown in Table 1. Measurements were made both in oxygen and air saturated solutions. The larger values in solutions saturated with oxygen compared with those saturated with air are due to competition between triplet decay and energy transfer from triplet PMHC-Q to molecular oxygen. Consequently the measured quantum yields (ΦΔ) should follow the relationship:-
| [7] |
where is the quantum yield extrapolated to infinite oxygen concentration, kq is the second order rate constant for triplet quenching by oxygen and kT is the first order rate of decay of the triplet in deaerated solution. It is evident that the triplet yields are all relatively high, being similar in the three polar solvents studied (methanol, ethanol and acetonitrile). The singlet oxygen yield appears slightly higher in cyclohexane where quinone methide formation from the triplet state is not a competing process as noted above. Table 1 also shows that in ethanol the singlet oxygen yield from α-TQ is similar to that from PMHC-Q. According to equation (7) a plot of 1/ΦΔ versus 1/[O2] should yield as the intercept. Examples for PMHC-Q and perinaphthenone in methanol are shown in Figure 6, from which is 0.89. Our laser flash photolysis experiments show that the lifetime of the perinaphthenone triplet is about 20 μs in methanol, allowing efficient energy transfer to oxygen even at comparatively low oxygen concentrations. In contrast the shorter triplet lifetime (ca. 1 μs) of PMHC-Q allows triplet deactivation to compete more effectively with energy transfer and the resulting reduction in singlet oxygen yield from PMHC-Q at lower oxygen concentrations is evident. The short lifetime of the tocopherolquinone triplet state may therefore limit the amount of singlet oxygen formed under physiological oxygen concentrations.
Table 1.
Quantum yields for singlet oxygen (ΦΔ) measured pulsed laser 355 nm excitation of tocopherolquinones. Perinaphthenone in oxygen-saturated solutions in the same solvents was used as the quantum yield standard taking ΦΔ = 0.95 in all cases.
| Compound | Solvent | Saturating Gas |
ΦΔ ± SD |
|---|---|---|---|
| PMHC-Q | Ethanol | O2 | 0.76 ± 0.03 |
| Air | 0.59 ± 0.03 | ||
| Methanol | O2 | 0.85 ± 0.03 | |
| Air | 0.70 ± 0.03 | ||
| Acetonitrile | O2 | 0.72 ± 0.05 | |
| Cyclohexane | O2 | 0.93 ± 0.08 | |
| α-TQ | Ethanol | O2 | 0.80 ± 0.03 |
| Air | 0.63 ± 0.04 |
Figure 6.
Effect of oxygen concentration on the singlet oxygen yield from PMHC-Q (■) and perinaphthenone (□) in methanol, plotted according to equation (7).
3.4 Reactivity of the triplet state of PMHC-Q
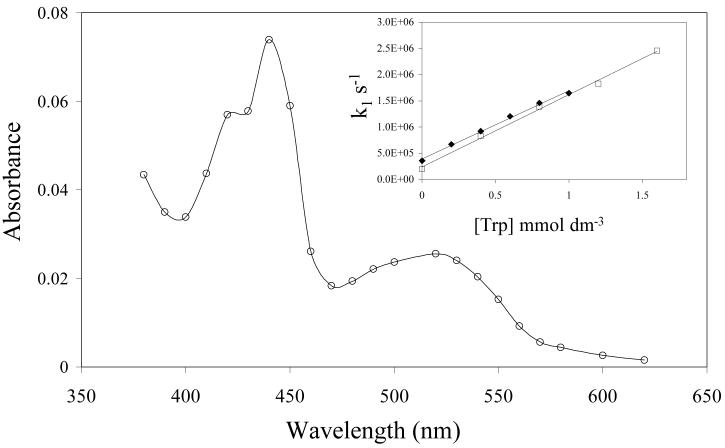
Scheerer and Grätzel [41] estimated the reduction potential of triplet duroquinone [E(3DQ/DQ•−)] to be +2.17 V, showing it to be highly oxidising. We have previously shown that 3DQ is very rapidly reduced by antioxidants including ascorbate, tocopherol [45], lipoate [46] and 4-hydroxycinnamates [47]. This suggests that triplet tocopherolquinones also likely to be highly oxidising and be capable of damaging biological targets such as proteins unless efficiently scavenged by antioxidants. The second order rate constant for reaction of triplet PMHC-Q (3[PMHC-Q]) with tryptophan, reaction (8), was measured from a plot of the first order rate constant for triplet absorption decay at 470 nm versus tryptophan concentration (insert to Fig 7) and found to be (1.30 ± 0.05) × 109 dm3 mol−1 s−1, compared with an almost identical value of (1.38 ± 0.05) × 109 dm3 mol−1 s−1 measured similarly for the reaction of 3DQ with tryptophan.
| .....(8) |
Figure 7.
Transient spectrum measured 10 μs after laser flash photolysis of a deaerated solution of PMHC-Q (200 μmol dm−3) and tryptophan (2 mmol dm−3) in ethanol-water (1:1 v/v buffered to pH 7 with 10 mmol dm−3 phosphate). Inset: Effect of tryptophan concentration on the first order rate constant for decay of triplet states of DQ (□) and PMHC-Q (◆) in the same solvent.
Figure 7 shows the transient absorption spectrum formed after reaction of triplet PMHC-Q with tryptophan in an ethanol-water (1:1 v/v) solution at pH 7. After the rapid decay of 3[PMHC-Q] the spectrum shows the formation of the semiquinone radical anion, [PMHC-Q] •− , λmax 440 nm and the neutral tryptophan radical, λmax 520-530 nm [48]. This result clearly demonstrates the one-electron oxidation of tryptophan by 3[PMHC-Q] occurring with a second order rate constant approaching the diffusion-controlled limit.
4. CONCLUSIONS
Overall the free radical and triplet excited state properties of model tocopherolquinones such as Trol-Q and PMHC-Q reflects those of duroquinone. The major difference is in the behaviour of the triplet state where the triplet lifetime is generally significantly shorter than that of dururoquinone, especially in nonpolar solvents such as hexane and liquid paraffin, and formation of the methide is observed in protic solvents such as methanol. The results obtained here demonstrate the potential for α-TQ to generate biologically-damaging reactive oxygen species as summarised in Scheme 1. One-electron reduction of the water soluble analogue of α-TQ results in the formation of the semiquinone radical anion (α-TQ•− ) at physiological pH. In vivo this is likely to involve enzymes such as cytochrome P450 reductase [49]. α-TQ•− reacts rapidly with oxygen to form superoxide radical, the favourable equilibrium constant of this reaction in the case of duroquinone having already been demonstrated [39]. Superoxide may then disproportionate (catalysed by SOD) to give hydrogen peroxide and potentially the hydroxyl radical via a Fenton-type reaction. These results appear to contradict the assertion [50] that under physiological conditions superoxide may reduce α-TQ to α-TQ•− and thereby generate α-TQH2 by disproportionation of the semiquinone.
The excited triplet state of α-TQ also reacts with oygen to form the highly oxidising excited singlet (1Δg) oxygen. At high oxygen concentration the overall yield singlet oxygen approaches unity. However, the short lifetime of the triplet state and competing processes such as methide formation in solvents such as methanol may reduce the actual yield of singlet oxygen. Such limitation may apply to α-tocopherolquinone within the hydrophobic environment of a lipid bilayer, although this may be offset by the higher oxygen concentrations expected in lipid bilayers due to the favourable partitioning of oxygen from the aqueous phase [51]. The reactive oxygen species resulting from these processes (O2•−, •OH and 1O2) are all capable of producing oxidative damage to biological targets such as nucleic acids, proteins and lipids [52]. We have also now shown that the triplet excited state of α-TQ has the potential to directly oxidise a biological target, in this case tryptophan. Such one-electron oxidation of tryptophan residues in proteins is known to affect their activity and structure [53] and it is postulated that certain tryptophan-rich integral membrane proteins may offer antioxidant protection [54].
The quantum yields for singlet oxygen formation from α-TQ are considerably higher than those recently observed from α-tocopherol (vitamin E) itself [29]. Excitation of α-tocopherol requires light of wavelengths < 310 nm in the UVB (290-320 nm) and UVC (< 290 nm) regions of the spectrum where the flux from sunlight is low. In contrast α-TQ has a broad absorption in the region of 320-380 nm within the UVA spectral region where the amount of energy within the solar spectrum is higher. α-TQ is therefore more likely to be important as as potential sensitizer of singlet oxygen than α-tocopherol. α-TQ has been found as a photoproduct of vitamin E applied topically to mouse skin [55] . Excited states of carbonyls and quinones can be formed by chemoexcitation in dark reactions, some of them involving enzyme catalysis, associated with electron or oxygen transfer [56,57]. The mechanisms described here for oxidative damage arising from formation of triplet α-TQ are therefore potentially more broadly significant than simply in terms of phototoxicity.
5. ACKNOWLEDGEMENTS
The authors thank the CCLRC Biomed Network and the University of Salford for a studentship to AGC and Dr Ruth Edge for assistance with the pulse radiolysis experiments. The authors also thank CCLRC Daresbury Laboratory for access to the Free Radical Research Facility.
REFERENCES
- 1.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systemic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 2.Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high dose vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B. Polyphenols: antioxidant treats for healthy living or covert toxins? J Sci Food Agric. 2006;86:1992–1995. [Google Scholar]
- 4.Brigelius-Flohé R, Traber MG, Vitamin E. function and metabolism. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 5.Pryor WA. Vitamin E and heart disease: Basic science to clinical intervention trials. Free Rad Biol Med. 2000;28:141–164. doi: 10.1016/s0891-5849(99)00224-5. [DOI] [PubMed] [Google Scholar]
- 6.Schneider C. Chemistry and biology of vitamin E. Mol Nutr Food Res. 2005;49:7–30. doi: 10.1002/mnfr.200400049. [DOI] [PubMed] [Google Scholar]
- 7.Tucker JM, Townsend DM. Alpha-tocopherol: roles in prevention and therapy of human disease. Biomed Pharmacotherap. 2005;59:380–387. doi: 10.1016/j.biopha.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. γ-Tocopherol traps mutagenic electrophiles such as NOx and complements α-tocopherol: physiological implications. Proc Natl Acad Sci USA. 1997;94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh RJ, Goss SPA, Joseph J, Kalyanaraman B. Nitration of γ-tocopherol and oxidation of α-tocopherol by copper-zinc superoxide dismutase/H2O2/NO2−: role of the nitrogen dioxide free radical. Proc Natl Acad Sci USA. 1998;95:12912–12917. doi: 10.1073/pnas.95.22.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton GW, Ingold KU. Vitamin E: application of the principles of physical organic chemistry to the exploration of its structure and function. Acc Chem Res. 1986;19:194–201. [Google Scholar]
- 11.Esterbauer H, Gebicki J, Puhl H, Jürgens G. The role of lipid-peroxidation and antioxidants in oxidative modifications of LDL. Free Rad Biol Med. 1992;13:341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 12.Ricciarelli R, Zingg J-M, Azzi A. Vitamin E: protective role of a Janus molecule. FASEB J. 2001;15:2314–2325. doi: 10.1096/fj.01-0258rev. [DOI] [PubMed] [Google Scholar]
- 13.Azzi A, Stocker A. Vitamin E: non-antioxidant roles. Prog Lipid Res. 2001;39:231–255. doi: 10.1016/s0163-7827(00)00006-0. [DOI] [PubMed] [Google Scholar]
- 14.Liebler DC, Burr JA, Matsumoto S, Matsuo M. Reactions of the vitamin E model compound 2,2,5,7,8-pentamethylchroman-6-ol with peroxyl radicals. Chem Res Toxicol. 1993;6:351–355. doi: 10.1021/tx00033a016. [DOI] [PubMed] [Google Scholar]
- 15.Liebler DC, Burr JA, Philips L, Ham AJL. Gas chromatography-mass spectrometry analysis of vitamin E and its oxidation products. Anal Biochem. 1996;236:27–34. doi: 10.1006/abio.1996.0127. [DOI] [PubMed] [Google Scholar]
- 16.Moore ANJ, Ingold KU. α-Tocopheryl quinone is converted into vitamin E in man. Free Rad Biol Med. 1997;22:931–934. doi: 10.1016/s0891-5849(96)00276-6. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi R. Oxidation products of vitamin E in the peroxidation of liposomal and biological systems. J Clin Biochem Nutr. 2003;34:111–120. [Google Scholar]
- 18.Cornwell DG, Williams MV, Wani AA, Wani G, Shen E, Jones KH. Mutagenicity of tocopheryl quinones: evolutionary advantage of selective accumulation of dietary alpha-tocopherol. Nutr Cancer. 2002;43:111–118. doi: 10.1207/S15327914NC431_13. [DOI] [PubMed] [Google Scholar]
- 19.Sachdeva R, Thomas B, Wang X, Ma J, Jones KH, Hatcher PG, Cornwell DG. Tocopherol metabolism using thermochemolysis: chemical and biological properties of γ-tocopherol, γ-carboxyethyl-hydroxychroman, and their quinones. Chem Res Toxicol. 2005;18:1018–1025. doi: 10.1021/tx0496441. [DOI] [PubMed] [Google Scholar]
- 20.Jones KH, Liu JJ, Roehm JS, Eckel JJ, Eckel TT, Stickrath CR, Triola CA, Jiang ZC, Bartoli GM, Cornwell DG. Gamma-tocopheryl quinone stimulates apoptosis in drug-sensitive and multidrug-resistant cancer cells. Lipids. 2002;37:173–184. doi: 10.1007/s11745-002-0878-2. [DOI] [PubMed] [Google Scholar]
- 21.Maroz A, Brede O. Reaction of radicals with benzoquinone – addition or electron transfer? Radiat Phys Chem. 2003;67:275–278. [Google Scholar]
- 22.Mukai K, Itoh S, Morimoto H. Stopped flow kinetics of vitamin E regeneration reaction with biological hydroquinones (reduced forms of ubiquinone, vitamin K, and tocopherolquinone) in solution. J Biol Chem. 1992;267:22277–22281. [PubMed] [Google Scholar]
- 23.Shi H, Noguchi N, Niki E. Comparative study on dynamics of antioxidative action of α-tocopheryl hydroquinone, ubiquinol and α-tocopherol against lipid peroxidation. Free Rad Biol Med. 1999;27:334–346. doi: 10.1016/s0891-5849(99)00053-2. [DOI] [PubMed] [Google Scholar]
- 24.Liebler DC, Burr JA. Antioxidant reactions of α-tocopherolhydroquinone. Lipids. 2000;35:1045–1047. doi: 10.1007/s11745-000-0617-8. [DOI] [PubMed] [Google Scholar]
- 25.Siegel D, Bolton EM, Burr JA, Liebler DC, Ross D. The reduction of α-tocopherolquinone by human NAD(P)H:quinone oxidoreductase: the role of α-tocopherolhydroquinone as a cellular antioxidant. Mol Pharmacol. 1997;52:300–305. doi: 10.1124/mol.52.2.300. [DOI] [PubMed] [Google Scholar]
- 26.Infante JP. A function for the vitamin E metabolite α-tocopherol quinone as an essential enzyme cofactor for the mitochondrial fatty acid desaturases. FEBS Lett. 1999;446:1–5. doi: 10.1016/s0014-5793(99)00170-2. [DOI] [PubMed] [Google Scholar]
- 27.Dowd P, Zheng ZB. On the mechanism of the anticlotting action of vitamin E quinone. Proc Natl Acad Sci USA. 1995;92:8171–8175. doi: 10.1073/pnas.92.18.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuz̆il J, Thomas SR, Stocker R. Requirement for, promotion, or inhibition by α-tocopherol of radical-induced initiation of plasma lipoprotein lipid peroxidation. Free Rad Biol Med. 1997;22:57–71. doi: 10.1016/s0891-5849(96)00224-9. [DOI] [PubMed] [Google Scholar]
- 29.Dad S, Bisby RH, Clark IP, Parker AW. Formation of singlet oxygen from solutions of vitamin E. Free Rad Res. 2006;40:333–338. doi: 10.1080/10715760500491174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holder DJ, Allen D, Land EJ, Navaratnam S. Establishment of pulse radiolysis facility on the SRS linac at Daresbury Laboratory. In: Garvey T, Duff LJ, Roux LP, Petit JC, Poole J, Riviki L, editors. Proceedings of the 8th European Particle Accelerator Conference; Paris: European Physical Society; 2002. pp. 2804–2806. [Google Scholar]
- 31.Navaratnam S. Photochemical and photophysical methods used in the study of drug photoreactivity. In: Tonnesen HH, editor. Photostability of Drugs & Drug Formulations. Boca Raton: CRC Press; 2004. pp. 255–284. [Google Scholar]
- 32.Smith LI, Ungnade HE, Hoehn HH, Wawzonek S. The chemistry of vitamin E. VI. The addition of dienes to phenols and hydroquinones. J Org Chem. 1939;4:311–317. [Google Scholar]
- 33.Cohen N, Lopresti RJ, Neukom C. Studies on the total synthesis of (2R, 4′R, 8′R)-α-tocopherol (vitamin E). Stereospecific cyclizations leading to optically active chromans. J Org Chem. 1981;46:2445–2450. [Google Scholar]
- 34.Battino R, Rettich TR, Tominaga T. The solubility of oxygen and ozone in liquids. J Phys Chem Ref Data. 1983;12:163–178. [Google Scholar]
- 35.Cargill RW. Solubility of oxygen in some water + alcohol systems. J Chem Soc Faraday Trans I. 1976;72:2296–2300. [Google Scholar]
- 36.Nonell S, Braslavsky SE. Time-resolved singlet oxygen detection. Meth Enzymol. 2000;319:37–49. doi: 10.1016/s0076-6879(00)19006-8. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt R, Tanielian C, Dunsbach R, Wolff C. Phenalenone, a universal reference compound for the determination of quantum yields of singlet oxygen O2(1Δg) sensitization. J Photochem Photobiol A: Chemistry. 1994;79:11–17. [Google Scholar]
- 38.Buxton GV, Greenstock CL, Helman WP, Ross AB. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radical (.OH/.O−) in aqueous solution. J Phys Chem Ref Data. 1988;17:513–886. [Google Scholar]
- 39.Patel KP, Willson RL. Semiquinone free radicals and oxygen. J Chem Soc Faraday Trans I. 1973;69:814–825. [Google Scholar]
- 40.Amouyal E, Bensasson R. Duroquinione triplet reduction, in cyclohexane, ethanol and water, and by durohydroquinone. J Chem Soc Faraday Trans I. 1976;72:1274–1287. [Google Scholar]
- 41.Scheerer R, Grätzel M. Laser photolysis studies of duroquinone triplet state electron transfer reactions. J Am Chem Soc. 1977;99:865–871. [Google Scholar]
- 42.Darmanyan AP, Foote CS. Solvent effects on singlet oxygen yield from n,π* and π,π* triplet carbonyl compounds. J Phys Chem. 1993;97:5032–5035. [Google Scholar]
- 43.Kemp DR, Porter G. Nanosecond flash photolysis of α-tocopherylquinone. J Chem Soc A. 1971:3510–3513. [Google Scholar]
- 44.Creed D. The role of an o-quinone methide in the photochemistry of duroquinone. Chem Comm. 1976;4:121–122. [Google Scholar]
- 45.Bisby RH, Johnson SA, Parker AW. Quenching of reactive oxidative species by probucol and comparison with other antioxidants. Free Rad Biol Med. 1996;20:411–420. doi: 10.1016/0891-5849(95)02094-2. [DOI] [PubMed] [Google Scholar]
- 46.Bisby RH, Parker AW. Antioxidant reactions of dihydrolipoic acid and lipoamide with triplet duroquinone. Biochem Biophys Res Commun. 1998;244:263–267. doi: 10.1006/bbrc.1998.8245. [DOI] [PubMed] [Google Scholar]
- 47.Bisby RH, Parker AW. Structure of the radical from one-electron oxidation of 4-hydroxycinnamate. Free Rad Res. 2001;35:85091. doi: 10.1080/10715760100300621. [DOI] [PubMed] [Google Scholar]
- 48.Posener ML, Adams GE, Wardman P, Cundall RB. Mechanism of tryptophan oxidation by some inorganic radical-anions - pulse-radiolysis study. J Chem Soc Faraday Trans I. 1976;72:2231–2239. [Google Scholar]
- 49.Thor H, Smith MT, Hartzell P, Bellomo G, Jewell SA, Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. J Biol Chem. 1982;257:12419–12425. [PubMed] [Google Scholar]
- 50.Thornton DE, Jones KH, Jiang Z, Zhang H, Liu G, Cornwell DG. Antioxidant and cytotoxic tocopheryl quinones in normal and cancer cells. Free Rad Biol Med. 1995;18:963–976. doi: 10.1016/0891-5849(94)00210-b. [DOI] [PubMed] [Google Scholar]
- 51.Al-Abdul-Wahid MS, Yu C-H, Batruch I, Evanics F, Pomés R, Prosser RS. A combined NMR and molecular dynamics study of the transmembrane solubility and diffusion rate profile of dioxygen in lipid bilayers. Biochemistry. 2006;45:10719–10728. doi: 10.1021/bi060270f. [DOI] [PubMed] [Google Scholar]
- 52.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3rd ed. Oxford: Oxford University Press; 1999. [Google Scholar]
- 53.Koufen P, Rück A, Brdiczka D, Wendt S, Wallimann T, Stark G. Free radical-induced inactivation of creatine kinase: influence on the octameric and dimeric states of the mitochondrial enzyme (Mib-CK) Biochem J. 1999;344:413–417. [PMC free article] [PubMed] [Google Scholar]
- 54.Moosmann B, Behl C. Cytoprotective antioxidant function of tyrosine and tryptophan residues in transmembrane proteins. Eur J Biochem. 2000;267:5687–5692. doi: 10.1046/j.1432-1327.2000.01658.x. [DOI] [PubMed] [Google Scholar]
- 55.Kramer-Strickland K, Krol ES, Liebler DC. UV-B-induced photooxidation of vitamin E in mouse skin. Chem Res Toxicol. 1999;12:187–191. doi: 10.1021/tx980204h. [DOI] [PubMed] [Google Scholar]
- 56.Cilento G, Baptista RC, Brunetti IL. Triplet carbonyls: from photophysics to biochemistry. J Mol Struct. 1994;324:45–48. [Google Scholar]
- 57.Cilento G, Adam W. From free radicals to electronically excited species. Free Rad Biol Med. 1995;19:103–114. doi: 10.1016/0891-5849(95)00002-f. [DOI] [PubMed] [Google Scholar]