Abstract
Background
The quality and quantity of dietary carbohydrate intake, measured as dietary glycemic load (GL), is associated with a number of cardiovascular disease (CVD) risk factors and, in healthy young women, is related to increased high sensitivity C-reactive protein (hsCRP) concentrations. Our objective was to determine if GL is related to hsCRP and other measures of CVD risk in a population of sedentary, overweight, dyslipidemic middle-aged women and men enrolled in an exercise intervention trial (STRRIDE).
Methods
This was a cross-sectional evaluation of the relationships between measures of dietary carbohydrate intake, calculated from food frequency questionnaire data, and CVD risk factors, including plasma hsCRP, measured in 171 subjects.
Results
After adjusting for energy intake, GL and other measures of carbohydrate intake were not independently related to hsCRP (P>0.05 for all). In analyses performed separately for each gender, only the quantity of carbohydrate intake was independently related to hsCRP (R2=0.28; P<0.04), and this relationship was present for women but not for men. The strongest relationship identified between GL and any CVD risk factor was for cardiorespiratory fitness (R2=0.12; P<0.02); an elevated GL was associated with a lower level of fitness in all subjects, and this relationship persisted even when the findings were adjusted for energy intake and gender (R2=0.48; P<0.03).
Conclusions
In middle-aged, sedentary, overweight to mildly obese, dyslipidemic individuals, consuming a diet with a low GL is associated with better cardiorespiratory fitness. Our findings suggest that the current literature relating carbohydrate intake and hsCRP should be viewed with skepticism, especially in the extension to at-risk populations that include men.
INTRODUCTION
A number of recent reports have shown that elevated post-prandial blood glucose concentrations lead to increased metabolic and cardiovascular risk [reviewed in 1]. Since diet is a major determinant of post-prandial glucose response, these findings have re-focused attention on a well-known method for characterizing the impact of dietary carbohydrate on blood glucose response—the dietary glycemic load (GL). GL describes the quality, characterized by the glycemic index (GI), as well as quantity of carbohydrates consumed in the diet. The terms GI and GL are often used interchangeably, but they are not synonymous2. The GI of a food reflects the post-prandial glucose response to a single serving of that food; the GI of a diet reflects the average post-prandial glucose effect of single servings of all foods in a diet. GL takes into account the both the type and amount of carbohydrate consumed in a diet to indicate the overall predicted impact of quantity and quality of the dietary carbohydrate intake on the post-prandial glucose response.
Dietary GL has been associated with coronary heart disease3 and a number of cardiovascular risk factors, including risk of type 2 diabetes mellitus4, 5, reduced HDL-cholesterol6–8, and elevated triacylglycerol concentrations8. These associations suggest that diets producing a high post-prandial glucose load may lead to detrimental metabolic alterations in lipid profiles and insulin sensitivity. Moreover, a report linking GL with serum concentrations of high sensitivity C-reactive protein (hsCRP) in healthy women9 adds strength to the accumulating evidence that determinants of insulin action and inflammatory pathways are connected10. As such, interventions comparing weight loss achieved by using a low GL diet versus a low fat diet11 or high GL diet12 suggest that a low GL diet imposes superior metabolic cardiovascular changes, including reductions in hsCRP concentrations. It is particularly notable that these interventions have been performed primarily in young, healthy, overweight, females (over 75%).
An acute phase reactant and independent predictor of cardiovascular disease risk, hsCRP shows promise as an indicator of metabolic responses to environmental influences such as dietary intake patterns. Since studies to date have included only healthy subjects and with a predominance of women, the primary objective of this work was to assess the relationship between dietary GL and serum hsCRP concentrations in a population of women and men at-risk for cardiovascular disease. A secondary objective was to assess the relationship between GL and other metabolic cardiovascular risk factors in this population.
METHODS
Study Design
This was a cross-sectional evaluation of subjects enrolled in the first Studies of Targeted Risk Reduction In Defined Exercise (STRRIDE I, ClinicalTrials.gov Identifier: NCT00200993), which examined individuals at-risk for cardiovascular disease living in Durham and Greeneville, NC, from 1999–2002. Relevant institutional review boards approved the research protocol, and informed consent was obtained for each subject. The data presented here were all collected at baseline, i.e., prior to treatment randomization.
Subjects
Inclusion criteria were inactivity (not participating in regular exercise), overweight to mild obesity (body mass index [BMI] 25–35 kg/m2), dyslipidemia (LDL-cholesterol of 130–190 mg/dl or HDL-cholesterol <40 mg/dl for men and <45 mg/dl for women), post-menopausal status for women, and age between 40 and 69. Exclusion criteria included the use of medications known to affect carbohydrate or lipid metabolism, participation in a dietary regimen designed to induce weight loss, presence of diabetes mellitus (fasting glucose>140 mg/dl), hypertension (BP>160/90 mmHg), known cardiovascular disease, tobacco use, and/or a musculoskeletal condition that would prohibit exercise training.
Metabolic and Cardiovascular Risk Factor Assessments
We measured anthropomorphic characteristics as previously described13, and visceral adiposity and subcutaneous adiposity with a single slice abdominal CT scan at L414, 15. Cardiorespiratory fitness was assessed with exercise treadmill testing and measured in maximal oxygen consumption (ml O2*min/kg), as previously described15.
Fasting plasma was collected from each participant and stored at −80° Celsius. Lipoprotein analyses were performed by LipoScience (Cary, NC) using nuclear magnetic resonance spectroscopy16. Fasting insulin (Access Immunoassay System, Beckman Coulter, Fullerton, CA) and fasting glucose (YSI model 2300 Stat Plus, Yellow Springs Instruments, Yellow Springs, OH) were measured as previously described17.
High sensitivity-CRP Measurement
We measured high sensitivity CRP concentrations with an ELISA (UBI) as previously described18. The manufacturer reported inter- and intra-assay variations of 6.6 to 10.8% and 3.9 to 9.2%, respectively. Each sample was assayed in duplicate with coefficients of variation less than 15%.
Assessment of dietary carbohydrate
Reported carbohydrate intake and dietary GL and GI were assessed using a semi-quantitative food frequency questionnaire, the Block 1998 Revision of the Health Habits and History Questionnaire or HHHQ, (Block Dietary Data Systems, Berkeley, California). HHHQs were completed by STRRIDE participants who indicated their consumption frequency and portion size for over 100 food items. Completed forms were analyzed by Block Dietary Data Systems for nutrient content. This information along with GI values for foods assigned by the Nutritional Epidemiology Core at the University of North Carolina (http://www.cnrc.unc.edu/core_b.htm) was used to calculate GL and GI according to the following formulae:
and Daily GI = [Daily GL/(Total g carbohydrate − Total g fiber)] × 100. GI was expressed relative to bread (Bread GI = 100).
The methodology for the development of the HHHQ19, and its validation for assessing the foods from which the GL and GI were derived20 have been previously reported. Data collection via a food frequency questionnaire such as the HHHQ is the preferred approach for GL/GI analysis in large studies where food by food calculation is impractical9.
Data analysis
We evaluated descriptive statistics including distributions for each variable. Variables with highly skewed distributions, fasting insulin, triacylglycerol, and hsCRP, were logarithmically transformed prior to statistical significance testing. Standard correlations were used to confirm assignment of GI values and to evaluate relationships between GL and cardiovascular risk factors. Analogous to the previously recommended residuals method,21 we used multivariable linear regression to account for the impact of HDL-cholesterol and energy intake on hsCRP and GL. For all models with more than a single predictor, two-way and three-way interaction terms were initially included in the full model. If the interaction term was not significant, it was removed, and the main effects model was refit. For all analyses, statistical significance was established at P<0.05, and statistics were performed using SAS Version 8.2 Enterprise Guide (SAS, Cary, NC). Power calculations were performed using Power and Precision 2.0 (Biostat, Englewood, NJ).
RESULTS
Relationships between carbohydrate intake and hsCRP
Descriptive data are presented in Table I. Our primary analysis of interest was to determine whether dietary carbohydrate quantity or quality is related to the surrogate of cardiovascular risk, hsCRP. Of the 171 STRRIDE participants who had completed HHHQs, 144 had plasma samples available for measurement of hsCRP concentrations. Since hsCRP screening recommendations for cardiovascular disease suggest excluding and repeating hsCRP values greater than 10 mg/L22, we evaluated the relationship between hsCRP and carbohydrate quality and quantity for only those subjects (n=136) with hsCRP concentrations less than 10 mg/L.
Table I.
Baseline Characteristics*
| All subjects | Women | Men | |
|---|---|---|---|
| n | 171 | 88 | 83 |
| Age† (y) | 53.0±6.6 (40–69) | 54.6±5.3 (43–66) | 51.3±7.4 (40–69) |
| Race | |||
| Asian | 4 | 3 | 1 |
| Black | 25 | 13 | 12 |
| Hispanic | 1 | 0 | 1 |
| White | 141 | 72 | 69 |
| BMI (kg/m2) | 29.6±3.0 (23.3–38.1) | 29.2±3.1 (23.3–38.1) | 29.9±2.8 (24.7–36.2) |
| hsCRP††,‡ (log) (mg/L) | 2.2±2.8 (0.1–58.5) | 2.9±2.9 (0.2–58.5) | 1.7±2.4 (0.1–22.8) |
| Total energy† (kcal) | 1789±706 (595–4045) | 1582±578 (595–3280) | 2008±765 (703–4045) |
| Glycemic load† | 155.3±65.9 (14.2–381.4) | 141.0±59.1 (14.2–381.4) | 170.5±69.5 (16.5–367.3) |
| Glycemic index (Bread = 100) | 75.9±5.9 (31.3–86.8) | 76.0±5.3 (57.0–86.8) | 75.8±6.6 (31.3–84.7) |
hsCRP = high sensitivity C-reactive protein
Data presented as means ± SD. Ranges are depicted in parentheses.
Significant gender differences present: P<0.05 (Unpaired Student’s t-test).
Geometric means presented.
First, we wished to determine if a relationship existed for GL and hsCRP concentrations. Given the significant inverse relationship between HDL-cholesterol concentrations and GL, and the fact that we have previously noted a positive relationship between HDL-cholesterol and hsCRP18, we hypothesized that HDL-cholesterol may be a confounder in the relationship between GL and hsCRP. Indeed, after adjusting for HDL-cholesterol concentrations, GL (P<0.04) was independently related to hsCRP and explained 14% of the variance in hsCRP (see Table II). After adjustment for energy intake and HDL-cholesterol, GL (P<0.18) was no longer an independent predictor of hsCRP concentrations, but together, GL, energy intake, and HDL-cholesterol accounted for 14% of the variance on hsCRP (see Table II).
Table II.
Relationships for hsCRP (log) (mg/L) and glycemic load, glycemic index, and daily carbohydrate.*
| All subjects n=136 | Women n=66 | Men n=76 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β coefficient | P value | Model R2 | β coefficient | P value | Model R2 | β coefficient | P value | Model R2 | |
| GL – | |||||||||
| Unadjusted | 0.000322 | 0.53 | <0.01 | 0.001354 | 0.12 | 0.04 | 0.000253 | 0.68 | <0.01 |
| Adjusted- HDLc | 0.001134 | 0.04 | 0.14† | 0.002126 | 0.01 | 0.25† | 0.000524 | 0.49 | 0.01 |
| Adjusted- HDLc + EI | 0.001610 | 0.18 | 0.14† | 0.002712 | 0.10 | 0.26† | −0.000352 | 0.85 | 0.01 |
|
| |||||||||
| GI (Bread = 100) – | 0.000067 | 0.99 | <0.01 | −0.009233 | 0.34 | 0.01 | 0.006140 | 0.36 | 0.01 |
| Unadjusted | |||||||||
| Adjusted- HDLc | 0.005164 | 0.36 | 0.11† | −0.0012543 | 0.89 | 0.14† | 0.006512 | 0.38 | 0.01 |
| Adjusted- HDLc + EI | 0.003686 | 0.52 | 0.13† | −0.0030889 | 0.74 | 0.21† | 0.005440 | 0.47 | 0.02 |
|
| |||||||||
| DC (g) – | 0.000273 | 0.47 | <0.01 | 0.001130 | 0.08 | 0.05 | 0.000152 | 0.75 | <0.01 |
| Unadjusted | |||||||||
| Adjusted- HDLc | 0.000862 | 0.03 | 0.14† | 0.001636 | 0.01 | 0.26† | 0.000375 | 0.52 | 0.01 |
| Adjusted- HDLc + EI | 0.001433 | 0.14 | 0.15† | 0.002995 | 0.04 | 0.28† | −0.0004429 | 0.75 | 0.02 |
hsCRP=high sensitivity C-reactive protein GL=glycemic load EI =energy intake GI=glycemic index DC=daily carbohydrate intake
Data analyzed with multivariable linear regression.
P<0.05 for the model, indicating a significant relationship between all “predictors” and hsCRP; P value listed within the table reflects whether the individual carbohydrate intake measure was significantly and independently related to hsCRP.
Second, we wished to determine if dietary carbohydrate quality, measured as GI, was related to hsCRP concentrations. In adjusted (HDL-cholesterol as well as energy intake + HDL-cholesterol) analyses, GI was not independently related to hsCRP concentrations, and the amount of variance in hsCRP was less than that found with GL (see Table II). When either GL or total daily carbohydrate were added to the energy intake and HDL-cholesterol-adjusted model containing GI, there was an improvement in the explanation of variance in hsCRP from 13% to 15%.
Next, we wished to determine whether the carbohydrate quantity alone, daily carbohydrate intake (DC), was related to hsCRP concentrations. Similar to the relationships seen for GL, after adjustment for HDL-cholesterol, total DC was significantly and independently related to hsCRP concentrations. Together, total DC (P<0.03) and HDL-cholesterol accounted for 14% of the variance in hsCRP in this subject population (see Table II). However, after adjustment for energy intake plus HDL-cholesterol, DC (P<0.14) was no longer an independent predictor of hsCRP concentrations (R2=0.15, model P<0.001). Furthermore, adding GL or GI to the model containing DC provided no improvement in its predictive capacity (R2=0.15 for all, model P<0.005 for all).
Relationships between carbohydrate intake and hsCRP for men and women
Taking note of published reports of gender differences in susceptibility to a number of cardiovascular risk factors, a key part of our primary objective for this analysis was to determine if the relationship between hsCRP and GL was different in men versus women. As part of this analysis, we tested the interaction term gender*GL; it was not found to be a significant independent predictor for hsCRP (unadjusted GL*gender: P<0.29; HDLc adjusted GL*gender: P<0.21; HDLc and EI adjusted GL*gender: P<0.21). However, based on the aforementioned a priori evidence of relationships between hsCRP and metabolic cardiovascular risks in women18 but no reports of these relationships in men, we did evaluate the associations between carbohydrate intake and hsCRP separately by gender group.
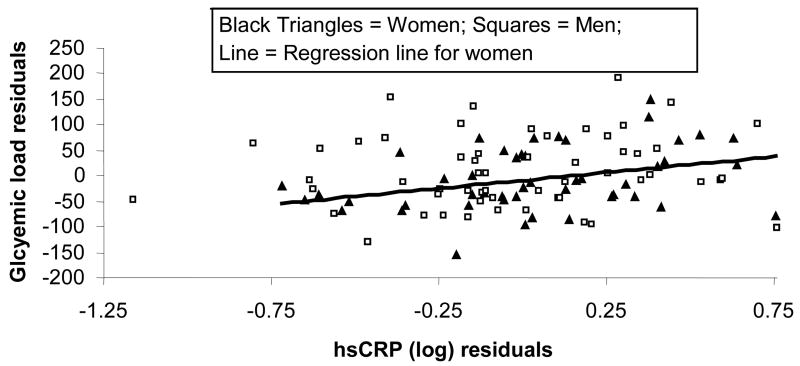
In women, both HDL-cholesterol and GL were independent predictors of hsCRP and accounted for 25% of the variance (P<0.002) (see Figure 1). In contrast, even after adjusting for HDL-cholesterol concentrations, we found no significant relationship between GL and hsCRP in men (P<0.78). GI was not an independent predictor of hsCRP in any analysis (see Table II). Interestingly, we found that total DC intake (P<0.01) and HDL-cholesterol (P<0.01) were each independently related to and together explained 26% of the variance in hsCRP in women (model P<0.001), but neither variable explained any portion of the variance in hsCRP in men (model P<0.81). When we controlled for both energy intake and HDL-cholesterol, total DC was independently (P<0.04) related to hsCRP in women and accounted for 28% of the variance in hsCRP (model P<0.003). These relationships were not apparent for men (model P<0.84).
Figure 1. Relationship between glycemic load and hsCRP after adjusting each for their relationship with HDL-cholesterol concentrations.
hsCRP (log) = 0.403*HDL-cholesterol + 0.001*Glycemic load − 0.361; R2=0.14; P<0.0005; hsCRPwomen (log) = 0.357*HDL-cholesterol + 0.002*Glycemic load − 0.397; R2=0.25; P<0.002. Relationship for men alone was not significant. hsCRP= high sensitivity C-reactive protein.
Relationships for carbohydrate intake and cardiovascular risk factors
In addition to evaluating the relationship between GL and hsCRP, a secondary objective of this study was to evaluate the relationship between GL and other cardiovascular risk factors. Prior to adjusting for energy intake, we found significant relationships between dietary GL and several known metabolic cardiovascular risk factors. GL was correlated with weight (r=0.24, P<0.001), minimal waist circumference (r=0.22, P<0.007), cardiorespiratory fitness (r=0.18, P<0.02), visceral adiposity (r=0.22, P<0.005), and HDL-cholesterol (r=−0.22, P<0.01). Additionally, we noted trends toward statistical significance in the relationships between GL and BMI (P<0.10) and triacylglycerol (P<0.06). We found no relationship between GL and age, subcutaneous adiposity, percent body fat, fasting plasma glucose, fasting insulin, mean blood pressure, or LDL-cholesterol.
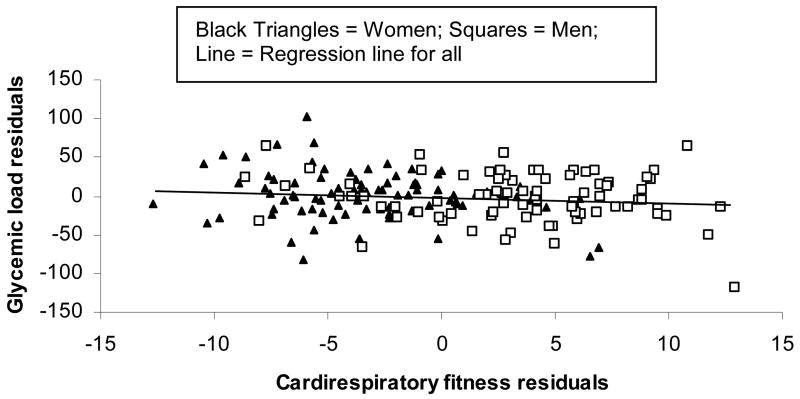
After adjustment for energy intake, GL (P<0.02) was independently and inversely related to cardiorespiratory fitness (VO2max, ml*min/kg) (R2=0.12, model P<0.0001) (see Figure 2). The association between energy intake adjusted-GL and fitness was also independent of gender. Together, GL, energy intake, and gender account for 48% of the variance in cardiorespiratory fitness (model P<0.0001, PGL<0.03). There was no significant gender*GL interaction for the relationship between GL and cardiorespiratory fitness (p<0.32). All other relationships between metabolic cardiovascular risk factors and GL were no longer apparent after adjusting for energy intake.
Figure 2. Energy intake adjusted relationship between glycemic load and cardiorespiratory fitness (VO2 max).
Multivariable linear regression was used to predict cardiorespiratory fitness after adjustment for energy intake: n = 171, Cardiorespiratory fitness (ml*min/kg) = −0.03*GL + 0.005*kcal/day + 23.2; R2 = 0.12, model P<0.0001, PGL<0.02. GL remains an independent predictor (PGL<0.03) of cardiorespiratory fitness after accounting for gender. GL = Glycemic load.
Fasting insulin was correlated with GI (P<0.05), but the relationship between GI and fasting insulin was no longer apparent after adjustment for energy intake. We found no significant relationship between GI and any other risk factor (age, weight, BMI, minimal waist circumference, percent body fat, subcutaneous adiposity, visceral adiposity, cardiorespiratory fitness, HDL-cholesterol, LDL-cholesterol, triacylglycerol, fasting plasma glucose, fasting insulin, or mean blood pressure, P>0.05 for all) with or without adjustment for energy intake.
Power of the study
We believed that a meaningful effect of GL would be one that would explain 10% of the variance in hsCRP or contribute 5% to a known amount of variance. For the primary analysis, which included GL, HDL-cholesterol, and EI, we had 91% power (α=0.05) to detect a significant relationship explaining 10% of the variance in hsCRP. If HDL-cholesterol and EI together accounted for 10% of the variance in hsCRP, we had 80% power (α=0.05) to detect that GL might explain an additional 5% of the variance in hsCRP. Additionally, we had 79% power (α=0.05) to detect a significant interaction between gender and GL that would improve the explanation of variance from 10% to 15%.
DISCUSSION
Our findings indicate that in a subject demographic of sedentary, overweight to mildly obese, dyslipidemic middle-aged men and women, GL was related to a number of cardiovascular risk factors, including hsCRP, weight, waist circumference, visceral adiposity, and low HDL-cholesterol concentrations. However, after adjustment for energy intake, GL was inversely related to cardiorespiratory fitness, but other no relationships between GL and metabolic risk factors were sustained. The only measure of carbohydrate intake serving as an independent predictor of hsCRP concentrations after adjustment for energy intake was DC, and this relationship appeared stronger for women than for men.
Previously, the relationship between GL and hsCRP was demonstrated in a cross-sectional investigation in a population with only women9. Similarly, prospective investigations demonstrating reductions in hsCRP with low GL diets have been performed in samples with predominantly women11, 12. Here, in a well-characterized mixed-gender population at-risk for cardiovascular disease, the relationship between GL and hsCRP concentrations did not persist when adjusted for energy intake. Most interestingly, the relationships presented for hsCRP and all measures of carbohydrate intake appeared much more robust for women than men. These results led us to speculate that the relationship between carbohydrate intake and hsCRP may be stronger or, perhaps, specific for women, which might explain the current lack of such relationships reported for mixed-gender or male only populations.
Spurred by this relative lack of published relationships between hsCRP and GL in men, which persists even at the conclusion of this analysis, we believe that our a priori hypothesis of a gender difference justified the stratified analyses presented here. However, it is worth noting that we did not detect a significant interaction between gender and GL for the relationship with hsCRP. This finding suggests that we were underpowered to evaluate a subtle gender difference for this relationship. As such, given that we do not have power to definitively argue that the relationship between GL and hsCRP occurs only for women, we believe the remarkable differences in the relationships for women and men merit attention and highlight the need for future observational and prospective studies to carefully evaluate gender differences in relationships between carbohydrate intake and cardiovascular risk.
Such a gender disparity in the relationship between GL and hsCRP may reflect a propensity for hsCRP to better represent metabolic risk in women than men. Consistent with this hypothesis, we have previously reported that metabolic cardiovascular risk factors could account for close to 50% of the variance in hsCRP in women but only 17% in men18. Similarly, Frost et al6 reported that the inverse association between GI and HDL-cholesterol was stronger in women than in men.
Somewhat surprisingly, the relationship between GL and hsCRP was driven primarily by the total amount of carbohydrate consumed by the subjects. One might ask why an elevated consumption of carbohydrates would promote higher concentrations of an inflammatory molecule, such as hsCRP, when a high dietary GI would not. One possible explanation follows: CRP is produced in the liver, primarily in response to interleukin-6 (IL-6), but also to tumor necrosis factor-α (TNF-α) and IL-1, each of which has been invoked in insulin resistance pathways10. A higher GL diet may induce higher rates of IL-6 secretion from adipocytes (and thus elevated hsCRP) primarily as a result the higher caloric (and carbohydrate) load.
In addition to potential mechanistic links between diet and hsCRP, as suggested above, relationships between GL and cardiovascular risk factors may simply indicate correlations between disease risk and heath-related lifestyle choices. More importantly, it has not been determined whether lifestyle modifications, such as improving diet and exercise, can actually modulate hsCRP. A recent report demonstrated that a diet high in sucrose, a simple carbohydrate with a high GI, did not impose significant changes in hsCRP concentrations23. Such a finding would be consistent with observations by our group in this same population during the intervention portion of STRRIDE. While STRRIDE did not include a nutrition intervention, the exercise interventions produced marked improvements in a number of metabolic cardiovascular risk factors without producing any clinically relevant change in hsCRP22. Further work addressing the true role of hsCRP as a surrogate for changing cardiovascular risk is needed to address this issue.
To our knowledge, we are the first to describe a relationship between GL and cardiorespiratory fitness. Other reports have examined the relationships of GL and GI with self reported physical activity4, 5, 7, 24, but cardiorespiratory fitness has not been previously assessed in this context. The inverse relationship between GL and cardiorespiratory fitness prompts the conclusion that individuals who are more physically fit consciously choose a lower GL diet. However, this supposition seems unlikely given that this is a sedentary, middle-aged population that is relatively unfit and untrained. Based on other studies of GI/GL effects on metabolism, we hypothesize that lower GL diets may promote a higher VO2max. When administered 3 hours prior to a submaximal exercise bout, low GI diets promote increased fat oxidation and muscle glycogen sparing during exercise25. In the context of a low GL diet, performance on a maximal exercise test, represented here as VO2max, may be enhanced by a longer duration of fat oxidation and subsequent delay in shift to anaerobic glycolysis. Further studies are necessary to support or refute this hypothesis.
To summarize, we have shown that, in sedentary, overweight to mildly obese, dyslipidemic individuals, GL is inversely related to cardiovascular fitness. Also, our work suggests a potential gender-specific relationship between elevated hsCRP concentrations and a diet high in simple carbohydrates and in carbohydrate quantity. In addition to our limited ability to detect interactions in a moderately sized sample, another potential limitation of this study is the use of a relatively homogenous population of sedentary, dyslipidemic, overweight to mildly obese individuals. This could explain the disappearance of the GL/hsCRP relationship after adjustment for energy intake. Finally, our cross-sectional design precludes our ability to infer causal relationships. However, while recognizing these limitations, we urge others to view current literature with skepticism and recommend that future investigations relating cardiovascular risk, specifically hsCRP, and dietary carbohydrate intake will carefully consider potential gender disparities.
Acknowledgments
We would like to acknowledge the STRRIDE I participants and the rest of the STRRIDE research team at Duke University Medical Center and East Carolina University. Each author participated in the conception, design, experimental conduction and data analysis of this work. The manuscript was drafted by KMH and CWB and edited by all authors. This work was supported by the NHLBI (NIH) R01HL-57354 and NIA (NIH) P30 AGO28716-01 and AG028930-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beisswenger P, Heine RJ, Leiter LA, et al. Prandial glucose regulation in the glucose triad: emerging evidence and insights. Endocrine. 2004;25(3):195–202. doi: 10.1385/ENDO:25:3:195. [DOI] [PubMed] [Google Scholar]
- 2.Barclay AW, Brand-Miller JC, Wolever TM. Glycemic index, glycemic load, and glycemic response are not the same. Diabetes Care. 2005;28(7):1839–40. doi: 10.2337/diacare.28.7.1839. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Willett WC, Stampfer MJ, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71(6):1455–61. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 4.Salmeron J, Manson JE, Stampfer MJ, et al. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277(6):472–7. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 5.Salmeron J, Ascherio A, Rimm EB, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20(4):545–50. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- 6.Frost G, Leeds AA, Dore CJ, et al. Glycaemic index as a determinant of serum HDL-cholesterol concentration. Lancet. 1999;353(9158):1045–8. doi: 10.1016/s0140-6736(98)07164-5. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES, Liu S. Glycemic index and serum high-density lipoprotein cholesterol concentration among us adults. Arch Intern Med. 2001;161(4):572–6. doi: 10.1001/archinte.161.4.572. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Manson JE, Stampfer MJ, et al. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am J Clin Nutr. 2001;73(3):560–6. doi: 10.1093/ajcn/73.3.560. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Manson JE, Buring JE, et al. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75(3):492–8. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 10.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira MA, Swain J, Goldfine AB, et al. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292(20):2482–90. doi: 10.1001/jama.292.20.2482. [DOI] [PubMed] [Google Scholar]
- 12.Pittas AG, Roberts SB, Das SK, et al. The effects of the dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity (Silver Spring) 2006;14(12):2200–9. doi: 10.1038/oby.2006.258. [DOI] [PubMed] [Google Scholar]
- 13.Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE--a randomized controlled study. Arch Intern Med. 2004;164(1):31–9. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 14.Ellis KJ. Human body composition: in vivo methods. Physiol Rev. 2000;80(2):649–80. doi: 10.1152/physrev.2000.80.2.649. [DOI] [PubMed] [Google Scholar]
- 15.Kraus WE, Torgan CE, Duscha BD, et al. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE) Med Sci Sports Exerc. 2001;33(10):1774–84. doi: 10.1097/00005768-200110000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347(19):1483–92. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 17.Houmard JA, Tanner CJ, Slentz CA, et al. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96(1):101–6. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- 18.Huffman KM, Samsa GP, Slentz CA, et al. Response of high-sensitivity C-reactive protein to exercise training in an at-risk population. Am Heart J. 2006;152(4):793–800. doi: 10.1016/j.ahj.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Block G, Hartman AM, Dresser CM, et al. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–69. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 20.Mares-Perlman JA, Klein BE, Klein R, et al. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. J Nutr. 1993;123(3):489–501. doi: 10.1093/jn/123.3.489. [DOI] [PubMed] [Google Scholar]
- 21.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 22.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 23.Sorensen LB, Raben A, Stender S, et al. Effect of sucrose on inflammatory markers in overweight humans. Am J Clin Nutr. 2005;82(2):421–7. doi: 10.1093/ajcn.82.2.421. [DOI] [PubMed] [Google Scholar]
- 24.Schulze MB, Liu S, Rimm EB, et al. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80(2):348–56. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 25.Wee SL, Williams C, Tsintzas K, et al. Ingestion of a high-glycemic index meal increases muscle glycogen storage at rest but augments its utilization during subsequent exercise. J Appl Physiol. 2005;99(2):707–14. doi: 10.1152/japplphysiol.01261.2004. [DOI] [PubMed] [Google Scholar]