Abstract
The conventional methods for producing recombinant adeno-associated virus (rAAV) rely on transient transfection of adherent mammalian cells. To gain acceptance and achieve current good manufacturing process (cGMP) compliance, clinical grade rAAV production process should have the following qualities: simplicity, consistency, cost effectiveness, and scalability. Currently, the only viable method for producing rAAV in large-scale, e.g.≥1016 particles per production run, utilizes Baculovirus Expression Vectors (BEVs) and insect cells suspension cultures. The previously described rAAV production in 40 L culture using a stirred tank bioreactor requires special conditions for implementation and operation not available in all laboratories. Alternatives to producing rAAV in stirred-tank bioreactors are single-use, disposable bioreactors, e.g. Wave™. The disposable bags are purchased pre-sterilized thereby eliminating the need for end-user sterilization and also avoiding cleaning steps between production runs thus facilitating the production process. In this study, rAAV production in stirred tank and Wave™ bioreactors was compared. The working volumes were 10 L and 40 L for the stirred tank bioreactors and 5 L and 20 L for the Wave™ bioreactors. Comparable yields of rAAV, ~2e+13 particles per liter of cell culture were obtained in all volumes and configurations. These results demonstrate that producing rAAV in large scale using BEVs is reproducible, scalable, and independent of the bioreactor configuration. Keywords: adeno-associated vectors; large-scale production; stirred tank bioreactor; wave bioreactor; gene therapy.
1. Introduction
Recombinant adeno-associated virus vectors (rAAV) are an attractive system for therapeutic gene transfer (Warrington and Herzog, 2006). The small icosahedral virus capsids (20–25 nm diameter) distribute better interstitially than larger viruses or non-virus particles. Physically, the non-enveloped AAV particles are extraordinarily robust withstanding relatively harsh treatment during production and processing without inactivation thereby facilitating downstream processing (Smith et al., 2003). Significantly, AAV vectors are devoid of all virus genes and contain only the cis-acting elements required for vector genome replication, packaging, and transgene expression (Smith and Kotin, 2001). To continue pre-clinical and clinical studies, large amounts of rAAV are required especially for the systemically disseminated diseases.
Conventional rAAV production processes involve transient transfection of mammalian cells, typically HEK 293 cells. Achieving efficient transduction levels requires cell growth on solid support, either flat tissue culture plastic ware or roller bottles (Grieger and Samulski, 2005; Liu et al, 2003). Regardless of the format, the expansion of cells is dependent on the surface area. Therefore, ten-fold increases in cell number are not feasible beyond a certain level, e.g. 1,000 to 10,000 plates. Alternatively, suspension cell expansion is achieved volumetrically permitting a large increase in cell number simply by increasing the amount of medium in the bioreactor. The main concern with suspension culture is the ability to introduce efficiently the genetic components to produce rAAV.
Recently, a method for producing rAAV exploiting the baculovirus expression vector (BEV) system in insect cells was developed. Typically, this system uses three BEVs to provide the trans and cis acting components required for rAAV production (Urabe et al., 2002). In contrast to mammalian cell-based production, no additional helper virus functions, e.g., adenovirus early gene products are required to render the cell permissive for efficient rAAV production. The three AAV structural proteins, VP1, VP2, and VP3, are expressed at stoichiometric levels from one BEV and the transcription cassettes for the AAV non-structural proteins, Rep 52 and Rep 78, are carried on a second BEV and are also expressed stoichiometrically (Urabe et al., 2002). BEVs are commonly used in laboratories to produce recombinant proteins and virus-like particles (VLPs) (Latham et al., 2001; van Oers, 2006) and recently, to produce proteins used as vaccines (e.g. Galarza et al., 2005; Ye, et al., 2006). From a processing perspective, advantages of the BEV - insect cell system include: growth in serum-free culture medium, high tolerance to a range of osmolalities, linear scalability in suspension cultures, and high protein expression levels. Biologically, insect cells are capable of post-translational modification, e.g. glycosylation, as well as protein folding that more closely resembles mammalian cell processes than prokaryotic or fungal systems. The insect cell-based production system has the potential to alleviate the major bottleneck in the manufacturing of rAAV, viz. the requirement for transient transfection of adherent cells (Grieger and Samulski, 2005). Despite the advances in BEV technology and the production of rAAV in insect cells (Zeiser et al., 2000; Meghrous et al., 2005; Negrete et al., 2007), the practical aspects of large-scale production and processing of vector remains unresolved.
Recombinant AAV production in 40 L stirred tank bioreactor (STB) using BEVs has been reported (Negrete et al., 2007). However, the sterilize-in-place (SIP) stirred tank bioreactor represents a significant up-front capital investment and is not feasible for most laboratories doing in vitro analysis and preclinical studies. Additionally, specialized training is required and a background in biochemical engineering is helpful to operate the equipment. Another more accessible option for the production of rAAV using suspension cultures is the single-use disposable bioreactor such as the Wave™. This technology has several advantages including ease of operation in volumes ranging from 0.1 L to 500 L and use of sterile disposable cell bag that eliminates the sterilization and cleaning steps required between runs.
This study was designed to compare the production of rAAV in stirred tank bioreactor and wave bioreactor at two different scales. The results indicate that the yields of rAAV produced using the two bioreactor formats are comparable. Researchers conducting pre-clinical studies requiring relatively large amounts of rAAV can produce sufficient quantities of vector using Wave™ or Wave™-type bioreactors. Additionally, these results will facilitate the large-scale production optimization and bioprocessing scale-up.
2. Materials and Methods
2.1 Insect cell culture and cell counting
Spodoptera frugiperda Sf9 cells (Invitrogen Corp., Carlsbad, CA) were grown in serum-free medium in suspension (HyQSFX medium, HyClone, Logan, UT). Cells were grown in polycarbonate vented-top shake flasks (Corning Corp., Corning, NY) with medium not exceeding 30% of the total flask volume. Cultures were inoculated with 1e+06 cells/ml and incubated at 27°C ± 0.5°C in an orbital platform shaker at 120–140 rpm in ambient atmosphere. Cell density was determined using automated cell counting program (EasyCyte Mini, Guava Technologies, Inc, Hayward, CA) as described elsewhere (Phi-Wilson et al., 2001).
2.2 Production of baculovirus
Three BEVs were used to produce rAAV: 1. Bac-VP - containing the cap gene of AAV serotype 2 under the AcMNPV polyhedrin promoter; 2. Bac-Rep - containing Rep 78 and Rep 52 genes regulated by the modified immediate early promoter of Orgyia pseudotsugata MNPV and the AcMNPV polyhedrin promoter, respectively; and 3. Bac-GFP - the rAAV vector genome consisting of the AAV2 terminal repeats flanking contains the transgene for enhanced green fluorescent protein (eGFP) regulated by the AcNPV p10 and cytomegalovirus (CMV) immediate early promoters, respectively (Urabe et al., 2002). In the presence of Rep 78 and Rep 52, the vector genome is “rescued” from the baculovirus genome and replicates via the AAV origins to very high copy numbers. When co-infected with the capsid protein-expressing baculovirus, the de novo replicated rAAV genomes are packaged into the empty, virus-like particles.
BEV stocks were amplified in Sf9 cells grown in suspension in HyQSFX serum-free media. Cells growing in exponential phase at cell density of 2e+06 cells/ml and viability higher than 90% were infected with one of the three BEVs at a multiplicity of infection (MOI) of 0.05. Infected cells were incubated at 27°C ± 0.5°C in an orbital shaker platform rotating at 120–140 rpm. At 72 hpi, the cells were removed from the culture by centrifugation at 2,000 × g for 10 min. The clarified medium was filtered (0.2 μm) and stored at 4°C. The BEVs were titered by infecting Sf9 cells and detecting the presence of the baculovirus gp64 on cell surfaces using flow cytometry and an anti - gp64 monoclonal antibody (Mulvania et al., 2004).
2.3 Production of rAAV in shake flask
The production of rAAV at 200 ml scale was carried out in 1 L polycarbonate vented-top shake flasks (Corning Corp.). Flasks containing the HyQSFX serum free media (HyClone) were inoculated with 1e+06 cells/ml and incubated at 27°C ± 0.5°C in an orbital shaker platform rotating at 120–140 rpm in ambient atmosphere. When the cell density doubled cells were infected with the three BEVs (MOI = 3) and incubated for 72 h and processed (see below).
2.4 Production of rAAV in stirred tank bioreactor
In this study, two stirred tank bioreactors were used for the production of rAAV at 10 L and 40 L scales, a BioFlo 110 Modular Benchtop bioreactor (New Brunswick Scientific, Edison, NJ), and a jacketed, stainless steel SIP bioreactor, respectively. The former was equipped with a temperature regulated 10 L working volume glass vessel, a gas mixer and a primary control unit for monitoring and/or control different parameters (New Brunswick Scientific, Edison, NJ). The latter, a 40 L capacity working volume bioreactor, was equipped with a TRYTON controller (Pierre Guerin Technologies, Mauze, France).
In both cases, a pitch blade impeller maintained agitation at 100 rpm. The dissolved oxygen concentration was controlled at 30% of air saturation. Temperature was maintained at 27°C and pH was monitored without adjustment. Bioreactors were batch fed with HyQSFX serum-free media (HyClone) supplemented with 25 μg/ml gentamicin (Quality Biological, Inc., Gaithersburg, MD) and inoculated with Sf9 cells in exponential phase to obtain a cell density of 1e+06 cells/ml (viability greater than 90%). The three different baculoviruses were added (MOI = 3) when the cell density doubled. Cell culture was harvested after 72 hpi.
2.5 Production of rAAV in Wave™ Bioreactor
Production of rAAV was also carried out in a Wave™ bioreactor (System 20/50) (Wave Biotech, Bridgewater, NJ). Two sizes, 10 L and 50 L, of disposable cell culture bags were used with maximum working volume of 5 L and 25 L, respectively. Using the oxygen-air mix controller the cell culture bags were inflated with air before adding the serum free media HyQSFX supplemented with 25 μg/ml gentamicin (Quality Biological, Inc.). The medium was equilibrated to 27°C then inoculated with exponentially growing Sf9 cells, viability greater than 90%, to obtain a density of 1e+06 cells/ml. The rocker platform providing agitation was set to 25 cycles per minute at an angle of 9 degrees from horizontal. The air-oxygen mixer was set to 30% oxygen and was pumped into the headspace at a flow rate of 0.3 L/min providing both bag pressure and oxygen for cell culture respiration. The cells were infected with three different BEVs (MOI = 3) when the cell density doubled. The cell culture was harvested after 72 hpi and processed as described (see below).
2.6 Poly Ethylene Glycol (PEG) Precipitation and Gradient Ultracentrifugation
At each time point, the cell cultures were sampled and processed as follows: to lyse the cells, each 200 ml aliquot was subjected to three cycles of freezing and thawing at −80°C and 37°C, respectively. Centrifugation at 2,000 × g for 10 min removed particulates and insoluble materials from the cell lysate/medium. A 50% solution (w:v) of polyethylene glycol (PEG), 8000 average molecular mass (Sigma-Aldrich, St. Louis, MO), was added to the supernatant to a final concentration of 2% (w:v) and incubated overnight at 4°C with gentle agitation. The PEG-precipitated material was pelleted by centrifugation at 5,000 × g for 30 min. The supernatant was decanted and the pellet was resuspended in 12 ml of CsCl solution (Invitrogen, Carlsbad, CA) (refractive index = 1.372; corresponding to 1.408 g/cm3). The samples were centrifuged to equilibrium at 38,000 rpm (178,305 × g average force) for approximately 72 h at 20 °C in a Beckman SW41 swinging bucket rotor. After centrifugation, the gradients were harvested by side-puncture into 1 ml fractions. Fraction aliquots were equilibrated with phosphate buffered saline, pH 7.4 (PBS) plus 2mM MgCl2 by dialysis using 10,000 MWCO membranes (Pierce, Rockford, IL).
2.7 SDS-PAGE and western blot
Dialyzed ultracentrifugation gradient fractions were analyzed by SDS-polyacrylamide electrophoresis (PAGE) using precast 4–12% acrylamide bis-Tris gels and MOPS SDS running buffer (NuPAGE System, Invitrogen Corp., Carlsbad, CA). Samples applied to each lane consisted of 13 μl gradient sample with 5 μl sample loading buffer and 2 μl sample reducing agent. After 10 min of heating at 70°C, only 10 μl of the prepared sample was loaded to the gel. Samples were fractionated by electrophoresis (190 V for 50 – 70 min) then stained for 1 h (Simply Blue Safe Stain, Invitrogen) and distained with distilled water for 1 h.
For western blot analysis, proteins separated by SDS-PAGE were transferred to a nitrocellulose membranes (Invitrogen) using NuPAGE Transfer buffer and 10% methyl alcohol at 28V for 1.5 h. Membranes were blocked with 5% non-fat milk in PBS (pH 7.4) and 0.05% Tween 20 (Sigma-Aldrich) (PBST) for 30 min. Rabbit anti-serum that recognizes multiple AAV serotype capsid proteins was produced in-house and anti-rabbit IgG horse radish peroxidase (HRP)-conjugated (whole molecule) antibody (Sigma-Aldrich) were used for detecting capsid antigens. Both primary and secondary antibodies were diluted 1:5000 in 5% non-fat milk/PBST prior to use. Membranes were incubated with antibodies for 1 h then washed 3 times with PBST. Chemiluminescent signal produced from HRP catalyzed oxidation of enhanced luminol substrate (Chemiluminescence Reagent Plus, PerkinElmer Life Sciences Inc., Boston, MA) was documented using Kodak scientific imaging film (Biomax MS, Kodak, Rochester, NY).
2.8 Infective rAAV determination by analysis of transducing units (tu)
Biological activity of rAAV was assessed by transducing adherent human embryonic kidney (HEK) 293 cells in Dulbecco’s modified Eagle nutrient mixture F12 (D-MEM/F-12) medium (Invitrogen) supplemented with heat inactivated 10% fetal bovine serum (HyClone). Twenty thousand cells per well in 0.5 ml medium were seeded into 24 well plates and maintained at 37°C for 24 h in a 5% CO2 humidified incubator overnight. The rAAV samples were diluted serially and 1μl was added per well and plates were incubated for an additional 48 h. The medium was removed and the cells were detached from the plates by adding 200 μl trypsin (0.05%) – EDTA (0.02%) (Invitrogen). Fresh D-MEM/F12 medium was added to complete 500 μl and the cell suspension was filtered using 5 ml polystyrene round bottom tubes with cell-strainer cap (Becton Dickinson). The percentage of GFP-positive cells in the sample was determined by analyzing 10,000 cells by flow cytometry (FACSCalibur, Becton Dickinson, Franklin Lakes, NJ) and ranged between 2% to 30%. The rAAV transducing titer was calculated from the number of GFP positive cells and the dilution factor. The result obtained is expressed in tu/ml. This result was multiplied by 5 to obtain the total yield of tu per litter.
2.9 Full particle number determination by analysis of vector genomes (vg)
The rAAV samples were diluted 1:100 in dH2O and copy number standards were made by serial dilutions of plasmid pFBGFPR in TE buffer, pH 8.0 (Quality Biological, Inc.) corresponding to a range of 104 to 108 single-stranded vector genomes per ml. Each 50μl PCR reaction consisted of supermix: 25 μl Quanti Tect™ SYBR® green (Qiagen, Valencia, CA), DNA primers 1 μM (final concentration) CMV upper primer 5′-AACGCCAATAGGGACTTTCC-3′ (Integrated DNA Technologies, Coralville, IA); 1 μM (final concentration) CMV lower primer 5′-GGGCGTACTTGGCATATGAT-3′ (Integrated DNA Technologies), and dH2O (Quality Biological, Inc.) to achieve a total volume of 49μl. One μl of diluted samples or standards were added to the supermix. Using the following qPCR cycling profile: initial denaturation at 95 °C for 3min, followed by 40 cycles of 95 °C, 55 °C, and 72 °C. Each temperature in the cycle was maintained for 30 s. Concentration of vector genomes in the sample was calculated from standard curve (vg/ml = copy number from PCR × 1000 μl/ml × 100:1 dilution). The result obtained is expressed in vg/ml. This result was multiplied by 5 to obtain the total yield of vg per litter.
3. Results and Discussion
3.1 Protein components of rAAV produced in different configuration of bioreactors
The density of AAV particles containing a vector genome is about 1.41 g/cm3 whereas at the density of particles consisting only of capsid protein is approximately 1.37 g/cm3. The rAAV capsids consist of three proteins: VP1, VP2 and VP3, with molecular masses of 87 KDa, 72 KDa and 62 KDa, respectively (van Vliet et al., 2006). VP3 is the AAV major structural protein constituting approximately 80% of the capsid mass (Xie et al., 2004). Isopycnic CsCl gradients were used to separate AAV empty capsids from vector genome-containing particles. For comparison purposes among the different production configurations, an aliquot of the fraction corresponding to about 1.41g/cm3 from each production was subjected to SDS-PAGE and western blot analysis (Fig. 1). In all cases, samples obtained at 72hpi were analyzed.
Fig. 1.
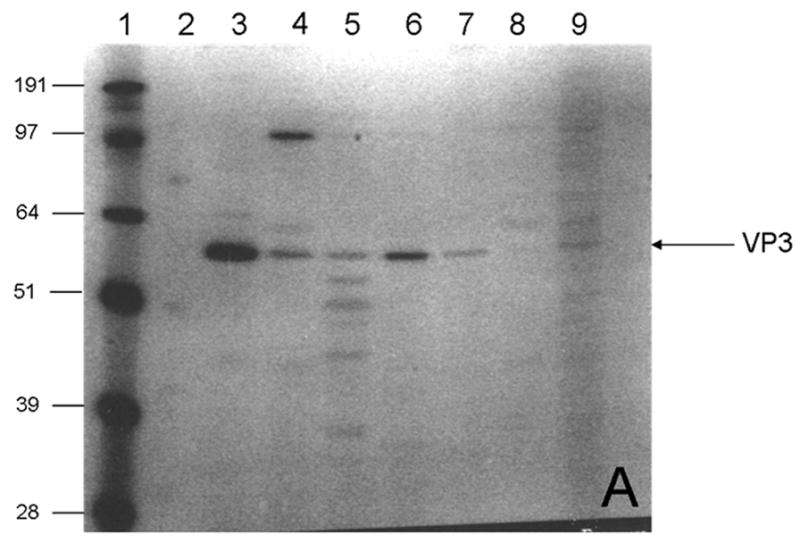
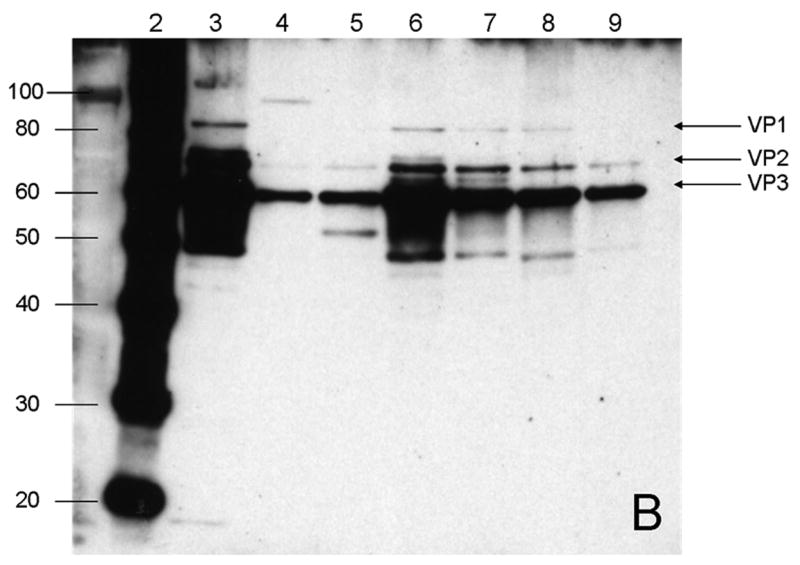
Distribution of rAAV capsid proteins (VP1, VP2 and VP3) analyzed by A) SDS-PAGE; and B) Western Blot. Only fractions with a density close to 1.41 g/cm3 of the CsCl gradient at 72hpi from each bioreactor configuration were analyzed. Lane 1) Low molecular weight marker; 2) Western blot marker. Densities in g/cm3 of the CsCl fractions for 3) 200ml, 1.4075; 4) 5L Wave, 1.4307; 5) 20 L Wave, 1.4128; 6) 10 L STB, 1.4180; 7) 40 L STB1, 1.4328; 8) 40L STB 2, 1.4159 and 9) 40L STB 3, 1.4117.
The rAAV major constituent protein VP3 is visible in stained gel (Fig. 1A, denoted by the arrow) for all bioreactor configurations. Western blots were used to detect more sensitively the capsid proteins of rAAV (Fig. 1B). The three capsid proteins of rAAV were observed in all the productions at different scales. This correlates with previous studies for the production of rAAV in insect cells (Urabe et al., 2002). The differences in intensities among the capsid protein bands obtained for different bioreactor configurations resulted from the accuracy in the selection of the initial point for fraction collection and volumes of the fractions collected after CsCl gradient ultracentrifugation.
To ascertain whether the rAAV-containing fractions also included baculovirus, the presence of the baculovirus envelope glycoprotein, gp64, was determined anti-gp64 antibody in a western blot of the gradient fractions. No gp64 antigen was detected in the rAAV-containing fractions; however, gp64 was detected in fractions at the top of the gradient at densities (≤1.31 g/cm3) lower than either filled rAAV or empty capsids. The density of BEV about 1.247 g/cm3 was determined previously (data not shown).
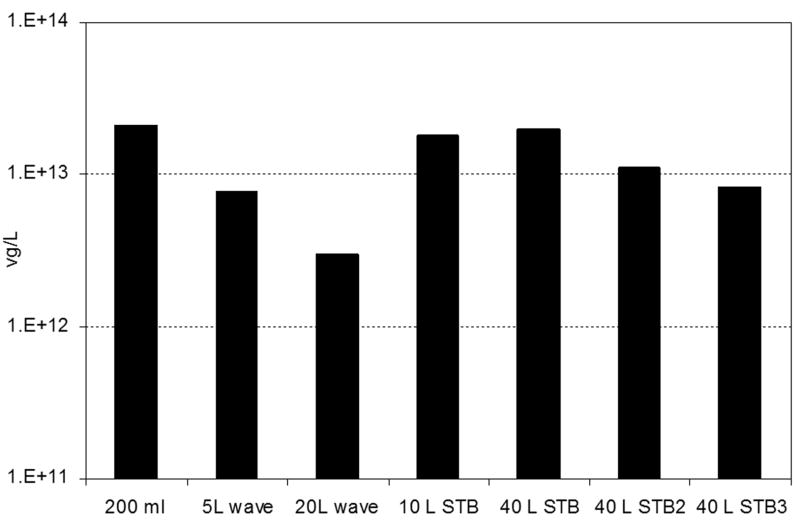
3.2 Yield of rAAV vector genomes (vg) in different configuration of bioreactors
The concentration of filled particles may be assessed using quantitative (q)PCR amplification of the vector genome. The qPCR results show the yield of vg per liter of cell culture harvested at 72 hpi (Fig. 2). The total amount of vg produced per liter of insect cell culture was calculated from the sum of vg in the CsCl gradient fractions corresponding to filled particles, i.e. 1.41 g/cm3 and flanking fractions, and normalized to 1L to compare among the different bioreactor configurations and scales. The average amount of vg produced corresponds to ~ 2e+13 particles per liter of cell culture. This value was comparable among the different configurations of bioreactors at each of the volumes studied except the yield from the 20 L Wave™. Maintaining the level of dissolved oxygen during the cell growth of Sf9 cells especially after infection is critical for recombinant protein production and correlates with the product yield (Saarinen and Murhammer, 2002; Gotoh et al., 2004). In the stirred tank bioreactor the dissolved oxygen can be controlled accurately, however, using the Wave™ bioreactor without a dissolved oxygen control loop e.g. where the signal from a dissolved oxygen probe in the medium is used to regulate the flow of O2 to the culture, the percentage of oxygen introduced to the headspace not always corresponds to the dissolved oxygen value. This may explain the lower amounts of rAAV produced in Wave bioreactors compared to stirred tank bioreactors. Despite the lower yields in the Wave™ bioreactor, it represents a reasonable alternative for producing large amounts of rAAV without the complex infrastructure required for stirred tank SIP bioreactor. It is not unreasonable to speculate that optimizing the O2 levels in the Wave™ would increase the rAAV production.
Fig. 2.
Yield of vector genomes of rAAV produced in different bioreactor configurations and scales. The yields correspond to 1L of cell culture harvested at 72hpi. The vg titers were obtained by qPCR.
The reproducibility between rAAV production batches was studied in stirred tank bioreactors. Similar yields (e.g. 1013 vg per liter of cell culture) of rAAV particles were obtained in three independent runs at 40L scale. For the replicates of the 40L productions, different batches of BEVs were used. One of the limitations of large scale production of rAAV exploiting the BEV - insect cell system is the genetic instability of the BEV upon passaging and amplification (Pijlman et al., 2003). However, in this case the use of different batches of baculovirus with similar passage number 3 did not affect the yields of rAAV. The yield of vg obtained in stirred tank bioreactor and wave bioreactor are comparable to the production at small scale in shake flask. This confirms that the system for producing rAAV by BEV is scalable.
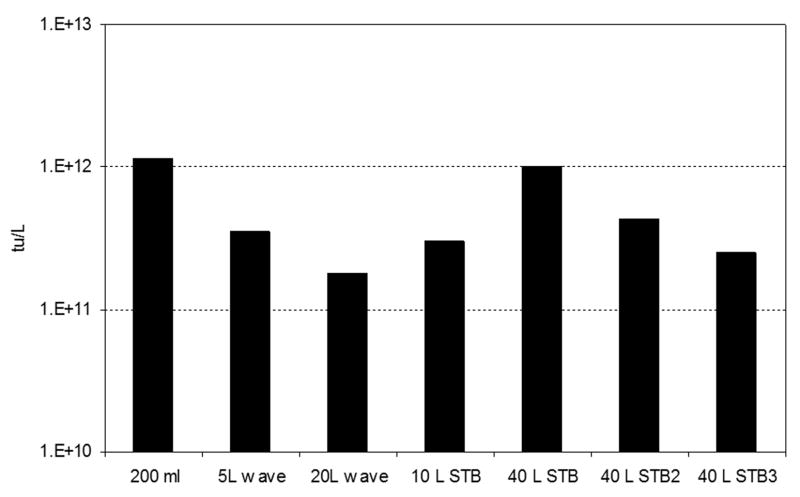
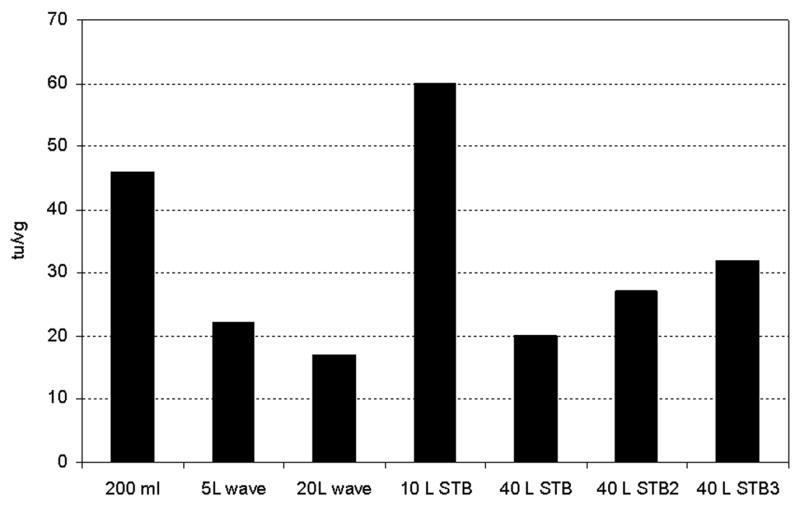
3.3 Yield of rAAV transducing units in different configuration of bioreactors
For gene therapy applications, the biological activity of the product is crucial. For this reason, the transducing activities (or biological activities) of the different rAAV preparations were determined using HEK 293 cells. Cells that produce the reporter gene product, eGFP, may be detected using flow cytometry. The yield of transducing units (tu) per liter of cell culture harvested at 72hpi is shown in Fig. 3. The amount of tu per liter was composed by the sum of tu across the CsCl gradient and normalized to 1L to compare among the different bioreactor configurations and scales. The average amount of tu obtained corresponded to ~1e+12 infective particles per liter of cell culture. This correlates to the yields reported previously (Negrete et al., 2007). The tu yields obtained were comparable among each bioreactor configuration and the 200ml production in shake flask. As expected, the tu profile agrees with that from the vg determined by qPCR (Fig. 2).
Fig. 3.
Yield of transducing units of rAAV produced in different bioreactor configurations and scales. The yields correspond to 1L of cell culture harvested at 72hpi. The tu titers were obtained by transducing HEK-293 cells.
3.4 Ratio of vg/tu in different configuration of bioreactors
One of the parameters useful for evaluating the quality of the rAAV is the vg/tu ratio (Fig. 4). This ratio represents the number of vector-containing particles required to produce a biologically measurable effect. Although the theoretical limit for vg/tu is 1, in practice this ratio may exceed 1000 even for good productions (Meghrous et al., 2005). However, it should be noted that measuring biological activity of a vector is not absolute and is affected by the sensitivity of the assay and the reporter gene product. In the present study, using HEK293 cells and flow cytometry to measure green fluorescent cells, the vg/tu ratio obtained was < 60 for vector produced in each of the culture configurations. This result demonstrates the consistency of the quality of the rAAV and is independent of the configuration of bioreactor and comparable to the production in shake flask. High quality product was also obtained in the production with the lowest yield.
Fig. 4.
Ratio of tu/vg for the rAAV produced in different configuration of bioreactors and scales.
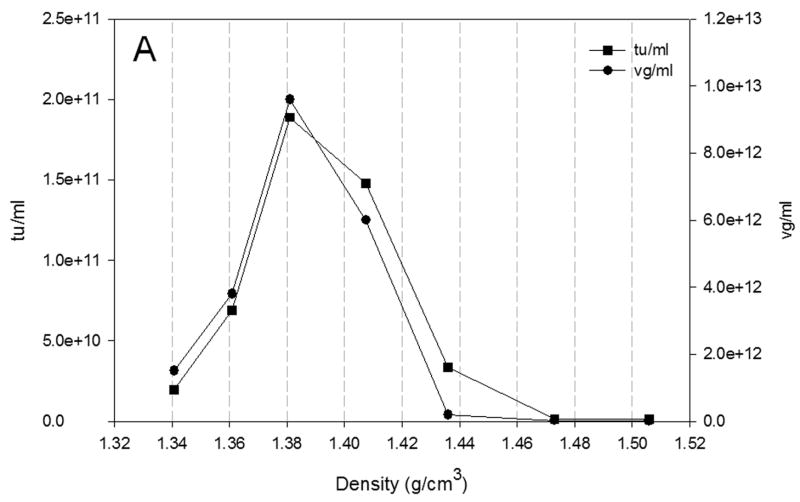
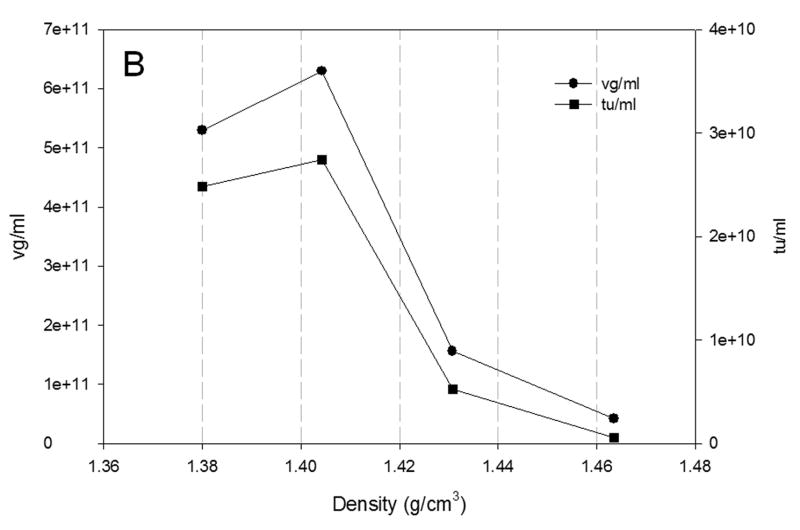
3.5 Distribution of rAAV across the CsCl gradient
The distribution of rAAV across the CsCl gradient is shown in Fig 5. The results of the production of rAAV at the largest scale studied in both configurations of bioreactors are presented. Fig. 5A shows the vg and tu from the production of rAAV at 200ml scale in shake flask. The distribution of vg and tu correlates with that reported previously at 40 L scale (Negrete et al., 2007). In Fig. 5A, the distribution profiles across the CsCl gradient of vg and tu are similar. The highest amounts of vg and tu were found at approximately 1.41 g/cm3 corresponding to full particles. This agrees with the results observed in Fig. 4 where the ratio vg/tu is less than 20. Similar pattern was observed for the production of rAAV at 20L scale in wave bioreactor (Fig. 5B) and at 40 L scale in stirred tank bioreactor (Fig. 5C). The difference in densities of the rAAV-containing fractions resulted from the slight variations in gradients and also in the selection of the initial point for fraction collection and volume of the fraction collected.
Fig. 5.
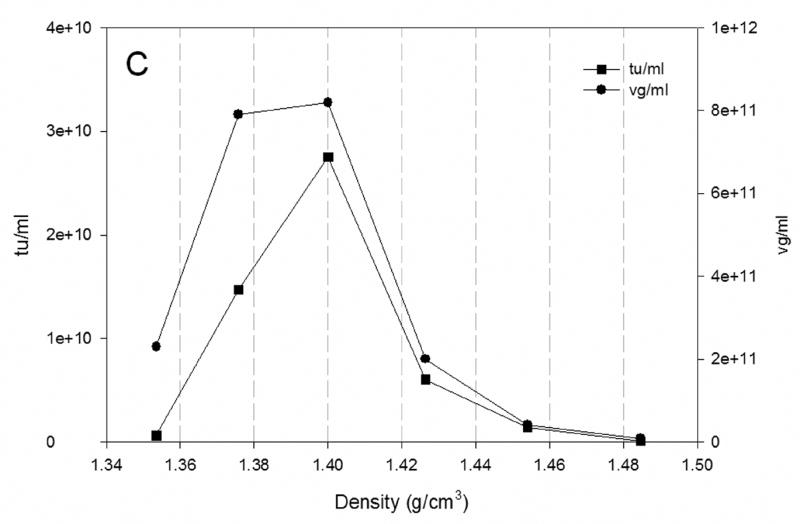
Distribution of vg (●) and tu (■) across the CsCl gradient from rAAV produced in A) shake flask; B) 20L wave bioreactor and C) 40L stirred tank bioreactor at 72 hpi. The vg titer was obtained by qPCR and the tu by transduction assay in HEK-293 cells.
In the present paper, the production of rAAV using wave bioreactor and stirred tank bioreactor were analyzed at 5 L and 20 L for the former and 10 L and 40L for the latter. The yields of biologically active rAAV obtained in each bioreactor configuration and volume was comparable to the yield obtained at the bench scale 200 ml shake flask. The vg/tu ratio obtained in each production was < 60 establishing consistency of vector quality. The use of this technology will facilitate the production of rAAV to satisfy the current and future demands of these vectors for gene therapy pre-clinical studies and clinical studies as well as potential commercial production. Because wave bioreactor can produce similar amounts of vector per liter as the stirred tank bioreactor, the relatively low capital investment required for setting-up a wave system permits many end-user laboratories access to large scale rAAV production. In addition to the relatively low cost of the Wave™ system, other attributes include: short training period for operators, use of disposable bags, accommodates a large range of culture volumes in a single instrument, e.g. 0.5 L to 25 L as determined by tissue culture bag volume, and scalability – Wave™ bioreactors are available up to 500L working volume. However, producing rAAV in stirred tank bioreactors has significant advantages particularly relevant to large- and commercial- scale production. Since the production costs are proportional to volume, at the largest volumes, detailed measurements and control of culture parameters ensure consistency between production runs. In addition, multi-thousand liter stirred tank bioreactors are in use and may be used for insect cell culture.
Acknowledgments
The authors wish to acknowledge J. Shiloach and L. Trinh from NIDDK for facilitating the use of the 40 L bioreactor and L. Yang from NHLBI for providing the baculovirus stocks.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Galarza JM, Latham T, Cupo A. Virus-like particle vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18:365–372. doi: 10.1089/vim.2005.18.365. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Chiba K, Kikuchi KI. Oxygen consumption profiles of SF-9 insect cells and their culture at low temperature to circumvent oxygen starvation. Biochem Eng J. 2004;17:71–78. [Google Scholar]
- Grieger JC, Samulski RJ. Adeno-associated virus as a gene therapy vector: development, production and clinical applications. Adv Biochem Eng Biotechnol. 2005;99:119–145. [PubMed] [Google Scholar]
- Latham T, Galarza JM. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J Virol. 2001;75:6154–6165. doi: 10.1128/JVI.75.13.6154-6165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Wagner K, Robinson N, Sabatino D, Margaritis P, Xiao W, Herzog RW. Optimized production of high-titer recombinant adeno-associated virus in roller bottles. Biotechniques. 2003;34:184–189. doi: 10.2144/03341dd07. [DOI] [PubMed] [Google Scholar]
- Meghrous J, Aucoin MG, Jacob D, Chahal PS, Arcand N, Kamen AA. Production of recombinant adeno-associated viral vectors using a baculovirus/insect cell suspension culture system: from shake flasks to a 20-L bioreactor. Biotechnol Prog. 2005;21:154–160. doi: 10.1021/bp049802e. [DOI] [PubMed] [Google Scholar]
- Mulvania T, Haynes B, Hedin D. A flow cytometric assay for rapid, accurate determination of baculovirus titers. Bioprocessing Journal. 2004;3:47–53. [Google Scholar]
- Negrete A, Esteban G, Kotin RM. On-line scanning spectroscopy for process optimization of large scale production of recombinant adeno-associated vectors. J Biotechnol. 2007 doi: 10.1007/s00253-007-1030-9. Submitted. [DOI] [PubMed] [Google Scholar]
- Phi-Wilson J, Harvey J, Goix P, O’Neill R. A technology for rapid acquisition of cell number and viability. Am Biotechnol Lab. 2001:34–36. [Google Scholar]
- Pijlman GP, van Schijndel JE, Vlak JM. Spontaneous excision of BAC vector sequences from bacmid-derived baculovirus expression vectors upon passage in insect cells. J Gen Virol. 2003;84:2669–2678. doi: 10.1099/vir.0.19438-0. [DOI] [PubMed] [Google Scholar]
- Saarinen MA, Murhammer DW. The response of virally infected insect cells to dissolved oxygen concentration: Recombinant protein production and oxidative damage. Biotechnol Bioeng. 2002;81:106–114. doi: 10.1002/bit.10460. [DOI] [PubMed] [Google Scholar]
- Smith RH, Ding C, Kotin RM. Serum-free production and column purification of adeno-associated virus type 5. J Virol Methods. 2003;114:115–124. doi: 10.1016/j.jviromet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Smith RH, Kotin RM. Adeno-associated viruses in. In: Tidona CA, Darai G, editors. The Springer index of viruses. Springer-Verlag New York; LCC: 2001. p. 1511. [Google Scholar]
- Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- van Oers MM. Vaccines for Viral and Parasitic Diseases Produced with Baculovirus Vectors. Adv Virus Res. 2006;68:193–253. doi: 10.1016/S0065-3527(06)68006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet K, Blouin V, Agbandje-McKenna M, Snyder RO. Proteolytic mapping of the adeno-associated virus capsid. Mol Ther. 2006;14:809–821. doi: 10.1016/j.ymthe.2006.08.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington KH, Jr, Herzog RW. Treatment of human disease by adeno-associated viral gene transfer. Hum Genet. 2006;119:571–603. doi: 10.1007/s00439-006-0165-6. [DOI] [PubMed] [Google Scholar]
- Xie Q, Hare J, Bu W, Jackson W, Turnigan J, Chapman MS. Large-scale preparation, purification and crystallization of wild-type adeno-associated virus 2. J Virol Methods. 2004;122:17–27. doi: 10.1016/j.jviromet.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Ye L, Lin J, Sun Y, Bennouna S, Lo M, Wu Q, Bu Z, Pulendran B, Compans RW, Yang C. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology. 2006;351:260–70. doi: 10.1016/j.virol.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Zeiser A, Elias CB, Voyer R, Jardin B, Kamen AA. On-line monitoring of physiological parameters of insect cell cultures during the growth and infection process. Biotechnol Prog. 2000;16:803–808. doi: 10.1021/bp000092w. [DOI] [PubMed] [Google Scholar]