Abstract
Many phytochemicals, the bioactive nonnutrient compounds found in plant foods, possess biologic effects associated with reduced risk of various diseases such as cancer. Genetic variation in pathways affecting absorption, metabolism, and distribution of phytochemicals is likely to influence exposure at the tissue level, thus modifying disease risk in individuals. Few studies have examined these gene-phytochemical interactions in humans. In this review, we discuss the sources of variation in metabolism and disposition of phytochemicals, and focus on two aspects of phytochemical handling that have received some attention: the impact of intestinal bacteria and genetically polymorphic phase II, conjugating enzymes.
Keywords: phytochemical, gut bacteria, genetic polymorphism, phase II biotransformation enzyme, interindividual variation
1. Introduction
Phytochemicals are bioactive nonnutrient chemical compounds found in plant foods, such as fruits, vegetables, grains, and other plant foods. They can be categorized into various groups, i.e., polyphenols, organosulfur compounds, carotenoids, alkaloids, and nitrogen-containing compounds. The polyphenols are some of the most studied compounds and can be further divided into flavonoids (including flavonols, flavones, catechins, flavanones, anthocyanidins, and isoflavones), phenolic acids, stilbenes, coumarins, and tannins [1].
Many phytochemicals are potent effectors of biologic processes and have the capacity to influence disease risk via several complementary and overlapping mechanisms [1–5]. In theory, genetic variation in pathways affecting absorption, metabolism, and distribution of phytochemicals is likely to influence exposure at the tissue level. Similarly, genetic variation in the pathways within which these compounds interact can alter biological response. However, beyond a few well-recognized conditions (e.g., glucose-6-phosphate dehydrogenase and vicine and covicine: favism), little is known about the biologic effects of genetic variation on these gene-phytochemical interactions in humans, particularly as it relates to cancer risk. Further, some phytochemicals undergo bacterial modification to produce metabolites that are more biologically active than the parent compounds. Few studies have systematically addressed the factors that contribute to the substantial variation in the metabolism and disposition of phytochemicals in vivo. Two aspects of phytochemical handling that are receiving some attention, particularly in relation to specific phytochemicals, are the impact of intestinal bacteria and genetically polymorphic phase II, conjugating enzymes.
2. Sources of variation in phytochemical metabolism and disposition
Researchers investigating the pharmacokinetics of phytochemicals in humans have observed substantial variation (reviewed in [6, 7]). Circulating concentrations of phytochemicals, such as psoralens, lignans, and the flavonoids naringenin and hesperitin, can vary widely among individuals even in the context of controlled feeding studies [8–10]. The process of phytochemical disposition, like that of disposition of drugs and other xenobiotics, involves absorption, metabolism, distribution, and excretion, and each of these parts may contribute to pharmacokinetic variability (Figure 1) [11].
Figure 1.
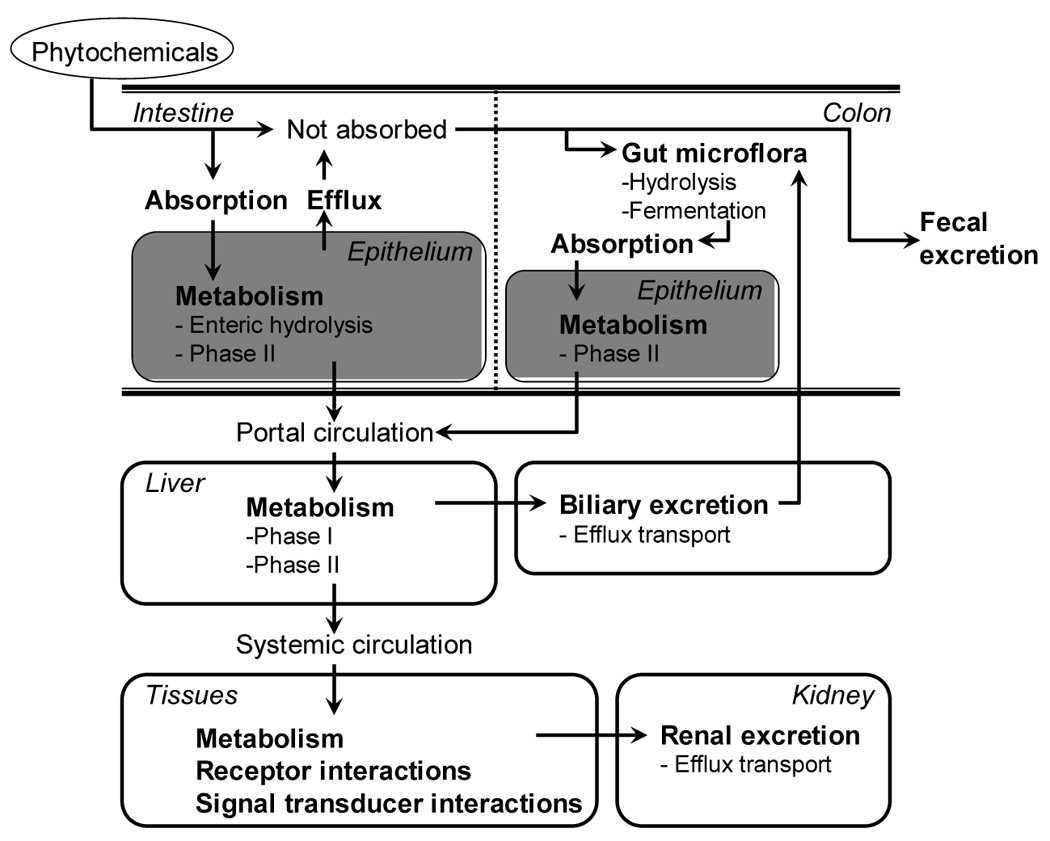
Sources of variation in phytochemical metabolism and disposition.
Many phytochemicals are present in plant foods as glycosides or other conjugates and need to be hydrolyzed in order to be absorbed [7]. This hydrolysis can be carried out by brush border membrane-bound β-glucosidases (e.g., lactase phlorizin hydrolase) or by gut bacterial β-glucosidases in the lower small intestine and colon. Once absorbed, aglycones undergo extensive first-pass metabolism in the gut epithelium or liver, with many compounds being conjugated with glutathione, glucuronic acid or, to a lesser extent, sulfate. Conjugation in the intestinal epithelium and liver by UDP-glucuronosyltransferases (UGT) and sulfotransferases (SULT) results in conjugates that are excreted in urine and bile. Those that are re-excreted through the bile duct are deconjugated by bacterial β-glucuronidase and can undergo enterohepatic recycling.
The transcellular transport of ingested food ingredients across the intestinal epithelium is another important factor determining bioavailability upon oral intake. For many phytochemicals and other xenobiotic compounds, this transcellular transport is dependent on the activity of membrane-bound, ATP-binding cassette (ABC) transport proteins, which are able to export the compounds from intestinal cells. ABC transporters can efflux a variety of conjugated and unconjugated compounds from the intestinal cells, either to the basolateral blood side, facilitating absorption, or back into the intestinal lumen, reducing bioavailability. The intestinal ABC transporters include P-glycoprotein (Pgp/MDR1/ABCB1), multidrug resistance proteins (MRPs/ABCCs) and breast cancer resistance protein (BCRP/ABCG2/ABCP/MXR) and these transporters are typically located specifically in the apical (intestinal luminal side) or basolateral (blood/plasma side) membrane of the enterocytes [12]. Animal and cell-based studies have demonstrated a role for P-gp and BCRP and other transporters in regulating the uptake of various flavonoids and other phytochemicals [13, 14]. Polymorphisms have been identified in ABCB1, ABCC1, ABCC2, and ABCG2; however, their impact on drug disposition in vivo are not well understood [15–17] and the implications for their effects on phytochemical efflux are unknown.
As with other xenobiotics, some phytochemicals undergo Phase I reactions in the liver. Several studies have shown, using human liver microsomes or monitoring metabolites in vivo in pharmacokinetic studies, that hydroxylation can occur at various positions on lignans, isoflavones, and other flavonoids, producing an array of novel secondary oxidation products [18–22]. However, oxidation products appear to be minor metabolites of most polyphenols, probably due to rapid conjugation of the would-be Phase I substrates in the intestinal epithelium and the liver. In contrast, isothiocyanates (ITC) derived from cruciferous vegetables have been shown to undergo extensive Phase I metabolism in rats [23]. Although it has not been determined to what extent these reactions occur in humans, theoretically, genetic variation in cytochrome P-450 (CYP) and other Phase I enzymes that can metabolize phytochemicals has the capacity to influence biologic response to phytochemicals [6].
In addition to the genetic variation that influences the degree of absorption, metabolism, and excretion of phytochemicals, variation in receptors and signal transducers that interact with phytochemicals has the potential to affect response to these plant constituents at the tissue level. For example, several estrogen receptor (ER) gene mutations resulted in receptors with altered binding to its natural agonist estradiol and concomitantly altered transcriptional properties [24–26]. Similarly, this may influence binding of other ER ligands, such as the phytoestrogens.
3. Phytochemical metabolism by gut bacteria
Gut bacteria can hydrolyze glycosides, glucuronides, sulfates, amides and esters [27]. They also carry out reduction, ring-cleavage, demethylation and dehydroxylation reactions [27–29]. The hydrolysis of glycosides and glucuronides typically results in metabolites that are more biologically active than the parent compounds. In contrast, further bacterial degradation and transformation of aglycones can lead to production of more or less active compounds, depending on the substrate being metabolized and the products formed.
Plant polyphenols, including phytoestrogens such as the isoflavones and lignans, are extensively metabolized in the gut by intestinal bacteria. To date, probably the most familiar example of interindividual differences in effects of host bacteria relates to isoflavone metabolism. Equol is produced from the soy isoflavone daidzein via the actions of intestinal bacteria. The production of equol in humans is of particular interest for several reasons, including: 1) there is substantial interindividual variation in the ability to produce equol, but the capacity to produce equol appears predominantly stable in an individual over time; 2) in vitro and in some animal models, equol is more biologically active than its precursor daidzein and the alternate metabolite O-desmethylangolensin; and 3) equol production has been associated with reduced risk of certain cancers and other diseases in humans (reviewed in [30]). In general, the proportion of equol-producing individuals is reported to be approximately one-third to one-half of the human population. Diet and host genetics may contribute to interindividual differences in equol production in humans; however, the reasons for such differences in the ability to harbor the equol-producing bacteria remain essentially unknown [30].
Wide interindividual differences in lignan metabolism also have been reported [31–36], but the reasons for such variation and the ultimate impact this may have on human health have not been fully evaluated. Plant lignans such as secoisolariciresinol diglucoside (SDG) and matairesinol, are metabolized by intestinal bacteria to the enterolignans (also known as mammalian lignans) enterodiol (END) and enterolactone (ENL). END can be further metabolized to ENL. Lignans are present in plants primarily as glycosides, and, upon ingestion, the sugar moieties are hydrolyzed to release the aglycones. Hydrolysis can be carried out by both bacterial β-glucosidases, and β-glucosidases in the human gut mucosal brush border [37]. The aglycones are absorbed or can be metabolized further by gut bacteria to the enterolignans. Matairesinol and SECO can be dehydroxylated and demethylated to form ENL and END, respectively [38]. Bacterial oxidation of END also occurs to yield ENL, but, while ENL can be reduced chemically to END, this reverse reaction does not appear to occur in vivo [39].
Matairesinol and SECO were long assumed to be the major plant lignans that could be converted to END and ENL; however more recently several studies have shown the capacity of gut bacteria to convert other plant lignans to enterolignans [38, 40]. Heinonen et al [38] reported that plant lignans including pinoresinol diglucoside, 7-hydroxymatairesinol, and lariciresinol could be converted to END and ENL. The efficiency of conversion of plant lignan precursors to END and ENL in 24-h incubations with human fecal inocula varied greatly depending on the compound and ranged from 0 to 100%. Studies have reported that urinary excretion of ENL is very low or nonexistent in some study participants challenged with SDG [9, 35, 36]; these results suggest that there may a subpopulation of individuals that lacks the bacteria or appropriate intestinal environment necessary for oxidation of END to ENL.
Interindividual differences in gut microbial community structure also affects metabolism of other polyphenols and ultimately human exposure to specific bioactive compounds. Isoxanthohumol, produced from the hop-derived prenylchalcone xanthohumol during the beer-making process, is the principal prenylflavonoid present in beer at concentrations ranging from 0.5 mg/L up to 4 mg/L [41]. It is metabolized by gut bacteria to 8-prenylnaringenin (8-PN), one of the most potent phytoestrogens currently known. Possemiers et al [42] reported that in vitro incubations of fecal samples from female volunteers resulted in varying degrees of conversion of isoxanthohumol to 8-PN ranging from approximately 7 to 79% after 72 h. The authors estimated that these interindividual differences in gut microbial activity could result in a 10-fold difference in intestinal 8-PN production after moderate beer consumption. Similarly, in another in vitro study, the intestinal bacterial communities from some individuals did not degrade the flavonoid naringenin, whereas those from others produced a range of intermediates, and those from some individuals completely degraded the compound [29]. Thus, differences in microbial biotransformation potential may result in the production of biologically-relevant concentrations of bacterially-derived phytochemicals in some individuals but not others, exerting differential health effect in the hosts.
Another group of compounds that undergo gut bacterial metabolism are the glucosinolates (β-thioglycoside N-hydroxysulfates) present in cruciferous vegetables. As with other plant compounds, the glucosinolates themselves are not biologically active, but some of their hydrolysis products, e.g. ITC and indole, are. The plant enzyme myrosinase, a β-thioglucosidase, co-occurs in plants producing glucosinolates and in intact plant tissues is located in a compartment separate from the glucosinolates. When the cells in plants are damaged (e.g., cut, ground, or chewed), enzyme and substrate come in contact, releasing the biologically active ITC. If myrosinase has been inactivated (e.g., with cooking), intestinal microbial metabolism of glucosinolates contributes to ITC exposure, albeit at a lower level [43]. Getahun and Chung (1999) observed large interindividual variation in excretion of dithiocarbamates (metabolites of ITC), ranging from 1 to 7% of the ingested glucosinolates for cooked watercress in which the plant myrosinase had been inactivated. Similarly, in a randomized, parallel arm of 400 µmol glucoraphanin/d (provided as a broccoli-sprout hot water extract, where ITC bioavailability is highly dependent on bacterial hydrolysis of the glucosinolates) vs. placebo, Kensler et al [44] reported large interindividual variation in overnight dithiocarbamate excretion, ranging from 1 to 45% of administered dose. Although these studies did not test the direct contribution of gut bacterial hydrolysis to the variation in response, the results suggest that interindividual differences in gut bacteria may play a role in availability of ITC.
4. Phytochemical metabolism by polymorphic phase II conjugating enzymes
Phytochemicals are metabolized in vivo by biotransformation enzymes in a manner similar to that of other xenobiotics. Many classes of phytochemicals are rapidly conjugated with glutathione, glucuronide, and sulfate moieties and excreted in urine and bile. Thus, in theory, polymorphisms in biotransformation enzymes, such as the glutathione S-transferases (GST), UGT, and SULT, have the capacity to affect phytochemical metabolism in the same fashion as they do carcinogens and other xenobiotics.
4.1. Glutathione S-Transferases
To date, probably, the most studied group of compounds in this regard is the ITC in cruciferous vegetables. The primary route of in vivo metabolism of ITC is by the mercapturic acid pathway, a major pathway for elimination of many xenobiotics [45]. Thiol conjugates of ITC are formed by conjugation with glutathione, catalyzed by GST. Subsequent, stepwise cleavage of glutamine and glycine yields L-cysteine-ITC, which are acetylated to produce N-acetyl-L-cysteine ITC conjugates (mercapturic acids); these are excreted in urine. Thus, GSTs play an important role in disposition of ITC in humans. Relationships between cruciferous vegetable intake and cancer risk may be influenced by genetic polymorphisms in biotransformation enzymes that metabolize ITC, as well as possibly in receptors and transcription factors that interact with these compounds.
In humans there are 3 major GST families: cytosolic GST, mitochondrial GST, and microsomal GST that are now referred to as membrane-associated proteins in eicosanoid and glutathione metabolism (MEPEG) [46]. Cytosolic GST are the largest GST family, containing 7 classes: α, ζ, θ, μ, π, σ, and ω GST. Two studies examined the ITC metabolism by different GST isozymes and found GSTM1-1 and GSTP1-1 to be the most efficient catalysts, GSTA1-1 to be less efficient, and GSTM2-2 and GSTM4-4 to be the least efficient [47, 48].
Depending on the population, 27 – 53% of people are GSTM1-null (no expression of GSTM1) and 20 – 47% of people in various ethnic groups are GSTT1-null (no expression of GSTT1) [49]. Investigators have hypothesized that individuals who are null for GSTM1 and GSTT1, and who therefore less readily conjugate and excrete ITC, would have greater amounts of ITC at the tissue level, and hence would experience a greater protective effect of glucosinolates consumption [49]. Results of one population-based study of ITC excretion among Chinese showed that urinary ITC was higher among GSTT1-positive, relative to GSTT1-null, individuals, but that GSTM1 and P1 genotypes had no effect [50]. In contrast, a recent pharmacokinetic study of sulforaphane disposition showed that GSTM1-null, relative to GSTM1-positive, individuals, had greater areas under the curve for plasma sulforaphane metabolite concentrations, faster rates of urinary sulforaphane metabolite excretion in the first 6 hours following consumption, and higher total excretion of sulforaphane and its metabolites over 24 h [51]. In a larger feeding study of a single meal containing 2.5 g broccoli/kg body weight, urinary ITC concentration did not differ by GSTM1, GSTP1, and GSTA1 genotypes except there was a tendency toward higher ITC excretion in GSTT1-positive individuals. Using a chi-square analysis, they observed a higher proportion of GSTM1-null individuals with high urinary ITC excretion compared to the proportion of GSTM1-positive individuals with high urinary ITC excretion [52]. Both of these studies were conducted using a single dose of broccoli, rather than more prolonged feeding that would be more comparable to habitual dietary practices among populations that routinely consume crucifers. Whether these differential responses are a function of acute versus chronic feeding or differences in the varieties of ITC present in broccoli versus crucifers commonly consumed in China remains to be established [51]; however, they speak to the further need to understand how genotype influences ITC disposition.
Polymorphisms in ITC-metabolizing enzymes may affect response of other biotransformation enzymes to ITC exposure. In one cross-sectional study, among frequent consumers of broccoli, GSTM1-null, relative to GSTM1-positive, individuals had a 21% higher CYP1A2 activity [53]. This relationship was not observed in a controlled feeding study designed to test a priori the effect of GSTM1 genotype on response to a diet high in cruciferous vegetables; increased CYP1A2 activity on the crucifer-containing diet was not affected by GSTM1 genotype [54]. However, in this same feeding study [55], serum GSTα concentration, a surrogate measure of hepatic GSTα and an enzyme also induced by ITC, increased significantly in response to cruciferous vegetable feeding, but only in GSTM1-null individuals.
4.2. UDP-Glucuronosyltransferases
The substrate-binding regions of the UGT genes are highly polymorphic and many result in amino acid changes that alter enzyme function to varying degrees in in vitro systems [56, 57]. The functional impact of an alteration in UGT protein is often substrate-specific. In vivo, where multiple UGTs are expressed in the same tissue, the overall impact is often less clear. UGT1A1 polymorphisms associated with Gilbert syndrome (fasting hyperbilirubinemia) have been most strongly associated with altered xenobiotic glucuronidation in vivo.
Dietary flavonoids are a structurally diverse class of compounds. This class includes the flavones and flavonols (e.g., apigenin, chrysin, galangin, luteolin, quercetin, etc.), flavanes (e.g., catechin, hesperetin, naringenin, etc.) and isoflavonoids (e.g., genistein, daidzein, etc.). The selectivity of glucuronosyl conjugation of the flavonoids is dependent both on the structure of a particular flavonoid as well as on the UGT enzyme involved in its conjugation. For example, UGT1A1, UGT1A8, and UGT1A9 have been shown to be especially active in conjugating luteolin and quercetin, whereas UGT1A4, UGT1A10, and UGT2B7 and UGT2B15 in the UGT2B family are less efficient [58]. The isoflavone genistein is conjugated by UGT1A3, UGT1A8, and, with less efficiency, by UGT1A1 and UGT1A10, but not by UGT2B15 [59, 60].
The effects of UGT polymorphisms on flavonoid clearance have not been examined; however, studies showing that polymorphisms affect glucuronidation and clearance of drugs and other xenobiotic compounds suggest that it is possible that similar effects may be seen for the dietary flavonoids. For example, the UGT1A1*28 polymorphism, which results in 30–40% lower UGT1A1 gene transcription among homozygous variant individuals, is associated with increased toxicity in colorectal cancer patients treated with the topoisomerase I inhibitor, irinotecan. Innocenti et al reported that, compared to *1/*1 and *1/*28 individuals, *28/*28 individuals had a higher prevalence of grade 4 neutropenia and a higher area under the curve (AUC) for the irinotecan metabolite SN-38 that requires glucuronidation in order to be cleared effectively [61]. Peters et al showed that, with exposure to well-cooked red meat (a source of mutagenic compounds) in a controlled feeding study, individuals with the *1/*28 and *28/*28 genotypes had a higher urinary mutagenicity index than did individuals with the *1/*1 genotypes [62]. The authors suggested that greater amounts of the mutagens were being excreted in the freeform, rather than being glucuronidated and deactivated. Further, Chung et al [63] showed that the UGT2B15 *2/*2 genotype was associated with a 0.58-fold lower systemic clearance compared with *1/*1 group for lorazepam in healthy volunteers. Although having this polymorphism may result in adverse responses in the context of exposure to toxic compounds or carcinogens, it may be beneficial in the context of reduced conjugation of phytochemicals. This has yet to be studied.
Other UGT polymorphisms have also been shown to speed drug clearance, but the impact on phytochemical metabolism remains unknown. For example, the UGT1A4 (L48V) variant glucuronidates tamoxifen and its active metabolites at a faster rate [64]. Women at high-risk for breast cancer who take tamoxifen as a chemopreventive agent, particularly those with the UGT1A4 (L48V) polymorphism, may experience reduced effectiveness of anti-estrogen therapy [64].
4.3. Sulfotransferases
Although the majority of flavonoid conjugates in circulation or excreted in urine are glucuronides, a fraction – 2 to 10% – are also sulfated by cytosolic SULT in the liver and gastrointestinal tract. Because sulfates can be deconjugated in target tissues, circulating sulfate conjugates of phytochemicals may act as a source of tissue aglycones. SULT1A1 has shown high sulfating activity with a variety of flavonoids, isoflavonoids, anti-oxidants, and other phenolic dietary compounds, and SULT1A3 also has high activity with the flavonoids, but not with isoflavonoids [65, 66]. Genetic variants in SULT genes with associated functional consequences on the translated protein have been identified. Single-nucleotide polymorphisms in SULT 1A1 and 2A1, are common and have been associated with altered response to therapeutic agents and sex steroid concentrations, respectively (reviewed in [67]). Similarly, this variability could influence the disposition of phytochemicals metabolized by SULT; however, this remains to be tested.
5. Summary
Intake of a particular phytochemical or its precursor does not necessarily equate with exposure at the tissue level. Interindividual differences in phytochemical metabolism and disposition may be affected by: gut microbial identity and activity; genetic determinants of biotransformation enzyme expression, stability, and activity; environmental exposures that influence gut microflora and biotransformation enzymes; and variation in levels of endogenous compounds that modulate biotransformation pathways. An enhanced understanding of the factors that contribute to interindividual differences in the metabolism and disposition of phytochemicals may allow for more comprehensive evaluation of the role of these dietary constituents in cancer prevention. With new molecular techniques available to characterize gut microbial communities, the bacteria involved in the rate-limiting steps of phytochemical metabolism can be characterized. Controlled pharmacokinetic studies that are powered sufficiently to test effects of genotype on phytochemical metabolism may help to identify and quantify the role of specific polymorphisms.
Acknowledgements
This work was supported in part by US NCI grants R01CA070913 and R01CA097366 and Fred Hutchinson Cancer Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134(12 Suppl):3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 2.Fresco P, Borges F, Diniz C, Marques MP. New insights on the anticancer properties of dietary polyphenols. Med Res Rev. 2006;26(6):747–766. doi: 10.1002/med.20060. [DOI] [PubMed] [Google Scholar]
- 3.Kar P, Laight D, Shaw KM, Cummings MH. Flavonoid-rich grapeseed extracts: a new approach in high cardiovascular risk patients? Int J Clin Pract. 2006;60(11):1484–1492. doi: 10.1111/j.1742-1241.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 4.Nichenametla SN, Taruscio TG, Barney DL, Exon JH. A review of the effects and mechanisms of polyphenolics in cancer. Crit Rev Food Sci Nutr. 2006;46(2):161–183. doi: 10.1080/10408390591000541. [DOI] [PubMed] [Google Scholar]
- 5.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55(3):207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Holst B, Williamson G. A critical review of the bioavailability of glucosinolates and related compounds. Nat Prod Rep. 2004;21(3):425–447. doi: 10.1039/b204039p. [DOI] [PubMed] [Google Scholar]
- 7.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 Suppl):230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 8.Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. 2001;131(2):235–241. doi: 10.1093/jn/131.2.235. [DOI] [PubMed] [Google Scholar]
- 9.Kuijsten A, Arts IC, Vree TB, Hollman PC. Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J Nutr. 2005;135(4):795–801. doi: 10.1093/jn/135.4.795. [DOI] [PubMed] [Google Scholar]
- 10.Shephard SE, Zogg M, Burg G, Panizzon RG. Measurement of 5-methoxypsoralen and 8-methoxypsoralen in saliva of PUVA patients as a noninvasive, clinically relevant alternative to monitoring in blood. Arch Dermatol Res. 1999;291(9):491–499. doi: 10.1007/s004030050443. [DOI] [PubMed] [Google Scholar]
- 11.Undevia SD, Gomez-Abuin G, Ratain MJ. Pharmacokinetic variability of anticancer agents. Nat Rev Cancer. 2005;5(6):447–458. doi: 10.1038/nrc1629. [DOI] [PubMed] [Google Scholar]
- 12.Brand W, Schutte ME, Williamson G, van Zanden JJ, Cnubben NH, Groten JP, et al. Flavonoid-mediated inhibition of intestinal ABC transporters may affect the oral bioavailability of drugs, food-borne toxic compounds and bioactive ingredients. Biomed Pharmacother. 2006;60(9):508–519. doi: 10.1016/j.biopha.2006.07.081. [DOI] [PubMed] [Google Scholar]
- 13.Sesink AL, Arts IC, de Boer VC, Breedveld P, Schellens JH, Hollman PC, et al. Breast cancer resistance protein (Bcrp1/Abcg2) limits net intestinal uptake of quercetin in rats by facilitating apical efflux of glucuronides. Mol Pharmacol. 2005;67(6):1999–2006. doi: 10.1124/mol.104.009753. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Cao J, Zeng S. Involvement of P-glycoprotein in regulating cellular levels of Ginkgo flavonols: quercetin, kaempferol, and isorhamnetin. J Pharm Pharmacol. 2005;57(6):751–758. doi: 10.1211/0022357056299. [DOI] [PubMed] [Google Scholar]
- 15.Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 2006;112(2):457–473. doi: 10.1016/j.pharmthera.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Ieiri I, Takane H, Hirota T, Otsubo K, Higuchi S. Genetic polymorphisms of drug transporters: pharmacokinetic and pharmacodynamic consequences in pharmacotherapy. Expert Opin Drug Metab Toxicol. 2006;2(5):651–674. doi: 10.1517/17425255.2.5.651. [DOI] [PubMed] [Google Scholar]
- 17.Kerb R. Implications of genetic polymorphisms in drug transporters for pharmacotherapy. Cancer Lett. 2006;234(1):4–33. doi: 10.1016/j.canlet.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs E, Kulling SE, Metzler M. Novel metabolites of the mammalian lignans enterolactone and enterodiol in human urine. J Steroid Biochem Mol Biol. 1999;68(56):211–218. doi: 10.1016/s0960-0760(99)00033-3. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs E, Metzler M. Oxidative metabolism of the mammalian lignans enterolactone and enterodiol by rat, pig, and human liver microsomes. J Agric Food Chem. 1999;47(3):1071–1077. doi: 10.1021/jf9809176. [DOI] [PubMed] [Google Scholar]
- 20.Otake Y, Walle T. Oxidation of the flavonoids galangin and kaempferide by human liver microsomes and CYP1A1, CYP1A2, and CYP2C9. Drug Metab Dispos. 2002;30(2):103–105. doi: 10.1124/dmd.30.2.103. [DOI] [PubMed] [Google Scholar]
- 21.Piver B, Fer M, Vitrac X, Merillon JM, Dreano Y, Berthou F, et al. Involvement of cytochrome P450 1A2 in the biotransformation of trans-resveratrol in human liver microsomes. Biochem Pharmacol. 2004;68(4):773–782. doi: 10.1016/j.bcp.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Potter GA, Patterson LH, Wanogho E, Perry PJ, Butler PC, Ijaz T, et al. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. Br J Cancer. 2002;86(5):774–778. doi: 10.1038/sj.bjc.6600197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kassahun K, Davis M, Hu P, Martin B, Baillie T. Biotransformation of the naturally occurring isothiocyanate sulforaphane in the rat: identification of phase I metabolites and glutathione conjugates. Chem Res Toxicol. 1997;10(11):1228–1233. doi: 10.1021/tx970080t. [DOI] [PubMed] [Google Scholar]
- 24.Miller N, Whelan J. Random mutagenesis of human estrogen receptor ligand binding domain identifies mutations that decrease sensitivity to estradiol and increase sensitivity to a diphenol indene-ol compound: basis for a regulatable expression system. J Steroid Biochem Mol Biol. 1998;64(3–4):129–135. doi: 10.1016/s0960-0760(97)00154-4. [DOI] [PubMed] [Google Scholar]
- 25.Murphy LC, Dotzlaw H, Leygue E, Douglas D, Coutts A, Watson PH. Estrogen receptor variants and mutations. J Steroid Biochem Mol Biol. 1997;62(5–6):363–372. doi: 10.1016/s0960-0760(97)00084-8. [DOI] [PubMed] [Google Scholar]
- 26.Oehler MK, Greschik H, Fischer DC, Tong X, Schuele R, Kieback DG. Functional characterization of somatic point mutations of the human estrogen receptor alpha (hERalpha) in adenomyosis uteri. Mol Hum Reprod. 2004;10(12):853–860. doi: 10.1093/molehr/gah113. [DOI] [PubMed] [Google Scholar]
- 27.Goldin BR. In situ bacterial metabolism and colon mutagens. Annu Rev Microbiol. 1986;40:367–393. doi: 10.1146/annurev.mi.40.100186.002055. [DOI] [PubMed] [Google Scholar]
- 28.Keppler K, Humpf HU. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg Med Chem. 2005;13(17):5195–5205. doi: 10.1016/j.bmc.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Rechner AR, Smith MA, Kuhnle G, Gibson GR, Debnam ES, Srai SK, et al. Colonic metabolism of dietary polyphenols: influence of structure on microbial fermentation products. Free Radic Biol Med. 2004;36(2):212–225. doi: 10.1016/j.freeradbiomed.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood) 2005;230(3):155–170. doi: 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]
- 31.Adlercreutz H, Fotsis T, Bannwart C, Wahala K, Makela T, Brunow G, et al. Determination of urinary lignans and phytoestrogen metabolites, potential antiestrogens and anticarcinogens, in urine of women on various habitual diets. J Steroid Biochem. 1986;25(5B):791–797. doi: 10.1016/0022-4731(86)90310-9. [DOI] [PubMed] [Google Scholar]
- 32.Adlercreutz H, Fotsis T, Heikkinen R, Dwyer JT, Goldin BR, Gorbach SL, et al. Diet and urinary excretion of lignans in female subjects. Med Biol. 1981;59(4):259–261. [PubMed] [Google Scholar]
- 33.Cunnane SC, Hamadeh MJ, Liede AC, Thompson LU, Wolever TM, Jenkins DJ. Nutritional attributes of traditional flaxseed in healthy young adults. Am J Clin Nutr. 1995;61(1):62–68. doi: 10.1093/ajcn/61.1.62. [DOI] [PubMed] [Google Scholar]
- 34.Hutchins AM, Martini MC, Olson BA, Thomas W, Slavin JL. Flaxseed consumption influences endogenous hormone concentrations in postmenopausal women. Nutr Cancer. 2001;39(1):58–65. doi: 10.1207/S15327914nc391_8. [DOI] [PubMed] [Google Scholar]
- 35.Lampe JW, Martini MC, Kurzer MS, Adlercreutz H, Slavin JL. Urinary lignan and isoflavonoid excretion in premenopausal women consuming flaxseed powder. Am J Clin Nutr. 1994;60(1):122–128. doi: 10.1093/ajcn/60.1.122. [DOI] [PubMed] [Google Scholar]
- 36.Nesbitt PD, Lam Y, Thompson LU. Human metabolism of mammalian lignan precursors in raw and processed flaxseed. Am J Clin Nutr. 1999;69(3):549–555. doi: 10.1093/ajcn/69.3.549. [DOI] [PubMed] [Google Scholar]
- 37.Rowland I, Faughnan M, Hoey L, Wahala K, Williamson G, Cassidy A. Bioavailability of phyto-oestrogens. Br J Nutr. 2003;89 Suppl 1:S45–S58. doi: 10.1079/BJN2002796. [DOI] [PubMed] [Google Scholar]
- 38.Heinonen S, Nurmi T, Liukkonen K, Poutanen K, Wahala K, Deyama T, et al. In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. J Agric Food Chem. 2001;49(7):3178–3186. doi: 10.1021/jf010038a. [DOI] [PubMed] [Google Scholar]
- 39.Setchell KDR, Adlercreutz H. In: Role of the Gut Flora in Toxicity and Cancer. Rowland I, editor. London: Academic Press; 1988. pp. 316–345. [Google Scholar]
- 40.Wang LQ, Meselhy MR, Li Y, Qin GW, Hattori M. Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chem Pharm Bull (Tokyo) 2000;48(11):1606–1610. doi: 10.1248/cpb.48.1606. [DOI] [PubMed] [Google Scholar]
- 41.Possemiers S, Heyerick A, Robbens V, De Keukeleire D, Verstraete W. Activation of proestrogens from hops (Humulus lupulus L.) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J Agric Food Chem. 2005;53(16):6281–6288. doi: 10.1021/jf0509714. [DOI] [PubMed] [Google Scholar]
- 42.Possemiers S, Bolca S, Grootaert C, Heyerick A, Decroos K, Dhooge W, et al. The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine. J Nutr. 2006;136(7):1862–1867. doi: 10.1093/jn/136.7.1862. [DOI] [PubMed] [Google Scholar]
- 43.Getahun SM, Chung FL. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol Biomarkers Prev. 1999;8(5):447–451. [PubMed] [Google Scholar]
- 44.Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14(11 Pt 1):2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 45.Conaway CC, Jiao D, Kohri T, Liebes L, Chung FL. Disposition and pharmacokinetics of phenethyl isothiocyanate and 6-phenylhexyl isothiocyanate in F344 rats. Drug Metab Dispos. 1999;27(1):13–20. [PubMed] [Google Scholar]
- 46.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 47.Kolm RH, Danielson UH, Zhang Y, Talalay P, Mannervik B. Isothiocyanates as substrates for human glutathione transferases: structure-activity studies. Biochem J. 1995;311(Pt 2):453–459. doi: 10.1042/bj3110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Kolm RH, Mannervik B, Talalay P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem Biophys Res Commun. 1995;206(2):748–755. doi: 10.1006/bbrc.1995.1106. [DOI] [PubMed] [Google Scholar]
- 49.Seow A, Vainio H, Yu MC. Effect of glutathione-S-transferase polymorphisms on the cancer preventive potential of isothiocyanates: an epidemiological perspective. Mutat Res. 2005;592(1–2):58–67. doi: 10.1016/j.mrfmmm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, et al. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev. 1998;7(9):775–781. [PubMed] [Google Scholar]
- 51.Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, et al. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82(6):1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 52.Steck SE, Gammon MD, Hebert JR, Wall DE, Zeisel SH. GSTM1, GSTT1, GSTP1, and GSTA1 polymorphisms and urinary isothiocyanate metabolites following broccoli consumption in humans. J Nutr. 2007;137(4):904–909. doi: 10.1093/jn/137.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Probst-Hensch NM, Tannenbaum SR, Chan KK, Coetzee GA, Ross RK, Yu MC. Absence of the glutathione S-transferase M1 gene increases cytochrome P4501A2 activity among frequent consumers of cruciferous vegetables in a Caucasian population. Cancer Epidemiol Biomarkers Prev. 1998;7(7):635–638. [PubMed] [Google Scholar]
- 54.Lampe JW, King IB, Li S, Grate MT, Barale KV, Chen C, et al. Brassica vegetables increase and apiaceous vegetables decrease cytochrome P450 1A2 activity in humans: changes in caffeine metabolite ratios in response to controlled vegetable diets. Carcinogenesis. 2000;21(6):1157–1162. [PubMed] [Google Scholar]
- 55.Lampe JW, Chen C, Li S, Prunty J, Grate MT, Meehan DE, et al. Modulation of human glutathione S-transferases by botanically defined vegetable diets. Cancer Epidemiol Biomarkers Prev. 2000;9(8):787–793. [PubMed] [Google Scholar]
- 56.Burchell B. Genetic variation of human UDP-glucuronosyltransferase: implications in disease and drug glucuronidation. Am J Pharmacogenomics. 2003;3(1):37–52. doi: 10.2165/00129785-200303010-00006. [DOI] [PubMed] [Google Scholar]
- 57.Maruo Y, Iwai M, Mori A, Sato H, Takeuchi Y. Polymorphism of UDP-glucuronosyltransferase and drug metabolism. Curr Drug Metab. 2005;6(2):91–99. doi: 10.2174/1389200053586064. [DOI] [PubMed] [Google Scholar]
- 58.Boersma MG, van der Woude H, Bogaards J, Boeren S, Vervoort J, Cnubben NH, et al. Regioselectivity of phase II metabolism of luteolin and quercetin by UDP-glucuronosyl transferases. Chem Res Toxicol. 2002;15(5):662–670. doi: 10.1021/tx0101705. [DOI] [PubMed] [Google Scholar]
- 59.Doerge DR, Chang HC, Churchwell MI, Holder CL. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos. 2000;28(3):298–307. [PubMed] [Google Scholar]
- 60.King CD, Rios GR, Green MD, Tephly TR. UDP-glucuronosyltransferases. Curr Drug Metab. 2000;1(2):143–161. doi: 10.2174/1389200003339171. [DOI] [PubMed] [Google Scholar]
- 61.Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol. 2004;22(8):1382–1388. doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 62.Peters U, Sinha R, Bell DA, Rothman N, Grant DJ, Watson MA, et al. Urinary mutagenesis and fried red meat intake: influence of cooking temperature, phenotype, and genotype of metabolizing enzymes in a controlled feeding study. Environ Mol Mutagen. 2004;43(1):53–74. doi: 10.1002/em.10205. [DOI] [PubMed] [Google Scholar]
- 63.Chung TW, Lee DI, Kim DS, Jin UH, Park C, Kim JG, et al. Molecular cloning and characterization of a large subunit of Salmonella typhimurium glutamate synthase (GOGAT) gene in Escherichia coli. J Microbiol. 2006;44(3):301–310. [PubMed] [Google Scholar]
- 64.Sun D, Chen G, Dellinger RW, Duncan K, Fang JL, Lazarus P. Characterization of tamoxifen and 4-hydroxytamoxifen glucuronidation by human UGT1A4 variants. Breast Cancer Res. 2006;8(4):R50. doi: 10.1186/bcr1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pai TG, Suiko M, Sakakibara Y, Liu MC. Sulfation of flavonoids and other phenolic dietary compounds by the human cytosolic sulfotransferases. Biochem Biophys Res Commun. 2001;285(5):1175–1179. doi: 10.1006/bbrc.2001.5316. [DOI] [PubMed] [Google Scholar]
- 66.Ronis MJ, Little JM, Barone GW, Chen G, Radominska-Pandya A, Badger TM. Sulfation of the isoflavones genistein and daidzein in human and rat liver and gastrointestinal tract. J Med Food. 2006;9(3):348–355. doi: 10.1089/jmf.2006.9.348. [DOI] [PubMed] [Google Scholar]
- 67.Nowell S, Falany CN. Pharmacogenetics of human cytosolic sulfotransferases. Oncogene. 2006;25(11):1673–1678. doi: 10.1038/sj.onc.1209376. [DOI] [PubMed] [Google Scholar]


