Abstract
Previously, we have shown that chronic exposure to environmental and social stimuli (ESS) during adolescence prevents the development of behavioral sensitization to amphetamine in adult rats. At the onset of the peripubertal-juvenile period (28-d) male rats were subjected to a 28-d long intermittent ESS protocol or handled as controls (NO-ESS). Twenty-four hours after the last session of ESS or NO-ESS, all rats started a regimen of behavioral sensitization to amphetamine (1 mg/kg, i.p.), in which rats were injected every third day with amphetamine or saline on four occasions. Then following one week abstinence all rats were challenged with a lower dose of amphetamine (0.5 mg/kg, i.p.) and their locomotor activity monitored for two hours. Our results showed that while NO-ESS rats developed behavioral sensitization to amphetamine, ESS rats did not develop this behavior. All rats were then sacrificed 3 days following the challenge to allow for amphetamine clearance.
Since mesolimbic dopamine has been implicated in behavioral sensitization to amphetamine we compared messenger RNA (mRNA) expression of key dopamine-related molecules in the mesolimbic circuitry in ESS and NO-ESS rats. A decrease in dopaminergic D1 receptor (D1R) gene expression in the caudate-putamen (CPu) was associated with amphetamine sensitization in the controls, possibly as a result of a chronic increase in DA release. In contrast, amphetamine treatment did not modulate D1R mRNA levels in ESS rats. No change has been detected in any other dopaminergic markers [D2R, D3R, tyrosine hydroxylase (TH) or dopamine transporter (DAT) mRNAs]. Consequently, we conclude that ESS may inhibit the development of behavioral sensitization to amphetamine through preventing the decrease in CPu D1R mRNA levels.
Introduction
Adolescence is the peak period in the human life span for development of addiction to various drugs. Biological as well as environmental factors experienced during development -such as sensory and environmental stimuli- play a major role in determining drug abuse vulnerability. Previous reports have shown that rats raised in impoverished environment have greater vulnerability to abuse drugs when compared to rats raised in social or enriched environments [1, 3, 12, 13, 30]. But, to our knowledge, there are no studies in rodents that have specifically explored adolescence as a critical developmental period where increased social interactions and exposures to novel environments may protect against drug taking behavior during adulthood. The incentive-sensitization theory of addiction states that addictive behavior is the consequence of progressive and persistent neuroadaptations caused by repeated drug use [22, 23]. Repeated intermittent administration of psychostimulants, such as amphetamine and cocaine, results in a progressive and long-lasting enhancement of behavioral responses elicited by a subsequent challenge injection of these drugs, which is the phenomenon termed behavioral sensitization. Behavioral sensitization to psychostimulants is thought to be relevant to addiction in humans because of the assumption that the neural substrate that mediates these effects is the same as, or at least overlaps with, the neural substrate responsible for the reinforcing effects of drugs [34].
A large body of literature implicates the mesolimbic dopaminergic (DA) circuitry in the development and the expression of behavioral sensitization to amphetamine [20, 25, 29]. The development of behavioral sensitization to amphetamine requires the action of this drug on DA cell bodies in the ventral tegmental area (VTA) [6, 20, 32]. The expression of behavioral sensitization to amphetamine is believed to occur at the level of the nucleus accumbens (NAc) where DA terminals are located [6], in part through an increase in DA release in this area [16, 17, 24]. In the current study, we have investigated mRNA regulation of genes modulating dopaminergic neural transmission in response to amphetamine treatment in the brain regions constituting the classic mesolimbic circuitry.
In a previous study we showed that rats subjected to 28 days of ESS during adolescence do not develop behavioral sensitization to amphetamine when compared to NO-ESS rats ([15]; figure 1A). Using the brain tissue from the 2002 study, we investigated changes in DA circuitry that may be the underlying mechanism(s) for the differences in the expression of behavioral sensitization between ESS and NO-ESS conditions. Specifically, we screen for changes in D1, D2 and D3 DA receptors, TH, and DAT mRNAs in the VTA, the NAc (shell and core), the CPu and the substantia nigra (SN) after repeated amphetamine or saline injections following a 28-d regimen of ESS or NO-ESS.
Figure1.
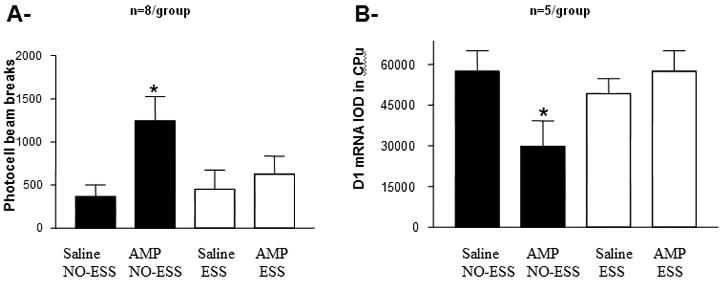
(A) modified figure from Kabbaj et al, 2002 showing that only control rats exhibit behavioral sensitization to amphetamine. ESS rats do not exhibit behavioral sensitization to the same drug. *=p<0.05 when compared to their respective saline pre-trained controls. (B) Control rats that exhibit behavioral sensitization to amphetamine show a decrease in D1 mRNA expression; ESS rats that do not exhibit sensitization to amphetamine do not show the decrease in D1 mRNA expression. *=p<0.05 when compared to their respective saline pre-trained controls.
Materials and methods
Thirty-two 21-d old male Sprague-Dawley rats (Charles River, Wilmington MA) were used in this study. Upon arrival, rats were housed four per cage, and kept on a 12h-12h light-dark cycle (lights on at 07.00 a.m.). Food and water were available adlibitum.
After 7 days of habituation to the housing conditions, 16 rats were subjected to a 28-day intermittent, ESS regimen consisting of five randomly assigned environmental or social stimuli. The other 16 rats were subjected to 1 min of handling every day for 28 days (NO-ESS). The exposure to ESS fell under a period roughly defined as the peripubertal-juvenile period (postnatal day 28-56). The day after the last ESS or NOESS sessions, rats were given 1 hr to habituate to the locomotor chambers before they get an injection of D-amphetamine sulfate (1 mg/kg, i.p.), or saline (1ml/kg, i.p.). Their locomotor response was recorded for 2 hrs individually. This procedure was repeated another 4 times at 3-d intervals. Following this treatment, to check for the expression of behavioral sensitization, all rats were challenged with a lower dose of D-amphetamine (0.5 mg/kg, i.p.) one week after the last drug treatment, and their locomotor response was monitored for 2 hrs. This protocol of intermittent injections was shown to induce strong behavioral sensitization [11, 21, 33]. Animals were sacrificed 3 days after the challenge test at 8:00 am in stress free conditions. The brains were immediately removed and frozen in isopentane cooled to - 40°C. The brains (n=5 per group) were then sectioned on a Bright-Hacker cryostat and 14-μm-thick coronal sections were mounted on polylysine-subbed slides. These slides were kept at -80°C until processed for in situ hybridization.
ESS protocol
Rats were subjected to one of the following randomly assigned ESS per day for 2 hours for 28 days: Brief isolation (animals were individually transferred into cages similar to home cages), novel environment (pairs of littermates were randomly introduced to novel environments that consist of boxes with different geometric shape), crowding (all animals in the ESS group were placed in one cage but as the animals grew in size, they were divided into two cages to avoid overcrowding), litter shifting (pairs of littermates were transferred to the home cages of same aged resident rats) and subordination (pairs of littermates were transferred to the home cages of pairs of older (8-months old) resident rats).
Drug
D-amphetamine sulfate was purchased from Sigma CO (St Louis). It was dissolved in 0.9% saline solution. The volume of injection was 0.1 ml per 100 g of body weight.
In situ hybridization histochemistry
As described previously [14], brain sections were fixed in 4% paraformaldehyde for 1 h, followed by 3 washes in 2×saline sodium citrate. The sections were then placed in a solution containing acetic anhydride (0.25%) in TEA (0.1 M, pH 8) for 10 min at room temperature, then rinsed in distilled water and dehydrated through graded alcohols. After air-drying, the sections were hybridized overnight with 35S-labeled cRNA probe. The D1, D2, D3, TH, DAT probes were 460 base pairs (bp), 495 bp, 326 bp, 300 bp and 529 bp respectively. The D1, D2 and D3 DA receptor probes were constructed by Dr. O. Civelli. The TH and DAT probes were cloned in Drs. Akil and Watson labs. Following hybridization, the coverslips were removed and the sections rinsed and washed twice in 2×SSC for 5 min each, then incubated for 1 h in RNase at 37°C. The sections were washed in increasingly stringent solutions of SSC, 2×, 1× and 0.5×, for 5 min each, followed by incubation for 1 h in 0.1×SSC at 65°C. After rinsing in distilled water, the sections were dehydrated through graded alcohols, air-dried and exposed to a Kodak XAR film (Eastman Kodak, Rochester, NY, USA). The DAT and TH probes were exposed to X-ray films (Eastman Kodak, Rochester, NY) for 3 days. The D1 and D2 probes were exposed for 5 days, and the D3 probe was exposed for 10 days.
Quantification of the radioactive signal
Optical density measurements were sampled from templates made for each brain region from both hemispheres and in rostro-caudal direction (figure 2). D1R mRNA was quantified in the CPu, the core and shell of the NAc. D2R mRNA was quantified in the CPu, the core and shell of the NAc, the VTA and the SN. D3R mRNA was quantified in the whole NAc. DAT mRNA and TH mRNA were quantified in the VTA and the SN (figure 2). For all the probes, 8 sections per region per rat were sampled. Optical density values were corrected for background and then averaged to produce one data point for each brain region for each animal. These data points were averaged per group and compared statistically. Optical densities measurements were quantified from X-ray film using National Institute of Health Image software.
Figure 2.
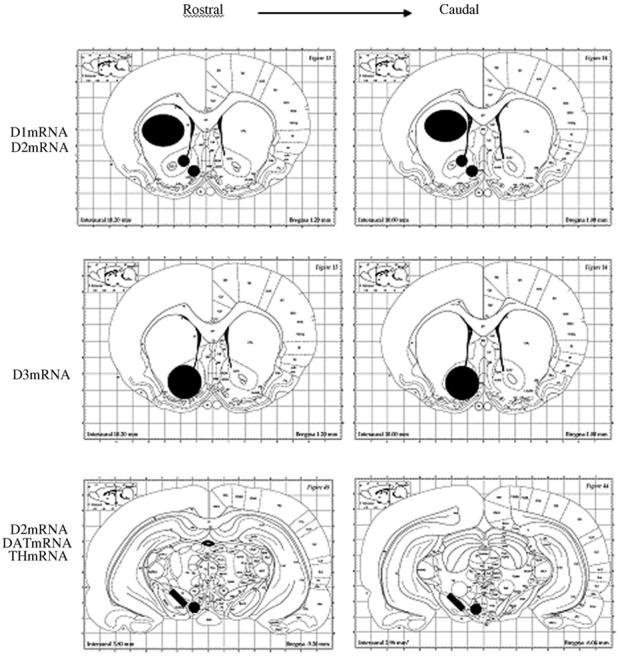
Location of templates used to sample optical density measurements within specific brain regions in each animal. The CPu and Acb sections were sampled from bregma +1.2mm rostrally to bregma +1.0 mm caudally according to Paxinos and Watson rat brain Atlas. The VTA and SN sections were sampled from bregma −5.2 rostrally to bregma −6.04 caudally according to the same Atlas.
Statistics
The mRNA expression results were analyzed with a two-way ANOVA. Saline vs. amphetamine was one between groups factor (treatment), NO-ESS vs. ESS (condition) was the other between groups factor. A simple regression analysis was conducted between the levels of D1 mRNA in the CPU and the locomotor activity on the amphetamine challenge day.
The statistical analyses for the behavioral data were described in the previous publication [15].
Results
A two-way ANOVA conducted on the integrated optical density for mRNA levels revealed a significant treatment effect for D1 DA receptor in the CPu [F(1,16)=7.20, p=0.01], whereas there was no significant condition effect detected [F(1,16)=1.33; p=0.26]. Moreover, there was a significant interaction between treatment × condition [F(1,16)=5.68, p=0.02]. Post-hoc Fisher comparisons showed that the amphetamine NO-ESS group exhibited significantly lower levels of D1 mRNA in the CPu when compared to the other 3 groups (ps<0.01). There were no other significant effects for D1 mRNA in the core, D1 mRNA in the shell; D2 in the CPu; D2 in the core, D2 in the shell; D2 in the VTA, D2 in the SN; D3 in the NAc; DAT in the VTA; DAT in the SN; TH in the VTA and TH in the SN (table 1; fig. 1b; fig. 3). Finally, there was no correlation between D1 mRNA levels in the CPU and locomotor activity to the low dose amphetamine challenge (r=0.08, p=0.20).
Table 1.
Optical density of the radioactive signal for D1, D2, D3, DAT and TH mRNAs in specific brain regions of amphetamine NO-ESS, amphetamine ESS, saline NO-ESS and saline ESS. Data are expressed as mean ± standard errors.
| AMPH NO-ESS (n=5) | AMPH ESS(n=5) | Saline NO-ESS (n=5) | Saline ESS (n=5) | |
|---|---|---|---|---|
| D1 mRNA CPu | 29739 ± 9513* | 55235 ± 6604 | 57440 ± 7572 | 49193 ± 5546 |
| D1 mRNA Core | 17921 ± 1716 | 17483 ± 1993 | 14974 ± 3455 | 14786 ± 2182 |
| D1 mRNA Shell | 20823 ± 2365 | 25709 ± 2024 | 19149 ± 3912 | 18413 ± 3249 |
| D2 mRNA VTA | 37685 ± 2508 | 39278 ± 3208 | 41820 ± 39615 | 40874 ± 3527 |
| D2 mRNA SN | 21960 ± 1967 | 22893 ± 1658 | 20550 ± 5822 | 21873 ± 2274 |
| D2 mRNA CPu | 293188 ± 33292 | 278171 ± 26233 | 317491 ± 35180 | 256768 ± 40118 |
| D2 mRNA Core | 81221 ± 10217 | 79341 ± 15317 | 83424 ± 13224 | 80224 ± 14114 |
| D2 mRNA Shell | 107395 ± 31037 | 90800 ± 22166 | 111057 ± 29521 | 104932 ± 29944 |
| D3 mRNA Acc | 78229 ± 18954 | 73519 ± 15518 | 69807 ± 14704 | 71385 ± 7919 |
| DAT mRNA VTA | 47357 ± 4301 | 49085 ± 8725 | 46726 ± 8905 | 47430 ± 5778 |
| DAT mRNA SN | 28660 ± 1433 | 31848 ± 3285 | 31280 ± 3568 | 33063 ± 4986 |
| TH mRNA VTA | 37248 ± 4033 | 41682 ± 1790 | 37123 ± 4668 | 43341 ± 5561 |
| TH mRNA SN | 19620 ± 1925 | 21414 ± 1083 | 19846 ± 1816 | 20094 ± 2900 |
=p<0.05.
Figure 3.
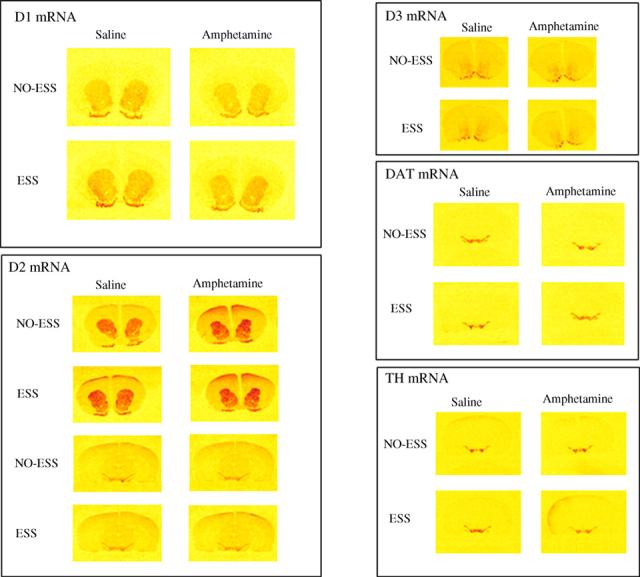
Color-enhanced pictures constructed from section images of x-ray films showing in situ hybridization with antisense cRNA probes against D1, D2, D3, DAT and TH mRNAs.
Discussion
Our previous study showed that ESS during adolescence blocked the expression of behavioral sensitization to amphetamine (figure 1A). Our present results show that ESS rats do not exhibit the decrease in CPu D1 receptor mRNA levels that accompanies behavioral sensitization to amphetamine observed in the NO-ESS controls rats (table 1; fig. 1b; fig. 3). These results suggest that D1 receptor in the CPu may be implicated in behavioral sensitization to amphetamine, and that the ESS pre-exposure may block this effect.
The dorsal striatum is classically considered as the “motor striatum”, and its possible role in behavioral sensitization has generally been minimized. It is becoming apparent that the caudate plays a role in complex psychological function. There is evidence of the role that the dorsal striatum plays during both amphetamine abstinence and during the expression of behavioral sensitization. Some authors reported no change in basal DA in the NAc [7, 8, 19, 27, 35], but a transient decrease in DA release in the CPu during amphetamine abstinence. For example, Paulson and Robinson (1996) pre-treated rats with escalating doses of amphetamine, after which the rats abstained from amphetamine for 3, 7 or 28 days. These investigators showed a decrease in DA and DA metabolites during early part of abstinence in the CPu but not in the NAc. After 28 days, basal DA in the CPu returned to normal, however there was an increase in basal DA metabolism in both the CPu and the NAc. Using a similar protocol but studying DA release after amphetamine challenge during expression of behavioral sensitization at 28 days, the same investigators reported an increase in DA release in both the CPu and the NAc in sensitized rats [18]. In the current study we show a decrease in the D1 mRNA specifically in the CPu after amphetamine treatment in the sensitized NO-ESS but not in the ESS group, which in turn show a behavioral inhibition to amphetamine. We propose that the decrease in the D1 mRNA in the CPu in sensitized rats is possibly mediated by an increase in DA release in the CPu. Present findings are in agreement with those of Paulson and Robinson (1995, 1996) in that, unlike the mainstream view, the CPu is critically implicated in the expression of behavioral sensitization to amphetamine. Thus the dorsal striatum may be involved in complex psychological function, which needs to be explored further.
In contrast, the decrease in D1 mRNA after behavioral sensitization to amphetamine in the sensitized NO-ESS rats did not occur in the ESS rats. Additionally, this group did not exhibit expression of behavioral sensitization to amphetamine. Although it is beyond the scope of this study to establish a causal relationship between the decrease in D1 mRNA in the CPu and the expression of behavioral sensitization to amphetamine, we suggest that DA activity via its D1 receptor in the CPu may be important in the expression of behavioral sensitization to amphetamine, which may be blocked by our ESS regimen during adolescence.
Although individual components of the ESS regimen are commonly employed as stress paradigms in the literature (e.g., isolation, crowding, subordination), what we have seen is that the combined ESS paradigm evokes a classic enrichment-like effect. It is known that although social encounters such as subordination are highly stressful and considered as “negative” experiences in adult animals, they may be rewarding in juveniles in specific contexts. For example, rough-and-tumble play behavior in juvenile animals induce positive social affect, and is rewarding [5], however it also induces secretion of glucocorticoids in juvenile animals resembling that of stressors [31]. Juvenile rats that were reared in enriched conditions display heightened locomotor activity to acute amphetamine, and in turn are less sensitive to repeated amphetamine [2]. We observed a similar inhibition in the expression of amphetamine sensitization, hence our ESS paradigm may be uniquely tapping into the “protective” pathway in the mesolimbic circuitry in a way similar to classic enrichment paradigms do. One plausible explanation presented in the literature for inhibited dopaminergic neurotransmission and behavioral effects in response to psychostimulants in enriched juvenile rats is a decrease in dopamine transporter function and decreased dopamine metabolism in the prefrontal cortex [36]. This is a hypothesis to be tested in the current ESS paradigm.
Glucocorticoid-dopamine interaction is a potential mechanism by which ESS causes an inhibition of behavioral sensitization to amphetamine. Shmidt et al., [26] reported that the expression of behavioral sensitization require the activation of glucocorticoid receptors (GR). Interestingly, corticosterone has been shown to modulate dopaminergic transmission through mesolimbic GR (for review see Piazza et al., 1996), and repeated injections of amphetamine were shown to down regulate hippocampal GR [28]. In fact, GR-mediated facilitation of striatal DA neurotransmission by way of an increase in the level of D1 receptor mRNA and protein in the striatum during behavioral sensitization to psychostimulants [4, 9, 10]. In the case of NO-ESS, amphetamine may render DA neurons of the CPU hyperactive towards CORT, and elevated DA levels may down regulate the post-synaptic D1 receptors. In the ESS however, the down regulation of CPu GR may counteract the normally-occurring blunting effect of amphetamine on D1 mRNA in the CPu observed during behavioral sensitization. Thus, we hypothesize that in ESS rats, less GR in the striatum may lead to a low sensitivity of these neurons to CORT, leaving D1 receptor levels unchanged. This hypothesis of course needs further investigations as many other factors (like growth factors) may be implicated.
To our knowledge this is the first comprehensive study to investigate the effects of chronic ESS during adolescence on the induction and expression of behavioral sensitization to amphetamine and its effects on the DA circuitry. These data further pave the way for understanding conditions where chronic ESS may result in a compensatory effect on the adaptive changes in the DA circuitry after psychostimulant administration especially during adolescence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmed SH, Stinus L, Le Moal M, Cador M. Social deprivation enhances the vulnerability of male Wistar rats to stressor- and amphetamine-induced behavioral sensitization. Psychopharmacology (Berl) 1995;117:116–24. doi: 10.1007/BF02245106. [DOI] [PubMed] [Google Scholar]
- 2.Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav. 1995;51:397–405. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- 3.Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–84. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- 4.Biron D, Dauphin C, Di Paolo T. Effects of adrenalectomy and glucocorticoids on rat brain dopamine receptors. Neuroendocrinology. 1992;55:468–76. doi: 10.1159/000126158. [DOI] [PubMed] [Google Scholar]
- 5.Burgdorf J, Panksepp J. Tickling induces reward in adolescent rats. Physiol Behav. 2001;72:167–73. doi: 10.1016/s0031-9384(00)00411-x. [DOI] [PubMed] [Google Scholar]
- 6.Cador M, Bjijou Y, Stinus L. Evidence of a complete independence of the neurobiological substrates for the induction and expression of behavioral sensitization to amphetamine. Neuroscience. 1995;65:385–95. doi: 10.1016/0306-4522(94)00524-9. [DOI] [PubMed] [Google Scholar]
- 7.Crippens D, Camp DM, Robinson TE. Basal extracellular dopamine in the nucleus accumbens during amphetamine withdrawal: a 'no net flux' microdialysis study. Neurosci Lett. 1993;164:145–8. doi: 10.1016/0304-3940(93)90878-o. [DOI] [PubMed] [Google Scholar]
- 8.Crippens D, Robinson TE. Withdrawal from morphine or amphetamine: different effects on dopamine in the ventral-medial striatum studied with microdialysis. Brain Res. 1994;650:56–62. doi: 10.1016/0006-8993(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 9.Czyrak A, Mackowiak M, Fijal K, Chocyk A, Wedzony K. Impact of metyrapone on MK-801-induced alterations in the rat dopamine D1 receptors. Pol J Pharmacol. 1997;49:305–16. [PubMed] [Google Scholar]
- 10.Czyrak A, Wedzony K, Michalska B, Fijal K, Dziedzicka-Wasylewska M, Mackowiak M. The corticosterone synthesis inhibitor metyrapone decreases dopamine D1 receptors in the rat brain. Neuroscience. 1997;79:489–95. doi: 10.1016/s0306-4522(96)00649-5. [DOI] [PubMed] [Google Scholar]
- 11.Dietz DM, Tapocik J, Gaval-Cruz M, Kabbaj M. Dopamine transporter, but not tyrosine hydroxylase, may be implicated in determining individual differences in behavioral sensitization to amphetamine. Physiol Behav. 2005;86:347–55. doi: 10.1016/j.physbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Green TA, Cain ME, Thompson M, Bardo MT. Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology (Berl) 2003;170:235–41. doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- 13.Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed-and progressive-ratio schedules. Psychopharmacology. 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- 14.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabbaj M, Isgor C, Watson SJ, Akil H. Stress during adolescence alters behavioral sensitization to amphetamine. Neuroscience. 2002;113:395–400. doi: 10.1016/s0306-4522(02)00188-4. [DOI] [PubMed] [Google Scholar]
- 16.Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- 17.Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993;13:266–75. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulson PE, Robinson TE. Regional differences in the effects of amphetamine withdrawal on dopamine dynamics in the striatum. Analysis of circadian patterns using automated on-line microdialysis. Neuropsychopharmacology. 1996;14:325–37. doi: 10.1016/0893-133X(95)00141-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 21.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–98. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 22.Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 23.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 24.Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain Res. 1988;462:211–22. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- 25.Rouge-Pont F, Marinelli M, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. II. Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7189–95. doi: 10.1523/JNEUROSCI.15-11-07189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt ED, Tilders FJ, Binnekade R, Schoffelmeer AN, De Vries TJ. Stressor- or drug-induced sensitization of the corticosterone response is not critically involved in the long-term expression of behavioural sensitization to amphetamine. Neuroscience. 1999;92:343–52. doi: 10.1016/s0306-4522(98)00725-8. [DOI] [PubMed] [Google Scholar]
- 27.Segal DS, Kuczenski R. In vivo microdialysis reveals a diminished amphetamine-induced DA response corresponding to behavioral sensitization produced by repeated amphetamine pretreatment. Brain Res. 1992;571:330–7. doi: 10.1016/0006-8993(92)90672-v. [DOI] [PubMed] [Google Scholar]
- 28.Shilling PD, Kelsoe JR, Segal DS. Hippocampal glucocorticoid receptor mRNA is up-regulated by acute and down-regulated by chronic amphetamine treatment. Brain Res Mol Brain Res. 1996;38:156–60. doi: 10.1016/0169-328x(96)00009-5. [DOI] [PubMed] [Google Scholar]
- 29.Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- 30.Stairs DJ, Klein ED, Bardo MT. Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behav Pharmacol. 2006;17:597–604. doi: 10.1097/01.fbp.0000236271.72300.0e. [DOI] [PubMed] [Google Scholar]
- 31.Terranova ML, Cirulli F, Laviola G. Behavioral and hormonal effects of partner familiarity in periadolescent rat pairs upon novelty exposure. Psychoneuroendocrinology. 1999;24:639–56. doi: 10.1016/s0306-4530(99)00019-0. [DOI] [PubMed] [Google Scholar]
- 32.Vezina P. Amphetamine injected into the ventral tegmental area sensitizes the nucleus accumbens dopaminergic response to systemic amphetamine: an in vivo microdialysis study in the rat. Brain Res. 1993;605:332–7. doi: 10.1016/0006-8993(93)91761-g. [DOI] [PubMed] [Google Scholar]
- 33.Vezina P, Queen AL. Induction of locomotor sensitization by amphetamine requires the activation of NMDA receptors in the rat ventral tegmental area [In Process Citation] Psychopharmacology (Berl) 2000;151:184–91. doi: 10.1007/s002130000463. [DOI] [PubMed] [Google Scholar]
- 34.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- 35.Wolf ME, White FJ, Nassar R, Brooderson RJ, Khansa MR. Differential development of autoreceptor subsensitivity and enhanced dopamine release during amphetamine sensitization. J Pharmacol Exp Ther. 1993;264:249–55. [PubMed] [Google Scholar]
- 36.Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behavioural Brain Research. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]


