Abstract
Chronic alcohol abuse by human beings has been shown to be associated with increased susceptibility to pulmonary infections and severity of inflammatory responses associated with pulmonary infection. On the basis of the higher likelihood of exposure to respiratory viruses, people who abuse alcohol would logically be susceptible to respiratory viral infections. To test this hypothesis, mice were provided alcohol in drinking water for 13–16 weeks with the Meadows–Cook protocol and infected intranasally with respiratory syncytial virus. At various times after infection, severity of infection was determined by evaluation of cellular and cytokine composition of bronchoalveolar lavage fluid (BALF) and histologic evaluation of inflammation. Infection was associated with neutrophil infiltration in both groups, but the proportion and number of neutrophils in BALF were significantly greater in the alcohol consumption group than in the control group. Concentrations of tumor necrosis factor-α and monocyte chemoattractant protein-1 in BALF in the alcohol consumption group were increased. Interferon-γ concentrations were lower in the alcohol consumption group at later times of infection. Pulmonary inflammation was cleared by 3–5 days after infection in the control group. In contrast, pulmonary inflammation was evident in the alcohol consumption group after 7 days of infection, and some mice showed severe inflammation with hemorrhage and edema. Interferon-α/β was evident in BALF at low concentrations in the alcohol consumption group for several days after infection, and increased mRNA for interferon-α/β was also evident in the alcohol consumption group. This was accompanied by the presence of virus in this group at these times of infection. Chronic alcohol consumption increased severity of pulmonary infection with a virus that naturally infects hosts by an aerosol route. Infection of mice that had consumed alcohol chronically was more severe in terms of increased proinflammatory cytokine production, inflammation, and a failure to clear the virus from the lungs.
Keywords: Respiratory viral infections, respiratory syncytial virus, pulmonary inflammation, MCP-1, TNF-α, IFN-γ
Introduction
Alcohol abuse by human beings has been associated with a greater incidence and prevalence of infections of the lung (Gamble et al., 2006; Guidot and Hart, 2005; Happel and Nelson, 2005; Shellito, 1998; Shellito and Olariu, 1998; Shellito et al., 2001; Vander Top et al., 2005). The effect of alcohol consumption on this phenomenon has been difficult to dissect for a number of reasons. Most important, however, is the fact that a large percentage of people who abuse alcohol smoke, which complicates the evaluation of mechanisms of alcohol-mediated changes in the lungs. To approach this problem experimentally, a number of animal studies have been conducted with the overarching goal of determining mechanistically how alcohol affects the lung. Specifically, it has been shown by others that alcohol consumption by experimental animals is associated with increased susceptibility to, and increased pathogenic effects mediated by, pulmonary bacterial infections, notably pulmonary infections with Streptococcus pneumoniae (Vander Top et al., 2005), Klebsiella pneumoniae (Shellito et al., 2001), and Pneumocystis carinii (Shellito, 1998; Shellito and Olariu, 1998). Increased susceptibility of human beings to pulmonary infections is associated with a number of co-factors, including aspiration, poor nutrition, and close exposure to other people. Impairments in mucociliary clearance of bacteria associated with alcohol and smoke exposure of experimental animals have also been reported (Vander Top et al., 2005). Along with these factors, it has been proposed that the well-described immunosuppression of host-defense mechanisms, both innate and acquired immune responses (Cook, 1998; Hoek et al., 2005; Jerrells, 2002; Jerrells and Sibley, 1995; Szabo, 1999), plays an important role in susceptibility to infections of the lung, especially viral infections.
The hypothesis that was tested in the studies described in this article is that a model of chronic (months) alcohol consumption with a mouse model increases susceptibility to, and pathogenic effects mediated by, a virus relevant to respiratory viral infections of human beings. Specifically, the well-described murine model of pulmonary infection with respiratory syncytial virus (RSV) was used to define the effects of alcohol on these pulmonary infections. This virus was chosen for several reasons. Most importantly, RSV is a pathogen of human beings, and infections in mice reproduce infections of human beings. Of importance are the study results, which will be discussed in the following sections, that show increased susceptibility of people who are immunocompromised and have compromised lung function and an association with alcohol abuse (de Roux et al., 2006).
As reviewed by Hall (2001), most RSV infections occur from November to May, with most activity in January and February. Frequent reinfection occurs, which is interpreted as a decrease in acquired immunity with time. The pathogenicity of RSV bronchiolitis includes necrosis of the small airway epithelium and sloughing of infected ciliated epithelium. The inflammation in the lung is associated with increased production of mucus, edema, and decreased lung function (Jafri et al., 2004). Although most infections occur in children, RSV also infects immunocompetent adults, producing mild “cold-like” infections, which has been suggested to be an important source of primary infections of children (Hall, 2000, 2001). These findings also support the concept that immunity to RSV is not total or lifelong and declines with age (Chala et al., 2003; de Bree et al., 2005; Mejias et al., 2005; Openshaw, 2005; Sethi and Murphy, 2005; Shlaes, 2000; Walsh and Falsey, 2004a, 2004b; Walsh et al., 2004). This has been suggested to be the result of poor immunogenicity of RSV and, in some cases, production of a T helper cell subtype 2 (TH2) immune response (Bukreyev et al., 2005; Hussell et al., 1996; Walsh and Falsey, 2004a; Waris et al., 1996). Respiratory syncytial virus infection is particularly problematic for adults with compromised immune function (Anaissie et al., 2004; Ebbert and Limper, 2005; Mejias et al., 2005; Walsh et al., 2004), cardiovascular disease (Sethi and Murphy, 2005; Shlaes, 2000; Walsh et al., 2004), and compromised lung function (especially chronic obstructive pulmonary disease) (Beckham et al., 2005; de Bree et al., 2005; Wilkinson et al., 2006), all of which have been associated with alcohol abuse. In addition, most people who abuse alcohol also smoke, and a history of smoking is an important co-factor in this picture. Together, the totality of risk factors for severe RSV infection is consistent with the findings of de Roux et al. (2006), which show that infections with respiratory viruses, including RSV, are important in the community-acquired pneumonia associated with alcohol abuse. One important characteristic of RSV infection is production of a robust TH2-type immune response when the innate immune response is altered, and the TH2 immune response to RSV has been implicated in the development of asthma and chronic pulmonary inflammation (Chala et al., 2003; de Bree et al., 2005; Mejias et al., 2005; Openshaw, 2005; Sethi and Murphy, 2005; Shlaes, 2000; Walsh and Falsey, 2004a, 2004b; Walsh et al., 2004). It has also been shown that there are several immunodominant viral proteins and, specifically, that the response to the G protein skews the pulmonary immune response to a strong TH2 response and fails to induce an antigen-specific CD8+ T-cell response, which is necessary for viral clearance (Bukreyev et al., 2005; Hussell et al., 1996; Waris et al., 1996; Zeng et al., 2006). Study findings have been interpreted to support the suggestion that RSV can establish persistent infections associated with chronic pulmonary inflammation and fibrosis (Ostler et al., 2001; Schwarze et al., 2004; Wilkinson et al., 2006). This effect has been suggested to be an important factor in loss of pulmonary function over time mediated by continued pulmonary inflammation in people with the persistent infection.
The study results presented in this article show that chronic alcohol consumption by BALB/c mice resulted in a more severe and prolonged viral pneumonitis in mice that consumed alcohol chronically in comparison with the infection of control mice. Further, the mice that consumed alcohol did not clear the virus as effectively as the control mice in spite of similar concentrations and continued production of type I IFN (IFN-α/β). There was an overproduction of tumor necrosis factor (TNF)-α and monocyte chemoattractant protein-1 (MCP-1) by the mice in the alcohol consumption group in comparison with findings for the control mice. In comparison with findings for control mice, concentrations of interferon (IFN)-γ were lower in the bronchoalveolar lavage fluid (BALF) obtained from mice that consumed alcohol at later times of infection. Together, these findings support the hypothesis that chronic alcohol consumption is associated with more severe respiratory viral infections through changes in innate and perhaps acquired immune responses in the lungs.
Materials and Methods
Animals and Alcohol Consumption
The studies described in this article were conducted with female BALB/c mice provided alcohol in drinking water [20% volume/volume (v/v)] as described by Song et al. (2002). In all experiments described, the mice were provided alcohol in drinking water for 13–16 weeks. We found providing alcohol with this protocol did not affect the weight gain of the mice provided alcohol, and there were no significant differences in weight between alcohol consumption and control groups throughout the experiments. The mean blood alcohol level in four experiments, which was determined between 8:00 and 9:00 a.m., was 0.241% (range, 0.120%–0.394%), which is consistent with the blood alcohol levels obtained by Dr. Robert Cook and colleagues with this model (Song et al. 2002), as well as in further work by Dr. Robert Cook (unpublished observation, 2007).
BALB/c mice were chosen for study on the basis of the RSV literature, which shows that this strain of mouse is susceptible to RSV infection, and the resulting pulmonary infection faithfully models human pulmonary infections (Jafri et al., 2004).
The studies described in this article were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee. Care and treatment of mice were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) revised 1996.
Virus and Infection
Respiratory syncytial virus subtype A2 was initially purchased from Advanced Biotechnologies Inc (Columbia, MD). Infections with RSV were produced with a sublethal dose of virus [i.e., 106 plaque-forming units (PFU) per mouse by intranasal infection of BALB/c mice] that is generally less than the dose used by other groups.
Infection with virus was induced by administering the viral dose in sterile saline (50 μl) for injection by an intranasal route to mice anesthetized with tribromoethanol (Avertin). Control mice were given saline (50 μl intranasally).
Virus titers in lungs, obtained from infected mice and homogenized in phosphate-buffered saline (10% v/v), were determined by plaque assays with the use of Hep2 or Vero cells grown in six-well tissue culture plates. The lower limit of detection in these assays was 40 PFU.
Cytokine Assays
Serum and BALF concentrations of cytokines and chemokines were determined by using the BD Cytometric Bead Array (CBA) produced by BD Biosciences (San Jose, CA)—specifically, the Mouse Inflammation Kit [interleukin (IL)-6, IL-10, MCP-1, IFN-γ, TNF, IL-12p70] and Mouse Th1/Th2 kit (IL-2, IL-4, IL-5, IFN-γ, TNF)—according to the manufacturer’s instructions. Concentrations of IFN-α/β were obtained by using IFN-α and IFN-β–specific enzyme-linked immunosorbent assay (ELISA) (PBL Biomedical Laboratories, Piscataway, NJ) according to the manufacturer’s directions.
Production of IFN-α/β was also evaluated by using a quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) assay. Lungs obtained from mice infected for 24, 48, and 72 h were perfused with heparinized Hanks’ balanced salt solution (HBSS) through the right ventricle and immediately placed in RNA STAT-60 (Tel-Test, Inc, Friendswood, TX) and homogenized with a Dounce homogenizer. After chloroform extraction and centrifugation, the aqueous phase was collected, and an equal volume of alcohol was added. An RNeasy Midi/Maxi Kit (QIAGEN Inc., Valencia, CA) was used to isolate the RNA by following the QIAGEN protocol for animal tissues. After the RNA was isolated, the concentration was determined by using the NanoDrop ND-1000, and the 260/280 ratio was also determined at the same time. A formaldehyde gel was also run to further determine the quality of the RNA extracted.
Five micrograms of each RNA sample was centrifuged at 14,000 rpm for 30 min at 4°C, and the pellet was resuspended in 11 μl of DEPC water. One microliter of Oligo(dT)12–18 primer was added to each sample. This was heated at 70°C for 10 min in a thermal cycler. A sample mixture, containing the following (all obtained from Invitrogen Corporation, Carlsbad, CA), was subsequently prepared for each sample: 4 μl of 5X First-Strand Buffer, 2 μl of dithiothreitol (DTT), 1 μl of 10 mM dNTP mix, and 1 μl of SuperScript II Reverse Transcriptase. This mixture was added to the sample and incubated in the thermal cycler at 42°C for 60 min. The reaction was immediately inactivated at 70°C for 10 min and stored at 20°C to be used for RT-PCR. The RT product was first diluted 1:10 in molecular-grade water, and the reaction mixture was subsequently prepared. Each sample received 1 μl of a 10 μM forward and reverse primer mix (Integrated DNA Technologies, Coralville, IA), along with 7 μl of molecular-grade water and 10 μl of SYBR Green Master Mix (Roche Applied Science, Indianapolis, IN). The RT-PCR was subsequently completed by using the Bio-Rad MyiQ PCR thermal cycler with the program optimized for the primers being used.
The PCR was evaluated over 10 to 30 cycles, and the relative induction of the appropriate genes was determined from the relative threshold (Cτ) values and normalized for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. The primers for IFN-β were obtained from Integrated DNA Technologies and are as follows:
IFN-β-forward GGA GAT GAC GGA GAA GAT GC
IFN-β-reverse CCC AGT GCT GGA GAA ATT GT
As a control, RNA, isolated from uninfected mice in the control and alcohol consumption groups, was evaluated for IFN-α and IFN-β expression. For both, expression was below the level of detection in this assay.
Evaluation of Pulmonary Inflammation
Inflammatory cells in BALF were collected, washed, and spun onto microscope slides and stained with Diff-Quik (VWR Scientific, Batavia, IL). Cells were classified on the basis of cellular morphologic characteristics.
To evaluate the phenotypes of dendritic cells (DCs) responding to the pulmonary infection, cells recovered in the BALF were evaluated with the use of flow cytometric analyses. Briefly, cells were recovered from the BALF by centrifugation, resuspended in FACS buffer (phosphate-buffered saline supplemented with 2% fetal bovine serum and 0.1% sodium azide), and counted. They were subsequently adjusted to 106 viable cells and stained with the following monoclonal antibodies for the indicated cell membrane markers:
anti–mouse plasmacytoid dendritic cell antigen-1 (mPDCA-1)-phycoerythrin (PE) (clone JF05-1C2.4.1; Miltenyi Biotec, Auburn, CA) specific for murine plasmacytoid cells
PE-Cy7 anti-mouse CD11b (clone M1/70; eBioscience, San Diego, CA)
allophycocyanin (APC) anti-mouse CD11c (clone N418; eBioscience)
anti–CD45-fluorescein isothiocyanate (FITC) (clone 30-F11; BD Biosciences) specific for the pan hemopoietic surface marker.
Cells in the mononuclear gate (determined by forward and light scatter) were gated on CD45+ cells and gated further on CD11c+ cells and, subsequently, PDCA-1 and CD11b to determine the CD11c+/CD11b−/PDCA-1+ plasmacytoid DC population.
A minimum of 104 cells were analyzed, and the proportion of each cell type was determined from the flow cytometric analyses. Total cell numbers were calculated from the total recovered cell number and the percentage of each cell type.
To evaluate the inflammation in lung tissue, the lungs were inflated with 0.9 ml of 10% buffered formalin before removal, further fixed in 10% buffered formalin, and embedded in paraffin. Sections (5 mm) were subsequently prepared. Sections were stained with hematoxylin-eosin, and inflammation was evaluated by light microscopy.
Statistical Analyses
Where appropriate, a one-way analysis of variance (ANOVA) in association with the Student–Newman–Keuls multiple-comparison test was used to determine statistical significance of data that contained three or more groups. Percentage data were evaluated with the Wilcoxon–Mann–Whitney statistic. Data obtained from experiments from two groups that were normally distributed were analyzed with a two-tailed t test. Differences at a P value ≤ .05 were considered statistically significant. Calculations were carried out with GraphPad Prism 4 software (GraphPad Software, Inc., San Diego, CA).
Results
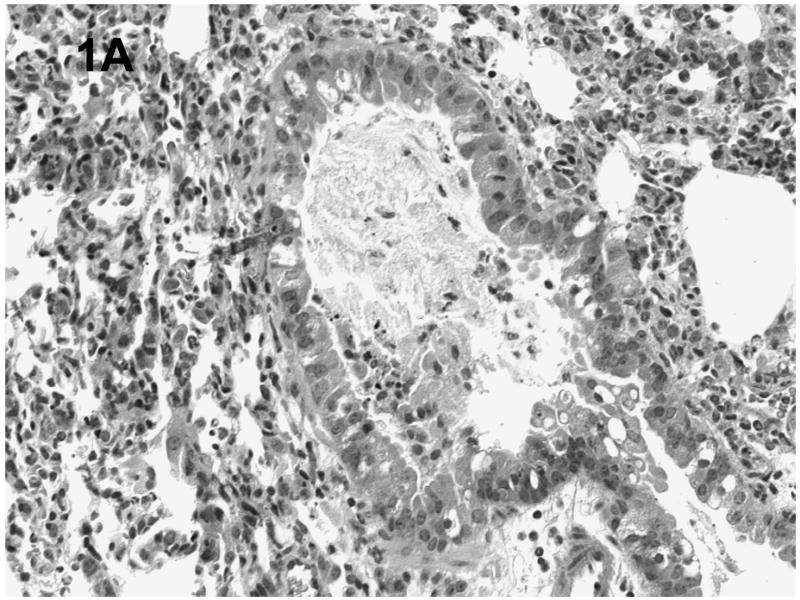
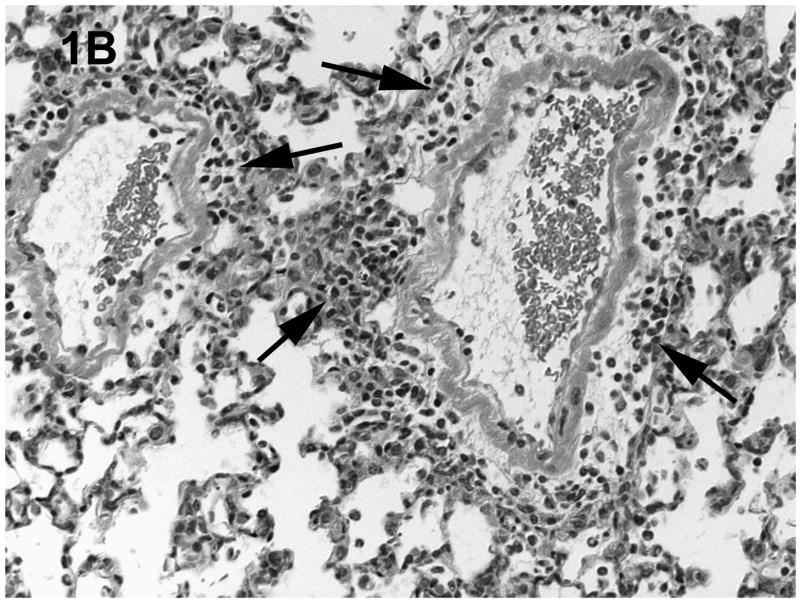
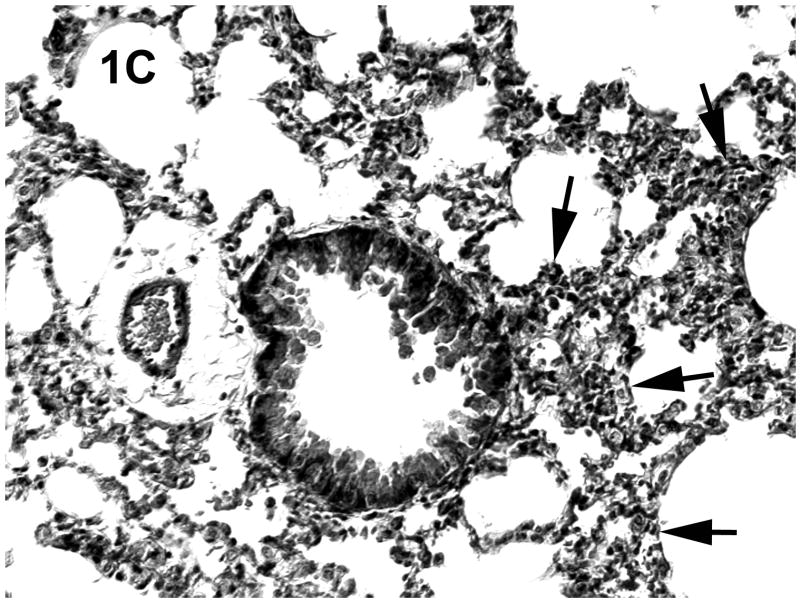
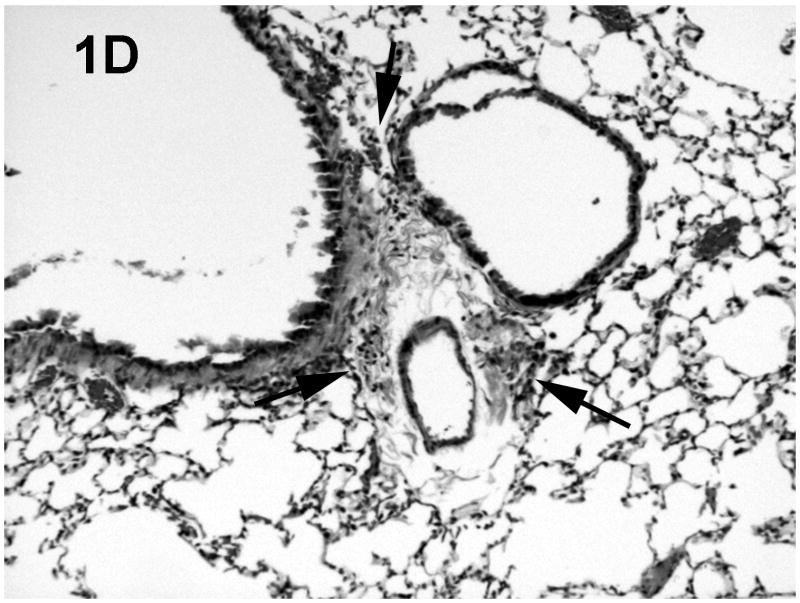
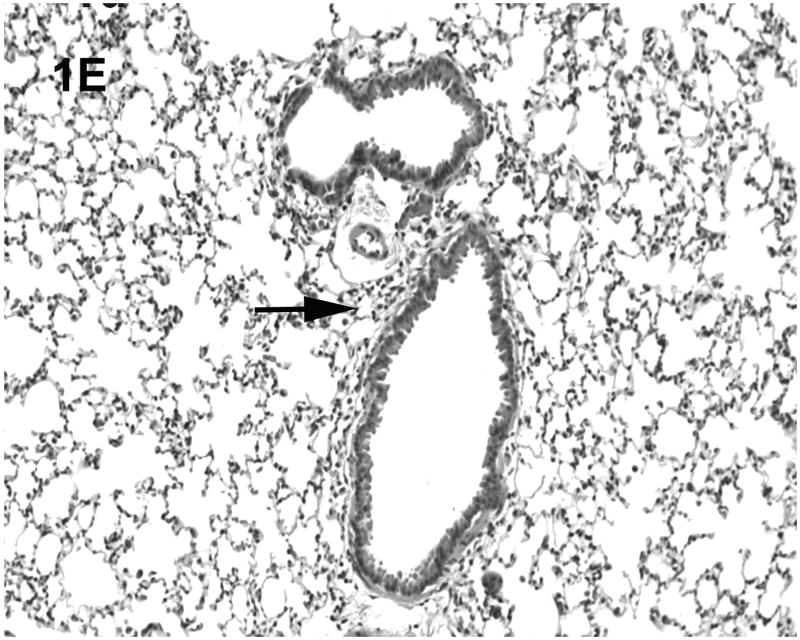
Histologic evaluation of lungs obtained from mice in the alcohol consumption group that were infected with RSV showed marked peribronchial inflammation 48 h after infection, which was predominantly mononuclear (Fig. 1), which is characteristic of RSV infection of mice (Jafri et al., 2004). In Fig. 1A, which shows a representative tissue section, there is evidence of inflammation, alveolar septa thickening, and mucus and inflammatory cells in the lumen of the bronchus. There are also changes in the bronchial epithelial cells, characteristic of RSV damage to the infected ciliated epithelium. Fig. 1B shows perivascular inflammation, also characteristic of RSV infection. Mice in the alcohol consumption group showed severe pneumonitis (Fig. 1C) and clear evidence of erythrocytes in the parenchyma of the lung 7 days after infection, which support the notion that there is both endothelial cell and epithelial cell damage. In contrast, control mice showed similar histologic changes in the lungs, but it appeared that the extent of the inflammation was less in control mice at the early times of infection. After 7 days of infection, essentially normal lung tissue or rare, minor areas of inflammation were observed for control mice (Fig. 1D), which is consistent with the clearance of the virus in this group noted in these experiments. The continued inflammation in mice in the group that consumed alcohol chronically is supported by the finding that these mice failed to clear the virus (see data in Fig. 3). Uninfected mice from the group that consumed alcohol chronically showed little to no changes in the lungs (Fig. 1E).
Fig. 1.
Effect of chronic consumption of alcohol on pulmonary pathogenesis of respiratory syncytial virus (RSV) infection. BALB/c mice were provided water or alcohol in drinking water [20% volume/volume (v/v)] for 13 weeks and infected with 106 plaque-forming units of RSV by an intranasal route. (A, B) Sections of lungs obtained from representative mice in the group provided alcohol 48 h after infection. Arrows (B) indicate perivascular inflammation. (C) Representative section of lung obtained from a mouse with a severe infection in the group provided alcohol 7 days after infection, showing (arrows) large areas of pneumonitis. (D) Representative section of lung obtained from a mouse in the control group, which shows minor areas of inflammation. (E) Sections of lung obtained from an uninfected mouse in the group provided alcohol for 13 weeks were also evaluated, and a representative stained section is presented. Arrow indicates inflammation. [Hematoxylin-eosin stain; final magnification X200 (A) and X100 (B, C, D, and E).]
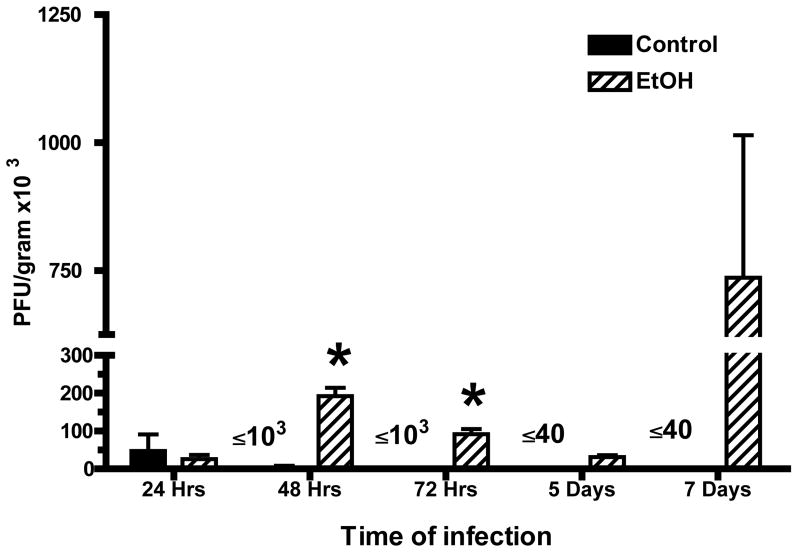
Fig. 3.
Concentration of respiratory syncytial virus (RSV) in lungs of mice provided alcohol chronically and control mice. Lungs were obtained from mice in each group and homogenized in Dulbecco’s Modification of Eagle’s Medium [(DMEM); Mediatech, Inc., Herndon, VA] supplemented with 2% fetal bovine serum. Ten-fold dilutions, prepared in DMEM with 2% fetal bovine serum, were added to duplicate subconfluent (80% to 90% confluent) monolayers of Hep2 cells in six-well cell culture plates (250 μl per well). After incubation at 37° for 30–45 min, each well was overlayed with 0.5% agarose in DMEM with 2% fetal bovine serum. After 5–7 days of incubation, plaques and areas of syncytia were counted. Each point represents mean ± SD of plaque-forming units (PFU) per gram of tissue of 3–5 mice per group per time and is datum obtained from two separate experiments. [*P ≤ .05 (analysis of variance post hoc t test).] EtOH = Alcohol.
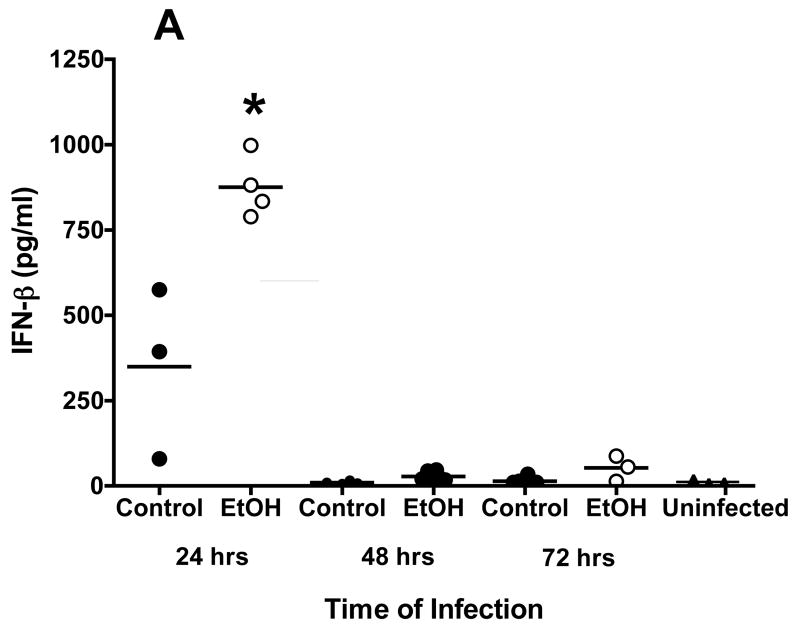
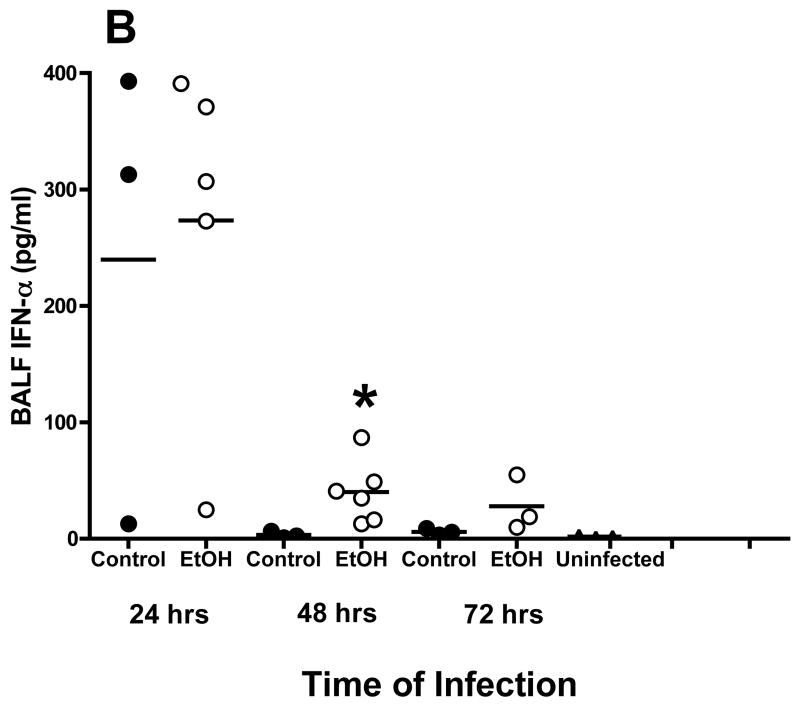
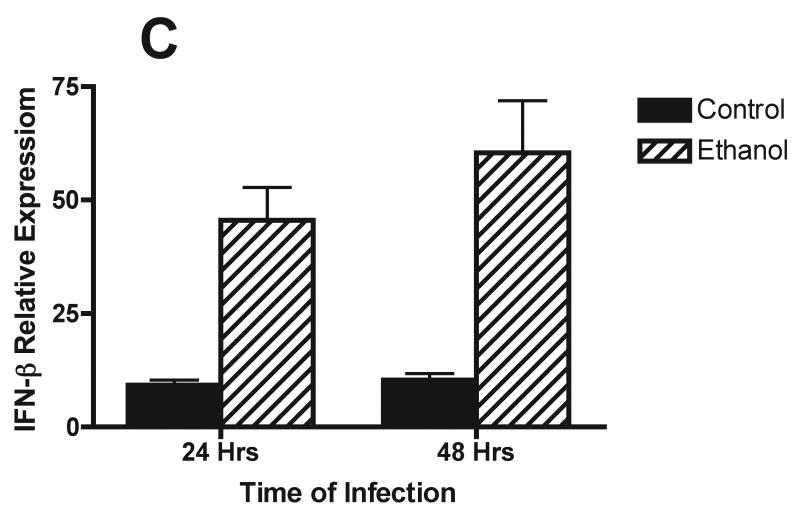
Studies also were conducted to define the effects of alcohol on cells and cytokine/chemokine responses after respiratory viral infection by evaluation of BALF obtained from mice infected with RSV. Because of numerous studies whose results reveal the critical role of type I IFN in control of RSV, induction of the appropriate T helper cell subtype 1 (TH1)-type immune response viruses, and activation of natural killer (NK) cell responses (Johnson et al., 2005), the concentrations of IFN-α and IFN-β in BALF obtained early in 72 h) were determined with an ELISA. Although there is variability in the assays, the findings show that IFN-β and IFN-α concentrations in BALF peaked at 24 h after infection in both control mice and mice in the alcohol consumption group. However, it appears that the IFN-β concentration was higher in the BALF obtained from mice in the alcohol consumption group than in the BALF obtained from control mice (Fig. 2A). Also, IFN-α concentrations above the baseline values of uninfected mice and control mice were noted in the BALF obtained from mice in the alcohol consumption group 48 and 72 h after infection (Fig. 2B). A similar increase in IFN-β production at 24 and 48 h after infection by cells in the lungs was demonstrated with the use of quantitative RT-PCR analyses of total RNA isolated from lung tissue at the same times (Fig. 2C). It is clear from the quantitative RT-PCR assay and the ELISA that both IFN-β gene expression and IFN-β production were greater for the group of mice that consumed alcohol for 16 weeks before the infection.
Fig. 2.
Effect of chronic consumption of alcohol on production of type I IFN (IFN-α/β). Scatter plots show individual values of IFN-β (A) and IFN-α (B) in bronchoalveolar lavage fluid (BALF), and the horizontal line represents mean value. (A, B) *P ≤ .05 (analysis of variance post hoc t test). (C) Quantitative reverse transcriptase-polymerase chain reaction for IFN-β mRNA isolated from the lungs of infected mice from each group (n = 4 mice per group) was also carried out and presented as IFN-β response relative to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA. Each point is mean ± SD fold change. The increased expression of IFN-β mRNA in RNA obtained from the lungs of mice at 24 and 48 h after infection in the group of mice provided alcohol chronically is significantly different [P ≤ .01 (Wilcoxon–Mann–Whitney)] from the level of IFN-β mRNA expression in RNA obtained from control mice. EtOH = Alcohol.
Findings obtained with the use of plaque assays of lung homogenates obtained at 24, 48, and 72 h and at 5 and 7 days after infection showed that control mice cleared the virus (plaque-forming units below the limit of detection) by 5 days of infection. In contrast, plaque numbers in lung homogenates obtained from mice that consumed alcohol were demonstrable at 7 days after infection when the virus was undetectable in lung homogenates obtained from control mice (Fig. 3). At the other times of evaluation, the plaque-forming unit numbers were at least 10-fold higher in the lungs obtained from mice in the alcohol consumption group. The data shown in Fig. 3 are representative of two experiments with 3–5 mice per group per time in each experiment. The data obtained from mice in the group that consumed alcohol chronically 24 and 48 h after infection were highly significant (P ≤ .01) in comparison with findings for the control mice. Virus was still evident 5 and 7 days after infection, a time when viral numbers were below the level of detection in samples from control mice.
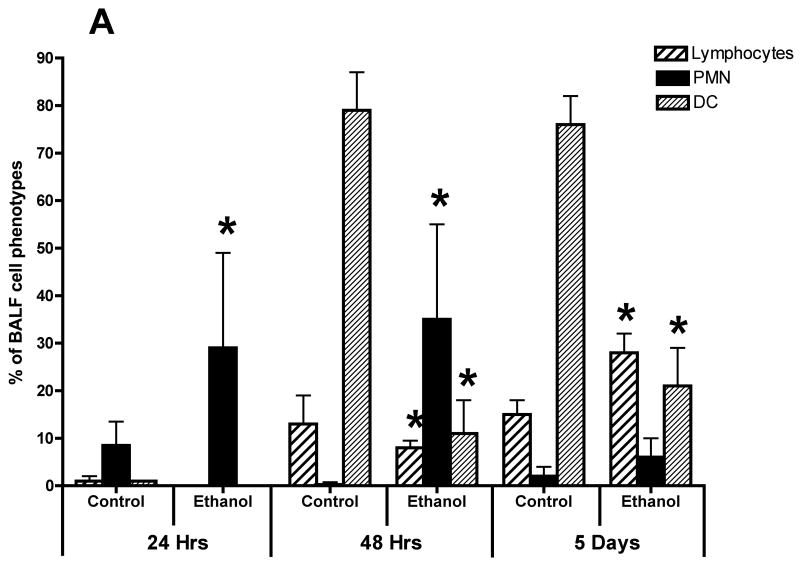
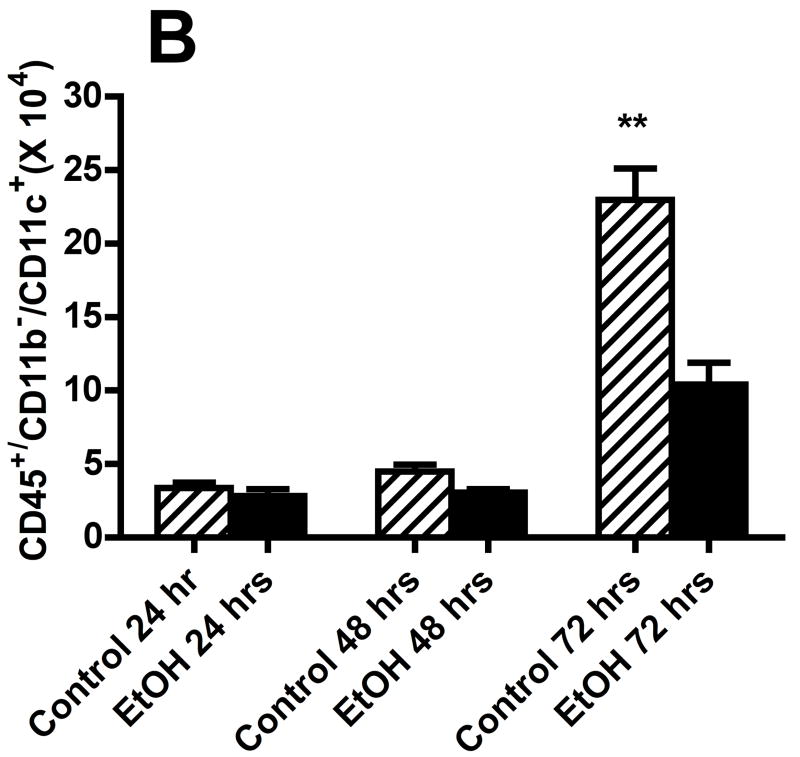
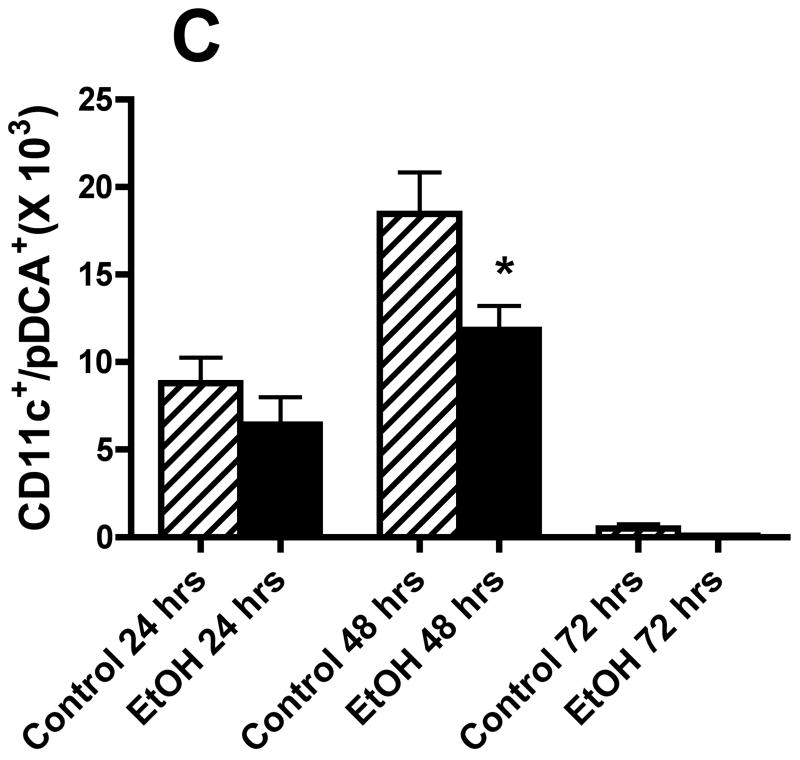
We also evaluated the types of cells recovered in the BALF obtained from control mice and mice in the alcohol consumption group after infection with RSV by staining cytospin preparations of BALF cells. The percentage of various cell morphologic phenotypes that were different between the two groups is presented in Fig. 4A. These findings show marked differences between the cells obtained from control mice and mice from the alcohol consumption group in the proportion of polymorphonuclear neutrophils (PMN) and DCs. The early (i.e., 24 and 48 h) infection of mice in the alcohol consumption group was associated with a marked influx of PMN, which was greater than the PMN response seen in control mice at the same times. It is interesting to note the large influx of DCs recovered in the BALF, which has been reported by others (Smit et al., 2006), and the marked differences between the proportions of cells with morphologic characteristics of DCs in the two groups of mice. Preliminary flow cytometric data (Fig. 4B) support the suggestion that there is a significant (P ≤ .05) decrease in the numbers of total CD45+/CD11b−/CD11c+ cells (which would include the DCs and a small negligible population of alveolar macrophages), and (Fig. 4C) the total number of plasmacytoid DCs (CD45+/CD11b−/CD11c+/PDCA-1+) in the BALF cells isolated from mice in the alcohol consumption groups.
Fig. 4.
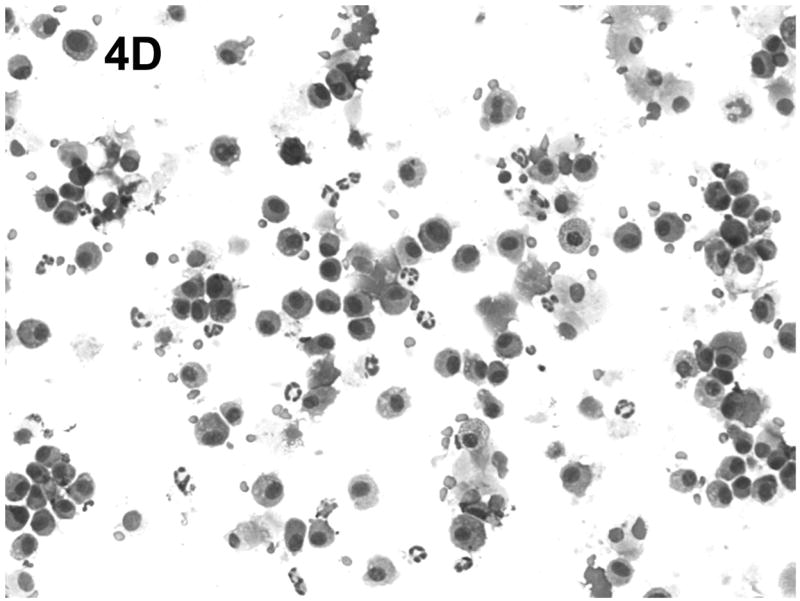
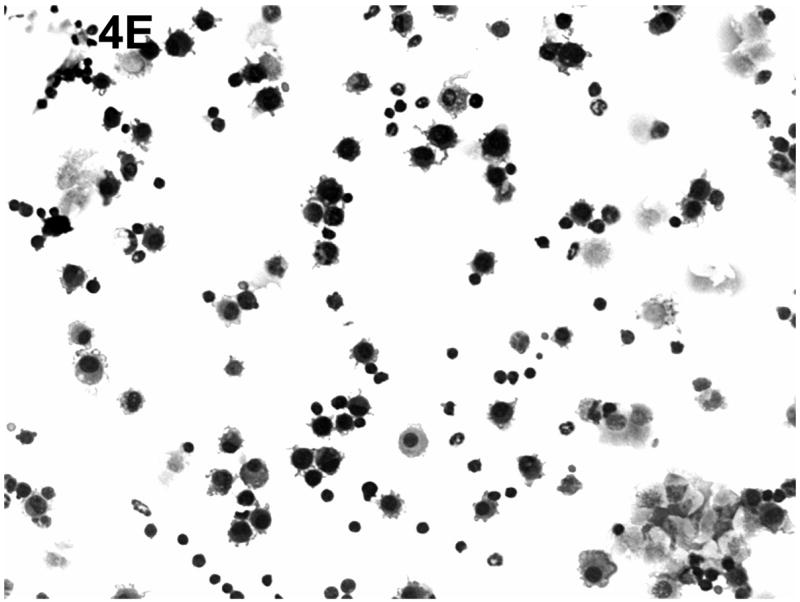
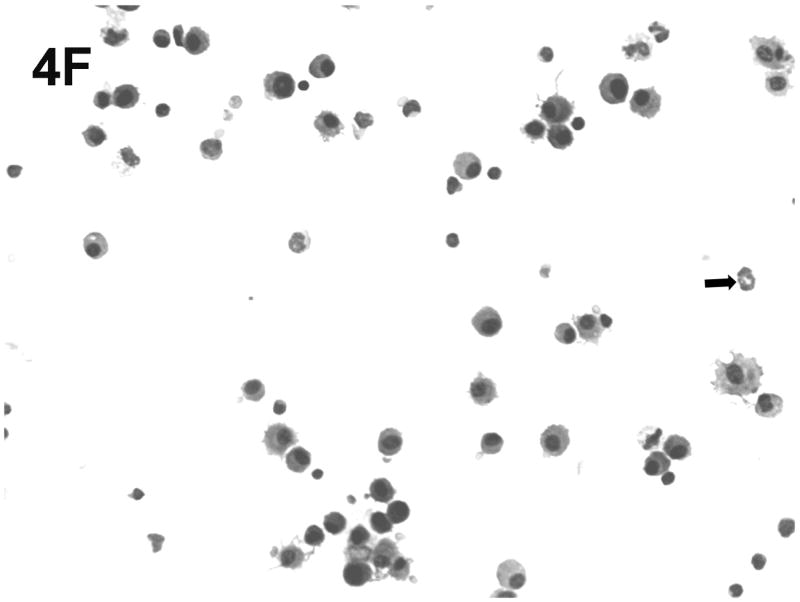
Effect of chronic consumption of alcohol on mononuclear cells recovered by bronchoalveolar lavage (BALF). Cells in lavage were collected by centrifugation, and cytospin preparations were prepared. Individually stained preparations, prepared by using a modified Wright-Giemsa stain, were evaluated by microscopy. Proportion of various cell types was determined by counting 200 cells, and percentage of each type was calculated (A). Cell phenotypes were identified by characteristic morphologic criteria. The BALF cells were also evaluated for dendritic cell (DC) phenotypes by flow cytometric analyses. The number of CD45+/CD11b−/CD11c+, which contains all DCs (B), or CD45+/CD11b−/CD11c+/PDCA-1+ (plasmacytoid DCs) (C) was determined as described in Materials and Methods. Representative photomicrographs of BALF cells obtained from mice in the alcohol consumption group 24 h (D) and 48 h (E) after infection are presented. Representative photomicrograph of BALF cells obtained from mice in the control group 48 h after infection is also presented (F). [Final magnification X100 (D, E) and X200 (F).] Each data point (A–C) represents mean ± SD differential count or cell number from 3–6 mice in each group at each time. (A) Significantly different [*P ≤ .05 (Wilcoxon–Mann–Whitney)] from cell percentages for control mice at same time after infection. (B, C) *P ≤ .05, **P ≤ .01 (analysis of variance post hoc t test). DC = Dendritic cells; Ethanol or EtOH = alcohol; PMN = polymorphonuclear neutrophils.
Representative cytospin-prepared cell populations were obtained 24 and 48 h after infection with RSV from mice from the group that consumed alcohol chronically (Figs. 4D and 4E) or from mice in the control group (Fig. 4F). As shown in the respective photomicrographs, there was an abundance of cells with obvious myeloid DC morphologic characteristics (presence of dendrites), neutrophils, alveolar macrophages, and erythrocytes in the BALF obtained from mice in the alcohol consumption group (Figs. 4D and 4E). The presence of erythrocytes in the BALF has been a consistent finding in the group of mice that consumed alcohol in the four experiments conducted to date, although erythrocytes were found in BALF at later times of infection (5 and 7 days of infection), but not consistently. In contrast, the photomicrograph presented in Fig. 4F, which was prepared with BALF obtained from mice in the control group 48 h after infection, shows the rare neutrophil, dendritic cells, lymphocytes, and alveolar macrophages, which supports the suggestion that the pulmonary inflammatory response was less in these animals.
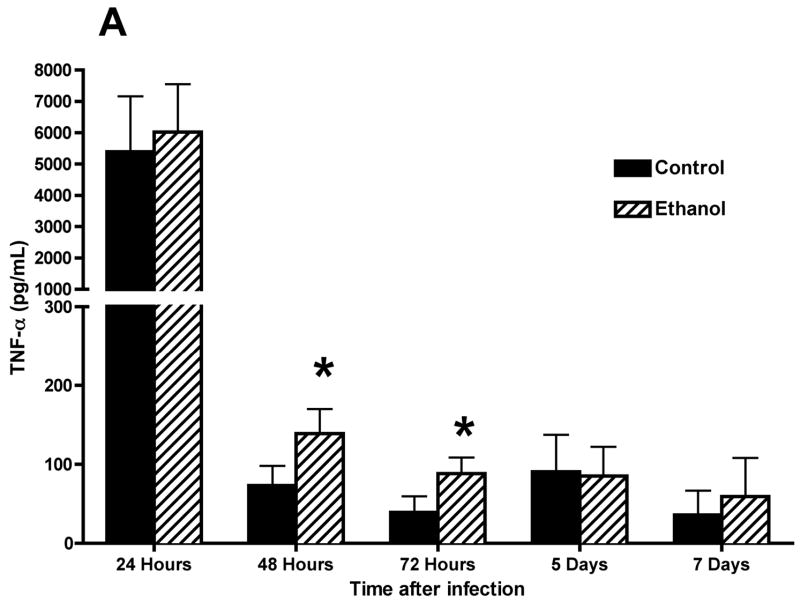
Concentrations of proinflammatory cytokines and chemokines in BALF were also determined with the use of flow BD Cytometric Bead Array (CBA) (BD Biosciences). These data (Fig. 5) show increased concentrations of TNF-α (Fig. 5A) and MCP-1 (Fig. 5B) and, importantly, decreased concentrations of IFN-γ at 7 days after infection (Fig. 5C) in BALF obtained from RSV-infected mice. The MCP-1 concentration remained elevated above control values through 7 days. It is interesting to note the higher concentration of IFN-γ at 72 h of infection in the alcohol consumption group, which may be associated with changes in the number of activated NK cells. This suggestion is supported by data obtained from a pulmonary infection of C57BL/6 mice with murine cytomegalovirus (MCMV) that were provided alcohol chronically with the same protocol as described in this article, as well as by study results obtained by others showing that RSV infection of a monocytic cell line induces IL-15 production, which is a cytokine that is involved in activation of NK cell proliferation and activation (Ennaciri et al., 2007). Results of the preliminary studies conducted with MCMV showed that the infection resulted in demonstrable increased numbers of activated NK cells in the lungs, which coincided with increased concentrations of IFN-γ (data not shown). It is also interesting to note the low concentration of IFN-γ 7 days after RSV infection in the alcohol consumption group, which may be a factor in the failure to clear the virus in this group. Similar data have been obtained for MCMV-infected mice in the alcohol consumption group in comparison with concentrations in BALF obtained from infected control mice (data not shown).
Fig. 5.
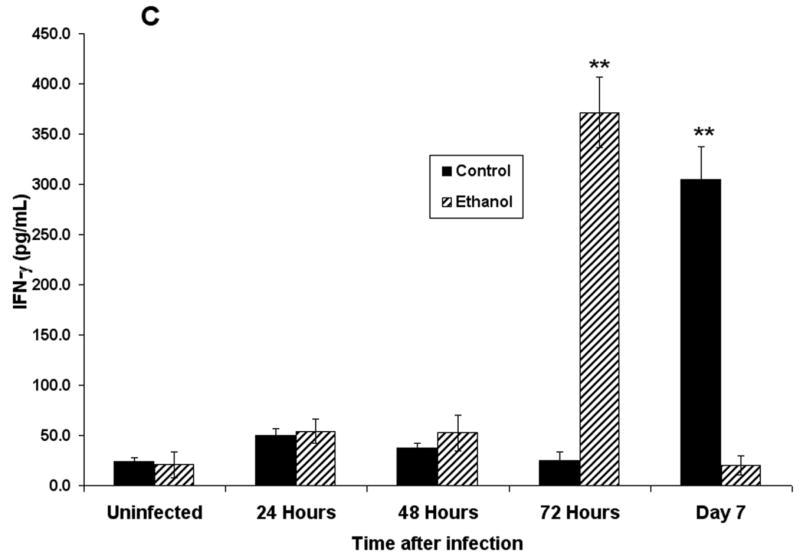
Effect of chronic consumption of alcohol on production of proinflammatory cytokines and chemokines. Concentrations of tumor necrosis factor (TNF)-α (A), monocyte chemoattractant protein-1 (MCP-1) (B), and interferon (IFN)-γ (C) were determined in bronchoalveolar lavage fluid obtained from BALB/c mice infected with respiratory syncytial virus. Each point represents mean ± SD values obtained from 3–6 mice per group. Significantly [*P ≤ .05, **P ≤ .01 (analysis of variance post hoc t test)] different from values for control mice at same time after infection. Ethanol = Alcohol.
Discussion
The findings presented in this article support the suggestion that chronic consumption of alcohol by mice results in several changes in the host response to respiratory viral infections. The experiments in these studies were conducted with the use of a virus that is an accepted model of infections of human beings. Respiratory syncytial virus was also chosen for these studies because of the study results that show that the virus is confined to the lungs and does not infect peripheral organs. Spread of virus to other organs, especially the spleen, would make interpretation of cytokine production and pathologic effects in the lungs difficult because of production of proinflammatory cytokines in secondary lymphoid organs and other effects of a systemic infection.
For several reasons, use of RSV for most of the work and future studies is a relevant model for evaluation of the effects of alcohol abuse by human beings on pulmonary innate and acquired host-defense and pathogenic responses. First, and most important, there is a large body of data that convincingly shows that pulmonary infections of mice faithfully reproduce infections of human beings (Jafri et al., 2004). Of importance are epidemiologic studies, whose results show that adults are susceptible to reinfection with this virus because of a proposed decay in the immunity induced in the primary infection and the relatively poor immunogenicity of RSV (Chala et al., 2003; de Bree et al., 2005; Mejias et al., 2005; Sethi and Murphy, 2005; Shlaes, 2000; Walsh and Falsey, 2004a, 2004b; Walsh et al., 2004). It has also been shown that adults with compromised immune function, chronic obstructive pulmonary disease, and cardiovascular disease, all of which have been associated with alcohol abuse, are more susceptible to pathogenic infections with RSV (Anaissie et al., 2004; Ebbert and Limper, 2005; Mejias et al., 2005; Walsh et al., 2004). In a recent publication, study results support the suggestion that viral infections (i.e., influenza virus, parainfluenza virus, and RSV infections) are important contributors to community-acquired pneumonia of people who abuse alcohol chronically (de Roux et al., 2006). Some study results have been interpreted to support the conclusion that alcohol consumption by mice results in a bias of the acquired immune response to a TH2-type response (Latif et al., 2002; Starkenburg et al., 2001), and it is of interest that a predominant response to the G protein of RSV will bias the pulmonary immune response to a TH2 response and a failure to produce an antigen-specific immune response that is necessary to clear the virus (Bukreyev et al., 2005; Hussell et al., 1996; Sethi and Murphy, 2005; Zeng et al., 2006). The bias to a TH2 response would also predispose the individual to asthma induced by reinfection with RSV (de Roux et al., 2006). If chronic consumption of alcohol skews the immune response in the lung to a TH2-type response, at least initially, the long-term consequences for pulmonary diseases would be of major public health importance. This possibility is supported by the role of IFN-α/β in changes in the immune response to RSV that would limit the production of the TH2-type immune response and, potentially, the development of asthma (Johnson et al., 2005). It will be important to extend the studies reported in this article to include in-depth studies of IFN-α/β production and responses.
In addition, RSV seems, on the basis of recent study findings (Ostler et al., 2001; Schwarze et al., 2004; Wilkinson et al., 2006), to be an attractive model for changes in pulmonary host-defense mechanisms associated with chronic alcohol abuse by human beings. These findings have been interpreted to suggest that RSV can establish persistent infections that are associated with chronic pulmonary inflammation and fibrosis (Ostler et al., 2001; Schwarze et al., 2004; Wilkinson et al., 2006), which would also be of great importance.
The study results presented in this article support the suggestion that relatively long-term consumption of alcohol in drinking water results in exaggerated inflammatory responses in the lungs of mice infected with RSV, as well as with an unrelated virus (i.e., MCMV; data not shown), which shows that alcohol consumption may have the same effects on unrelated viruses in experimentally induced respiratory viral infections. The continued inflammation is hypothesized to be the result of a failure to clear the virus from the lungs, which is supported by the study findings revealing large numbers of virus (reaching 107 PFU in some infected mice in the alcohol consumption group) as long as 7 days after infection. Results of the plaque assays (Fig. 3) and quantitative RT-PCR analyses (data not shown) show that the virus is cleared in control mice at this time, and the inflammation is cleared in the lungs of control mice. Of interest is the finding that RSV infection in the group of mice provided alcohol with the Meadows–Cook model is associated with severe pathologic effects in the lungs of some mice, which includes continued inflammation and the presence of erythrocytes in the lungs. There are several studies whose results show that alcohol consumption is associated with changes in permeability in the lungs as well as in other tissues (Brown et al., 2001; Guidot and Hart, 2005), and this seems to be exaggerated in the early responses to the viral infection, evidenced by what appears to be fluid and erythrocytes in the lung parenchyma (see Figs. 1, 4D, and 4E). However, it is unclear what disrupts the endothelium in the lungs of mice in the group provided alcohol with the chronic consumption model, which show continued viral infection and inflammation. A potential mediator of this effect is the increased production of proinflammatory cytokines, which would potentially affect the tight junctions of the pulmonary endothelium and epithelium. This is a focus of current studies.
It seems paradoxical that large concentrations of TNF-α are seen early in the infection regardless of the group evaluated (Fig. 5A) when most inflammatory cells were noted at later times. This phenomenon is still under investigation, but it is possible that the proinflammatory cytokines (especially TNF-α) are produced by infected epithelial cells or alveolar macrophages, or the early (within 24 h) infiltration of IL-8–activated neutrophils, which have been shown to produce a number of proinflammatory molecules, including TNF-α and MCP-1 (Antony et al., 1983; Daniels et al., 1992; Martinez et al., 2004; Sawant and McMurray, 2007). Findings in the literature as well as data presented in this article support the conclusion that RSV initially infects the bronchial epithelium, which has been shown to produce many proinflammatory cytokines, including IL-8 (Yoon et al., 2007). At this time, we do not favor the hypothesis that alveolar macrophages are involved in the early response to RSV infection. This is based partly on a search of the literature, which shows that the initial cytokine responses are produced predominantly by plasmacytoid DCs and epithelial cells. Also, it has been shown that depletion of lungs of phagocytic cells (alveolar macrophages and DCs), followed by reconstitution of lungs with alveolar macrophages, did not protect against infection with influenza virus (unpublished observation, K. Legge, 2007), which argues against a major protective role of alveolar macrophages in respiratory viral infections. It is clear that future studies with RSV and the alcohol model developed in this laboratory should include determination of the effects on alveolar macrophage–mediated regulation of the inflammatory process. The failure to control the viral infection seems to be independent of the production of IFN-α/β in response to RSV infection. Defects may exist in the signaling through the IFN receptors for the downstream production of the well-known antiviral molecules produced in tissue, including the lungs, in response to IFN-α/β (Su et al., 2007; Theofilopoulos et al., 2005). This suggestion is supported by published data, which show that RSV nonstructural proteins NS1 and NS2 inhibit a signaling molecule [signal transducer and activator of transcription 2 (STAT2)] expression, which results in inhibition of IFN-α/β signaling and production of antiviral mediators (i.e., 2′-5′ oligoadenylate synthetase) (Lo et al., 2005). The general finding by others that RSV infection inhibits the production of IFN-α (Guerrero-Plata et al., 2005; Ramaswamy et al., 2006; Schlender et al., 2005) also has to be considered when the pathogenesis of RSV is studied. It is unclear from the study results presented in this article and in the literature whether RSV inhibits production of IFN-β. This is also an area of active research in our laboratories.
The failure to control the virus in some mice later in the infection is associated with a relatively large increase in CD8+ T cells in the lungs of mice with a pulmonary MCMV infection (data not shown). It is currently unknown whether chronic consumption of alcohol affects the CD8+ T-cell response to RSV similar to the effect of chronic alcohol consumption on the CD8+ T-cell response to MCMV, but it is a distinct possibility.
Because of the well-described role of plasmacytoid DCs in limiting viral replication, as well as the pulmonary inflammatory response associated with RSV infection (Smit et al., 2006; Wang et al., 2006), it is interesting to note the changes in the population of cells with plasmacytoid DC surface markers in the BALF obtained from mice that consumed alcohol chronically. It is interesting that the bulk of the IFN-α/β is produced within 24 h of the infection, and the noted numbers of DCs in the BALF occurred somewhat later (48 and 72 h). Several possibilities may be involved in this observation. First, it is generally thought that plasmacytoid DCs can be considered a resident population in many tissues and function as a sentinel population, as well as a population of cells that is activated during infections (Wendland et al., 2007). It is also likely that most DCs in the BALF are myeloid DCs, and this is supported by data presented in this article. Finally, although plasmacytoid DCs are the most important producer of type I IFN, many other cell types, including epithelial cells and resident mononuclear cells, produce IFN-α/β. It is possible that all these factors are involved in this phenomenon.
It has been shown that the plasmacytoid DCs play an important role in regulation of inflammation and immune responses later in infection (Liu, 2005), and the finding that alcohol consumption is associated with prolonged inflammation and lung damage makes the effects of alcohol on this important function an important issue worthy of future study. These data also show a change in total cell numbers that would include the myeloid DC population. Because of the clear role of myeloid DCs in induction of CD8+ T cells, it is possible that these changes in the mice that consumed alcohol resulted in the inability to clear the virus and the low concentrations of IFN-γ noted in this group 7 days after infection. Further studies on DC function are planned.
Together, the findings presented in this article provide sufficient data to establish the Meadows–Cook model as a relevant model of alcohol consumption to study host-defense responses in the lungs of mice. It is especially important to note that the model of chronic alcohol consumption has been refined in the laboratory of Dr. Robert T. Cook (Song et al., 2002) to allow long-term consumption of alcohol by BALB/c mice, a strain that is not acceptable for other alcohol consumption protocols because of the noted toxicity of alcohol provided with liquid diets. We would submit further that pulmonary infection with RSV provides an excellent model system to define the mechanisms of alcohol-mediated changes in innate and acquired immune responses to respiratory viruses. The mouse model is also amenable to studies that would evaluate the effects of smoke exposure on susceptibility to respiratory viral infections, and the noted susceptibility to secondary bacterial infections noted with this model (Avadhanula et al., 2006), which are of particular importance in human beings who abuse alcohol. Because RSV is associated with a severe and continued loss of function of ciliated bronchial cells (see article by Wyatt et al. in this issue), the RSV model is a relevant model to evaluate the increased susceptibility to secondary bacterial infections that are so important in the pathogenesis of infections of people who abuse alcohol. In fact, we would suggest that a susceptibility of people who abuse alcohol to respiratory viral infections is a major factor in the well-characterized pulmonary bacterial infections associated with alcohol abuse simply because of an inability to effectively clear the bacteria associated with pathogenic infections of human beings who abuse alcohol.
Acknowledgments
The technical assistance of Michael Burrows is appreciated. The expert assistance of Dr. Charles A. Kuszynski with the flow cytometric analyses is gratefully acknowledged. Support for the research was provided by Research Developmental funds from the Department of Pathology and Microbiology, University of Nebraska Medical Center. The editorial assistance of Janice Jerrells, RN, BA, ELS, is gratefully acknowledged, and her support was critical for the development of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anaissie EJ, Mahfouz TH, Aslan T, Pouli A, Desikan R, Fassas A, Barlogie B. The natural history of respiratory syncytial virus infection in cancer and transplant patients: implications for management. Blood. 2004;103:1611–1617. doi: 10.1182/blood-2003-05-1425. [DOI] [PubMed] [Google Scholar]
- Antony VB, Sahn SA, Harada RN, Repine JE. Lung repair and granuloma formation. Tubercle bacilli stimulated neutrophils release chemotactic factors for monocytes. Chest. 1983;83:95–96. doi: 10.1378/chest.83.5.95s. [DOI] [PubMed] [Google Scholar]
- Avadhanula V, Rodriguez CA, DeVincenzo JP, Wang Y, Webby RJ, Ulett GC, Adderson EE. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol. 2006;80:1629–1636. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JD, Cadena A, Lin J, Piedra PA, Glezen WP, Greenberg SB, Atmar RL. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect. 2005;50:322–330. doi: 10.1016/j.jinf.2004.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, Harris FL, Bechara R, Guidot DM. Effect of chronic ethanol ingestion on alveolar type II cell: glutathione and inflammatory mediator-induced apoptosis. Alcohol Clin Exp Res. 2001;25:1078–1085. [PubMed] [Google Scholar]
- Bukreyev A, Belyakov IM, Prince GA, Yim KC, Harris KK, Berzofsky JA, Collins PL. Expression of interleukin-4 by recombinant respiratory syncytial virus is associated with accelerated inflammation and a nonfunctional cytotoxic T-lymphocyte response following primary infection but not following challenge with wild-type virus. J Virol. 2005;79:9515–9526. doi: 10.1128/JVI.79.15.9515-9526.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chala SR, Garcia-Barreno B, Melero JA, Palomo C. Prevalence of anti-human respiratory syncytial virus antibodies over three consecutive years in a healthy adult population. J Med Virol. 2003;71:298–304. doi: 10.1002/jmv.10483. [DOI] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Daniels RH, Finnen MJ, Hill ME, Lackie JM. Recombinant human monocyte IL-8 primes NADPH-oxidase and phospholipase A2 activation in human neutrophils. Immunology. 1992;75:157–163. [PMC free article] [PubMed] [Google Scholar]
- de Bree GJ, Heidema J, van Leeuwen EM, van Bleek GM, Jonkers RE, Jansen HM, van Lier RA, Out TA. Respiratory syncytial virus–specific CD8+ memory T cell responses in elderly persons. J Infect Dis. 2005;191:1710–1718. doi: 10.1086/429695. [DOI] [PubMed] [Google Scholar]
- de Roux A, Cavalcanti M, Marcos MA, Garcia E, Ewig S, Mensa J, Torres A. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest. 2006;129:1219–1225. doi: 10.1378/chest.129.5.1219. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Limper AH. Respiratory syncytial virus pneumonitis in immunocompromised adults: clinical features and outcome. Respiration. 2005;72:263–269. doi: 10.1159/000085367. [DOI] [PubMed] [Google Scholar]
- Ennaciri J, Ahmad R, Menezes J. Interaction of monocytic cells with respiratory syncytial virus results in activation of NF-κB and PKC-α/β leading to up-regulation of IL-15 gene expression. J Leukoc Biol. 2007;81:625–631. doi: 10.1189/jlb.0806507. [DOI] [PubMed] [Google Scholar]
- Gamble L, Mason CM, Nelson S. The effects of alcohol on immunity and bacterial infection in the lung. Med Mal Infect. 2006;36:72–77. doi: 10.1016/j.medmal.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Guerrero-Plata A, Baron S, Poast JS, Adegboyega PA, Casola A, Garofalo RP. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J Virol. 2005;79:10190–10199. doi: 10.1128/JVI.79.16.10190-10199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidot DM, Hart CM. Alcohol abuse and acute lung injury: epidemiology and pathophysiology of a recently recognized association. J Investig Med. 2005;53:235–245. doi: 10.2310/6650.2005.53506. [DOI] [PubMed] [Google Scholar]
- Hall CB. Nosocomial respiratory syncytial virus infections: the “Cold War” has not ended. Clin Infect Dis. 2000;31:590–596. doi: 10.1086/313960. [DOI] [PubMed] [Google Scholar]
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- Hoek J, Thiele GM, Klassen LW, Mandrekar P, Zakhari S, Cook RT, Ray NB, Happel KI, Kolls JK, Kovacs EJ, Szabo G. RSA 2004: combined basic research satellite symposium—mechanisms of alcohol-mediated organ and tissue damage: inflammation and immunity and alcohol and mitochondrial metabolism: at the crossroads of life and death session one: alcohol, cellular and organ damage. Alcohol Clin Exp Res. 2005;29:1735–1743. doi: 10.1097/01.alc.0000179313.64522.56. [DOI] [PubMed] [Google Scholar]
- Hussell T, Spender LC, Georgiou A, O’Garra A, Openshaw PJ. Th1 and Th2 cytokine induction in pulmonary T cells during infection with respiratory syncytial virus. J Gen Virol. 1996;77(Pt 10):2447–2455. doi: 10.1099/0022-1317-77-10-2447. [DOI] [PubMed] [Google Scholar]
- Jafri HS, Chavez-Bueno S, Mejias A, Gomez AM, Rios AM, Nassi SS, Yusuf M, Kapur P, Hardy RD, Hatfield J, et al. Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice. J Infect Dis. 2004;189:1856–1865. doi: 10.1086/386372. [DOI] [PubMed] [Google Scholar]
- Jerrells TR. Association of alcohol consumption and exaggerated immunopathologic effects in the liver induced by infectious organism. Front Biosci. 2002;7:d1487–d1493. doi: 10.2741/A855. [DOI] [PubMed] [Google Scholar]
- Jerrells TR, Sibley D. Effects of ethanol on cellular immunity to facultative intracellular bacteria. Alcohol Clin Exp Res. 1995;19:11–16. doi: 10.1111/j.1530-0277.1995.tb01466.x. [DOI] [PubMed] [Google Scholar]
- Johnson TR, Mertz SE, Gitiban N, Hammond S, Legallo R, Durbin RK, Durbin JE. Role for innate IFNs in determining respiratory syncytial virus immunopathology. J Immunol. 2005;174:7234–7241. doi: 10.4049/jimmunol.174.11.7234. [DOI] [PubMed] [Google Scholar]
- Latif O, Peterson JD, Waltenbaugh C. Alcohol-mediated polarization of type 1 and type 2 immune responses. Front Biosci. 2002;7:a135–a147. doi: 10.2741/latif. [DOI] [PubMed] [Google Scholar]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Lo MS, Brazas RM, Holtzman MJ. Respiratory syncytial virus nonstructural proteins NS1 and NS2 mediate inhibition of Stat2 expression and alpha/beta interferon responsiveness. J Virol. 2005;79:9315–9319. doi: 10.1128/JVI.79.14.9315-9319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Sironi M, Vecchi A, Colotta F, Mantovani A, Locati M. IL-8 induces a specific transcriptional profile in human neutrophils: synergism with LPS for IL-1 production. Eur J Immunol. 2004;34:2286–2292. doi: 10.1002/eji.200324481. [DOI] [PubMed] [Google Scholar]
- Mejias A, Chavez-Bueno S, Jafri HS, Ramilo O. Respiratory syncytial virus infections: old challenges and new opportunities. Pediatr Infect Dis J. 2005;24(11 Suppl):S189–S196. doi: 10.1097/01.inf.0000188196.87969.9a. discussion S196–S197. [DOI] [PubMed] [Google Scholar]
- Openshaw PJ. Antiviral immune responses and lung inflammation after respiratory syncytial virus infection. Proc Am Thorac Soc. 2005;2:121–125. doi: 10.1513/pats.200504-032AW. [DOI] [PubMed] [Google Scholar]
- Ostler T, Hussell T, Surh CD, Openshaw P, Ehl S. Long-term persistence and reactivation of T cell memory in the lung of mice infected with respiratory syncytial virus. Eur J Immunol. 2001;31:2574–2582. doi: 10.1002/1521-4141(200109)31:9<2574::aid-immu2574>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Ramaswamy M, Shi L, Varga SM, Barik S, Behlke MA, Look DC. Respiratory syncytial virus nonstructural protein 2 specifically inhibits type I interferon signal transduction. Virology. 2006;344:328–339. doi: 10.1016/j.virol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Sawant KV, McMurray DN. Guinea pig neutrophils infected with Mycobacterium tuberculosis produce cytokines which activate alveolar macrophages in noncontact cultures. Infect Immun. 2007;75:1870–1877. doi: 10.1128/IAI.00858-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlender J, Hornung V, Finke S, Günthner-Biller M, Marozin S, Brzózka K, Moghim S, Endres S, Hartmann G, Conzelmann KK. Inhibition of Toll-like receptor 7-and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol. 2005;79:5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze J, O’Donnell DR, Rohwedder A, Openshaw PJM. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am J Respir Crit Care Med. 2004;169:801–805. doi: 10.1164/rccm.200308-1203OC. [DOI] [PubMed] [Google Scholar]
- Sethi S, Murphy TF. RSV infection—not for kids only. N Engl J Med. 2005;352:1810–1812. doi: 10.1056/NEJMe058036. [DOI] [PubMed] [Google Scholar]
- Shellito JE. Alcohol and host defense against pulmonary infection with Pneumocystis carinii. Alcohol Clin Exp Res. 1998;22(5 Suppl):208S–211S. doi: 10.1111/j.1530-0277.1998.tb04003.x. [DOI] [PubMed] [Google Scholar]
- Shellito JE, Olariu R. Alcohol decreases T-lymphocyte migration into lung tissue in response to Pneumocystis carinii and depletes T-lymphocyte numbers in the spleens of mice. Alcohol Clin Exp Res. 1998;22:658–663. doi: 10.1111/j.1530-0277.1998.tb04308.x. [DOI] [PubMed] [Google Scholar]
- Shellito JE, quan Zheng M, Ye P, Ruan S, Shean MK, Kolls J. Effect of alcohol consumption on host release of interleukin-17 during pulmonary infection with Klebsiella pneumoniae. Alcohol Clin Exp Res. 2001;25:872–881. [PubMed] [Google Scholar]
- Shlaes DM. Respiratory syncytial virus infection: an emerging or unappreciated infection? Semin Respir Crit Care Med. 2000;21:305–311. doi: 10.1055/s-2000-9860. [DOI] [PubMed] [Google Scholar]
- Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med. 2006;203:1153–1159. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72:1109–1116. [PubMed] [Google Scholar]
- Starkenburg S, Munroe ME, Waltenbaugh C. Early alteration in leukocyte populations and Th1/Th2 function in ethanol-consuming mice. Alcohol Clin Exp Res. 2001;25:1221–1230. [PubMed] [Google Scholar]
- Su Q, Wang S, Baltzis D, Qu LK, Raven JF, Li S, Wong AHT, Koromilas AE. Interferons induce tyrosine phosphorylation of the eIF2α kinase PKR through activation of Jak1 and Tyk2. EMBO Rep. 2007;8:265–270. doi: 10.1038/sj.embor.7400891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (α/β) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- Vander Top EA, Wyatt TA, Gentry-Nielsen MJ. Smoke exposure exacerbates an ethanol-induced defect in mucociliary clearance of Streptococcus pneumoniae. Alcohol Clin Exp Res. 2005;29:882–887. doi: 10.1097/01.alc.0000164364.35682.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EE, Falsey AR. Age related differences in humoral immune response to respiratory syncytial virus infection in adults. J Med Virol. 2004a;73:295–299. doi: 10.1002/jmv.20090. [DOI] [PubMed] [Google Scholar]
- Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004b;190:373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis. 2004;189:233–238. doi: 10.1086/380907. [DOI] [PubMed] [Google Scholar]
- Wang H, Peters N, Schwarze J. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J Immunol. 2006;177:6263–6270. doi: 10.4049/jimmunol.177.9.6263. [DOI] [PubMed] [Google Scholar]
- Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, Pabst O, Förster R. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci USA. 2007;104:6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson TM, Donaldson GC, Johnston SL, Openshaw PJ, Wedzicha JA. Respiratory syncytial virus, airway inflammation, and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:871–876. doi: 10.1164/rccm.200509-1489OC. [DOI] [PubMed] [Google Scholar]
- Yoon JS, Kim HH, Lee Y, Lee JS. Cytokine induction by respiratory syncytial virus and adenovirus in bronchial epithelial cells. Pediatr Pulmonol. 2007;42:277–282. doi: 10.1002/ppul.20574. [DOI] [PubMed] [Google Scholar]
- Zeng R-h, Gong W, Fan C-f, Wang Y-f, Mei X-g. Induction of balanced immunity in BALB/c mice by vaccination with a recombinant fusion protein containing a respiratory syncytial virus G protein fragment and a CTL epitope. Vaccine. 2006;24:941–947. doi: 10.1016/j.vaccine.2005.08.064. [DOI] [PubMed] [Google Scholar]