Abstract
The shattered¹ (shtd¹) mutation disrupts Drosophila compound eye structure. In this report, we show that the shtd¹ eye defects are due to a failure to establish and maintain G1 arrest in the morphogenetic furrow (MF) and a defect in progression through mitosis. The observed cell cycle defects were correlated with an accumulation of Cyclin A (CycA) and String (Stg) proteins near the MF. Interestingly, the failure to maintain G1 arrest in the MF led to the specification of R8 photoreceptor cells that undergo mitosis, generating R8 doublets in shtd¹ mutant eye discs. We demonstrate that shtd encodes Apc1, the largest subunit of the Anaphase-promoting complex/Cyclosome (APC/C). Furthermore, we show that reducing the dosage of either CycA or stg suppressed the shtd¹ phenotype. While reducing the dosage of CycA is more effective in suppressing the premature S phase entry in the MF, reducing the dosage of stg is more effective in suppressing the progression through mitosis defect. These results indicate the importance of not only G1 arrest in the MF but also appropriate progression through mitosis for normal eye development during photoreceptor differentiation.
Keywords: Anaphase-Promoting Complex/Cyclosome, APC/C, Cdc25, Drosophila, shattered, String, CycA, eye development
Introduction
Proliferation and differentiation must be regulated coordinately to ensure normal development in multicellular organisms. An important mechanism in the control of these processes is the rapid degradation of proteins by ubiquitin-mediated proteolysis which is regulated by a large family of related ubiquitin protein ligases (Hershko and Ciechanover, 1998). The Anaphase-Promoting Complex/Cyclosome (APC/C) is one of the ubiquitin ligases that play a central role during cell cycle progression through the selective ubiquitination of cell cycle regulators that targets them for degradation via the proteasome (Page and Hieter, 1999; Peters, 1999).
The APC/C was originally identified as an activity required for anaphase onset in both yeast and higher eukaryotes (Irniger et al., 1995; King et al., 1995; Sudakin et al., 1995). The APC/C is composed of up to thirteen subunits, which are highly conserved from yeast to humans ((Yoon et al., 2002); reviewed in (Page and Hieter, 1999; Zachariae and Nasmyth, 1999)). Mutations in APC/C subunits in yeast lead to a failure to degrade both mitotic cyclins and the anaphase inhibitor securin (Cohen-Fix et al., 1996)(Irniger et al., 1995): reviewed in (Nasmyth, 2001) (Peters, 2002). Stabilization of APC/C substrates in mitosis results in cell cycle arrest at the metaphase-anaphase transition: reviewed in (Peters, 2006)‥ Mutations in only a few APC/C subunits have been characterized in Drosophila. Animals containing severe mutations in Apc2/morula (mr) or Apc5/imaginal discs aberrant (ida) die during development with reduced or absent imaginal discs, the diploid tissue that forms the adult structures of the fly. Mutant cells arrested with high levels of the mitotic cyclin, CycB, and contained aberrantly-condensed chromosomes (Bentley et al., 2002; Kashevsky et al., 2002; Reed and Orr-Weaver, 1997). Similarly, semi-lethal mutations in Cdc27/makos show metaphase arrest of larval neuroblasts, with a corresponding failure to degrade both CycB and CycA (Déak et al., 2003).
APC/C activity is also required for the continued degradation of mitotic cyclins in the subsequent G1 phase. In yeast, accumulation of mitotic cyclins in G1 leads to precocious S phase entry that is independent of G1 phase cyclins (Irniger and Nasmyth, 1997). In higher eukaryotes, the G1 function of the APC/C has been studied using mutations in Cdh1, a G1 phase-specific activator of the complex. A knockout of Cdh1 in vertebrate cells resulted in accumulation of mitotic cyclins in G1 phase and abrogation of a p21-dependent G1-arrest (Sudo et al., 2001). In Drosophila mutations of fizzy-related (fzr, FlyBase-rap), the Drosophila ortholog of Cdh1, cause quiescent G1 cells to re-accumulate mitotic cyclins and cells undergo an additional cell division cycle(Jacobs et al., 2002)(Sigrist and Lehner, 1997)(Pimentel and Venkatesh, 2005). Thus APC/C-Cdh1 regulates stability of mitotic cyclins during G1.
The compound eye of Drosophila is an excellent system to study the processes regulating cellular differentiation and proliferation. The adult compound eye develops from a monolayer epithelium, the eye imaginal disc. Differentiation and cell cycle synchronization occur coordinately in the morphogenetic furrow (MF), which moves from the posterior to the anterior of the eye epithelium (Fig. 1A). Anterior to the MF, cells are undifferentiated and proliferate asynchronously. At the leading (anterior) edge of the MF, uncommitted cells pass through mitosis within a region that expresses high levels of string (stg) mRNA (Thomas et al., 1994). String (Stg) is a phosphatase that is required in G2 phase to activate entry into mitosis. Stg protein is rapidly degraded upon exit from mitosis (Edgar et al., 1994). It was previously proposed that the high level of stg mRNA is required in this region to drive G2 cells through mitosis, resulting in the accumulation of cells in G1 in the MF (Thomas et al., 1994; Zavitz and Zipursky, 1997). In the MF, stg mRNA expression is regulated by the Hedgehog signaling cascade which also initiates patterning and differentiation of ommatidial precursors in the MF (Baonza and Freeman, 2005; Firth and Baker, 2005; Heberlein et al., 1995). Although most, if not all, cells in and ahead of the MF express stg mRNA, Stg protein is detected in only a small number of cells (Horsfield et al., 1998), indicating that Stg protein accumulation in this region is regulated post-transcriptionally. Immediately posterior to the MF, differentiating photoreceptor cells remain arrested in G1 while the rest of the cells enter a synchronous round of cell proliferation referred to as the “second mitotic wave ” (SMW). Blocking cell proliferation in the SMW or ectopic cell proliferation in the MF generally leads to developmental defects, indicating that precise cell cycle control in the MF and the SMW is critical for normal eye development (de Nooij and Hariharan, 1995; Thomas et al., 1994).
Figure 1. Abnormal photoreceptor cell differentiation in shtd¹.

(A) Schematic representation of cell division and differentiation in the eye disc. Anterior is to the right in these and subsequent panels. Differentiating photoreceptor cell precursors (purple), and mitotic figures (grey) are shown. Synchronous S phase entry domain and G2 domain are indicated by yellow and green bars, respectively. The morphogenetic furrow (MF) is shown by a black bar. Expression of string mRNA (dotted) is seen ahead the MF. At the anterior boundary of string mRNA expression, cells proceed through mitosis and become synchronized in G1. Behind the MF, cells either exit the cell cycle and differentiate or re-enter a synchronous S phase (SMW). (B, C) Expression of the neuronal marker Elav in differentiating photoreceptor cells in wild-type (B) and the shtd¹ mutant (C). The shtd¹ mutant shows disruptions both in the numbers of neuronal cells in the developing clusters and in spacing of the clusters. (D, E) Ato protein levels were decreased in the MF in shtd¹ (E, white line), however single Ato positive R8 cells were still observed behind the MF (E, white bracketed line). (F, G) Expression of LG51-lacZ in the R8 nucleus (purple) and CycB (green). Two R8-positive nuclei are seen in each photoreceptor cell cluster in shtd¹ (white arrowheads), instead of a single nucleus as in wild-type. (H, I) Sens expression identifies single R8 cells near the MF both in wild-type and shtd¹ mutant. Red arrowheads indicate large mitotic nuclei in shtd¹ mutant (I). More posteriorly, wild-type discs contain single R8 cells (H), while shtd¹ mutant discs contain R8 doublets (white arrowheads) (I). The MF is shown by a white bar.
In this report, we characterized the mutant phenotype of shattered (shtd). The shtd¹ allele was identified as a mutation causing visible abnormalities in the compound eye of Drosophila (Thomas et al., 1994). We show here that shtd¹ mutants fail to undergo G1 cell cycle arrest in the MF and exhibit additional cell divisions of the differentiating photoreceptor cells. In addition, the shtd¹ mutant is also defective in progression through mitosis and shows significantly increased apoptosis. We demonstrate that shtd encodes Apc1, the largest subunit of the APC/C and show that the developmental defects of shtd¹ are, at least in part, due to the cell cycle defects as a consequence of an accumulation of CycA and Stg in the mutants.
Materials and Methods
Fly genetics
Flies were cultured at 25°C. The EMS-induced shattered¹ (shtd¹) allele was identified as an X-chromosome linked rough eye mutation. The shtd¹ mutation was mapped genetically using the non-complementing deficiency Df(1)M10-A14 (13C7-8; 14A8-9) and the complementing deficiencies Df(1)M32-C13 (13C3-8; 14B14-18), and Df(1)M34-C1 (13B7-8; 14B15-18), which localized shtd to the cytological interval 13C3-8. The deficiency stocks were gifts from S. Frankel, S. Artavanis-Tsakonas and J. Carlson. The original shtd¹ chromosome carried a temperature sensitive mutation on the X chromosome that increased pupal lethality at 25°C, but had no effect on cell cycle control. This second site mutation was removed by recombination to w1118. For imprecise excision of the EP element, four EP lines that mapped to the 13C region were used: EP(X)1109 (13C5-6), EP(X)1593 (13C3), EP(X)1007 (13C2-3), and EP(X)491 (13C5-6). A total of 800 chromosomes were examined. The shtdEPΔ deficiency was recovered from EP(X)1109. For gamma-ray mutagenesis, isogenic y cv adult males were exposed to gamma irradiation (4000 Rads) using a Shepherd Model 143 137Cs irradiator. Two alleles, shtd² and shtd³, were recovered. The shtd² deficiency allele was mapped by amplifying genomic DNA fragments by PCR from homozygous mutants and wild-type across a region extending from STS Dm0467 at 13B4 to STS Dm3205 at 13E8. The LG51 and BB02 enhancer trap lines were generated in a screen (“Big M”) for insertions showing patterned expression of β-gal (J. Merriam, personal communication). The m(δ)0.5-lacZ was used as an R4 marker (Cooper and Bray, 1999). The CycE alleles were gifts from H. Richardson, the stg alleles from B. Edgar, the fzy alleles and fzy genomic rescue lines from I. Dawson, the rap/fzr alleles from T. Venkatesh, and the EP lines were from Exelixis Pharmaceuticals. All other Drosophila stocks were from the Bloomington Drosophila Stock Center at Indiana University, Bloomington, IN.
Histochemistry
Eye discs were directly dissected and fixed in iced-PLP containing (in 4ml) 1ml 16% Formaldehyde (Cat#18814, Polysciences, Inc.,) 3ml 0.1M Lysine pH7.4, 10mg Sodium meta-periodate for 20min. on ice, and washed in 1xPBS and a balanced salt solution (BSS) containing (in 1L) 2.21g NaCl, 3.98g KCl, 3.07g MgSO4·7H2O, 0.74g CaCl2·H2O, 1.79g Tricine, 3.60g Glucose, 17.12g Sucrose and 2.0g BSA, pH 6.95. Blocking was done with 3% normal goatserum, 0.2% saponin in BSS for 30 min. at RT. Primary antibody incubations were performed at 4°C overnight and secondary and dye-conjugated tertiary antibody reactions were done at room temperature for 4–5 hours and one hour, respectively. For BrdU incorporation, eye discs were dissected in 1xPBS and incubated with 75µg/ml BrdU in BSS for 45min at 25°C in dark. Eye discs were washed twice in 1xPBS and fixed in 4% Formaldehyde, 0.6%Tween-20 in 1xPBS for 20min. at RT and washed twice with 1xPBS. For denaturation, tissues were incubated with 100 unites of DNaseI (Roche) in 500µl DNaseI buffer (66mM Tris pH7.5, 5mM MgCl2) for 1hr at 25°C. then washed twice with 1xPBST (0.3% Tween-20). Blocking was done with 3% normal goat serum in 1xPBS for 30min. Primary antibody dilutions were as follows: mouse anti-CycA, 1:1, mouse anti-CycB, 1:5, rat anti-Elav, 1:10, mouse anti-β-galactosidase JIE7, 1:500 , mouse anti-Myc 9E10, 1:5 (Developmental Studies Hybridoma Bank, University of Iowa); rabbit anti-Stg, 1:20 (Edgar et al., 1994); sheep anti-BrdU, 1:1000 (Research Diagnostics, Inc.); rabbit anti-phosphohistone H3, 1:1000, mouse anti-MPM2, 1:500 (Upstate Biotechnology); rabbit anti-β-galactosidase, 1:1000 (ICN); mouse anti-CycE, 1:10 (Richardson, 1995), and rabbit anti-ACTIVE® Caspase-3, 1:200 (Promega). Dye-conjugated secondary and tertiary antibodies were from Jackson Immunoresearch, and were used at 1:500. Optical sections (1.2 µm) were collected on a BioRad MRC1024 confocal microscope. Tissue in situ hybridization was done following the E. Bier lab protocols (embryos and imaginal discs) using digoxygenin-labelled RNA probes (Roche DIG RNA labeling kit). TUNEL analysis was modified for eye discs from (Lisi et al., 2000). Acridine Orange staining was followed Protocol 12.7 in Drosophila Protocols (Wolff, 2000). For mosaic clone analysis, shtd³ FRT18A chromosomes were generated by recombination. Multiple lines of recombinant chromosomes were examined to yield consistency. To induce mosaic clones, flies were crossed with FRT18A pπMyc; hs-FLP (2nd), and 1hr heat shocks at 37°C were applied at 48hr and 60hr AEL. Prior to dissection, a single 30min heat shock at 37°C was applied to induce pπMyc.
Molecular biology
To generate the Shtd rescue construct, a 9.5 kb SalI-SpeI genomic DNA fragment from the P1 clone DS08954 was cloned into the XhoI-SpeI site of pCaSpeR4. P-element mediated transformation was performed in w1118 embryo following standard procedures. Three independent transgenic lines were established and used for rescue experiments. Sequencing was done with dRhodamin dye termination on an ABI 310 or ABI377 sequencer. Inverse PCR of the shtdEPΔ allele followed the Berkley Drosophila Genome Project protocol.
Results
R8 photoreceptor cells are frequently twinned in shtd¹ mutants
Flies containing the shtd¹ mutation have small eyes with highly disorganized ommatidia. To elucidate the function of Shtd, we set out to characterize the developmental defects of shtd¹ mutants. During development of the compound eye, the onset of photoreceptor cell differentiation occurs within the MF as groups of cells form clusters that are competent to assume a neuronal cell fate (Fig. 1A). Immunostaining using an antibody to the pan-neuronal protein Elav showed that differentiating photoreceptor cell clusters in shtd¹ displayed aberrant spacing and abnormal numbers of photoreceptor neurons as compared to wild-type (Fig. 1B and 1C). To further characterize shtd¹ eye phenotype, we examined specific differentiation markers that express in distinct developing photoreceptor neurons. We first examined R8 cell markers as R8 is the first cell to be specified in each ommatidial cluster and is required for the subsequent photoreceptor cell recruitment (Brennan and Moses, 2000; Frankfort and Mardon, 2002). Specification of the R8 cell fate is induced in the MF by expression of the proneural gene atonal (ato). Ato is first expressed in a broad stripe in the MF. Its expression is gradually restricted to a group of cells called the R8 equivalence group and finally to the R8 photoreceptors (Fig. 1D) (Baker et al., 1996; Dokucu et al., 1996; Jarman et al., 1994). ato loss-of-function mutations cause failure of both R8 differentiation and subsequent photoreceptor recruitment (Jarman et al., 1994; Jarman et al., 1995). In shtd¹ mutant eye discs, Ato protein as well as mRNA levels were slightly decreased in the MF (Fig. 1D and 1E, white line; data not shown). However, Ato positive R8 cells were still observed behind the MF with a slightly disorganized pattern in the shtd¹ mutants (Fig. 1D and 1E , white bracketed line).
Since ato expression persists only in the first 3–4 rows of differentiating R8 cells, the R8 specific enhancer trap lines, LG51 and BB02 were used to monitor R8 cells in the posterior of the eye disc. Interestingly, R8 doublets were observed with the R8 enhancer trap lines in shtd¹ eye discs even though only single R8 cells were specified in the first 3–4 rows as shown by anti-Ato staining. Double staining with Elav and Cyclin B showed that the twinned R8 cells were in the same clusters (Fig. 1F and 1G, clusters are circled with a white broken line, white arrowheads indicate R8). To further characterize the R8 twinning phenotype, we used Senseless (Sens) to mark the R8 cells. Sens is a good marker to identify R8 cells throughout third instar eye disc development, because Sens expression begins in the R8 equivalence group and later its expression is restricted and maintained only in the R8 cells (Frankfort et al., 2001)(Fig. 1H). We found Sens expressed in the R8 equivalence groups in shtd¹ with a slightly disorganized spacing pattern (data not shown, out of focus in Fig. 1H and 1I). Interestingly, single Sens-positive R8 cells were observed immediately behind the MF (Fig. 1H and 1I, white bracketed line ), while immediately posterior to this region, large single nuclei of Sens-positive cells were observed, followed by twinned R8 cells more posteriorly (Fig. 1I, red and white arrowheads). In contrast, Sens staining in the same region in wild-type eye discs did not detect either the large nuclei or the R8 doublets (Fig.1H).
Additional cell division of the developing photoreceptor cells in shtd¹ mutants
R8 twinning could be caused by a defect in the specification of R8 or by an additional round of cell division of already specified single R8 cells. The observation that single Sens-positive R8 cells were specified before the appearance of R8 doublets suggests the possibility of an additional round of cell division. To directly test this idea, we performed double labeling using Sens and the mitotic marker phosphorylated Histone H3 (PH3) (Goto et al., 1999). Double labeling showed that the large nuclei were also stained with PH3 antibody, indicating that these large R8 nuclei were in mitosis (Fig. 2A). Occasionally, Sens positive anaphase cells were also observed (Fig. 2A, inset), indicating that the twinned R8 cells in the posterior were derived from an additional cell division from the already specified single R8 cells. To determine if other photoreceptor types also undergo additional round of cell division, we carried out double staining of Elav and PH3. As shown in Figure 2B, a subset of Elav-positive cells colocalized with PH3 in shtd¹, but not in wild-type eye discs (Fig. 2B and 2C). Each cluster of Elav-positive cells in shtd¹ mutants frequently contains 1–2 cells labeled with PH3, suggesting that other types of photoreceptor cells also undergo an additional round of mitosis. In support of this observation, we found a subset of R2/R5 cells and R3/R4 cells were also labeled with mitotic marker PH3 in shtd¹ mutants when R2/R5, R3/R4 or R4 specific enhancer traps were used (data not shown). Therefore multiple types of photoreceptor cells undergo additional rounds of cell division in shtd¹ mutants. Interestingly, despite the observed additional mitosis, decreased number of R3 and R4 photoreceptor cells was observed in shtd¹ mutants. For example, there are only 41.5±6 (n=6) R4 cells (identified with m(µ)0.5-lacZ) observed in shtd¹ eye discs as compared to 275.3±23 (n=6) observed in wild-type discs. The decreased R3 and R4 cells in shtd¹ mutants could be due to increased apoptosis or due to defects in R cell recruitment (see discussion).
Figure 2. Loss of shtd¹ overrides G1 arrest of developing photoreceptor cells.

(A) shtd¹ mutant eye discs double stained with Sens (green) and PH3 (purple). White arrowheads indicate Sens-positive metaphase cells. Anaphase or telophase cells are occasionally seen (inset). Behind the band of metaphase cells, Sens-positive R8 doublets are seen (white arrows). (B, C) Elav labeled (green) neuronal cells colocalized with PH3 (purple) in the shtd¹ mutant (B), but not in wild-type (C). (D) Elav stained cells (green) co-labeled with BrdU in shtd¹ (purple, white arrows). (E, F) Acridine orange staining showed a few apoptotic cells in wild-type (E). In contrast, a dramatic increase of apoptotic cells was seen in the posterior of the eye disc in the shtd¹ mutant (F).
Premature S phase entry of G1 arrested cells and increased apoptosis in shtd¹ mutants
We found multiple R cell types undergo additional mitosis in shtd¹; therefore, we were interested to examine the effect of the shtd¹ mutation on the pattern of S phase. The MF spans about 8–9 cell widths and cells are arrest in G1 phase in this domain. Upon exiting the MF, the clusters of five photoreceptor precursor cells remain arrested in G1 while all cells that are not incorporated into preclusters re-enter S phase synchronously behind the MF, which is often referred to as the SMW (Fig. 3A) (Baker and Yu, 2001; Wolff and Ready, 1993). In shtd¹ eye discs, BrdU labeling showed that cells enter S phase precociously in the G1 arrest domain (Fig. 3A and 3B). We found that some Elav-positive neuronal cells incorporated BrdU posterior to the MF (Fig. 2D), indicating that differentiating photoreceptor cells enter an additional round of S phase. In contrast we did not observe BrdU incorporation into Sens-positive R8 cells (data not shown), indicating that the Sens-positive twinned R8 cells did not re-enter S phase prior to mitosis. These observations suggest that by the time R8 photoreceptors were determined and expressed Sens in shtd¹, the R8 cells had already completed S phase.
Figure 3. Loss of shtd results in ectopic mitotic Cyclins and String accumulation and failure to arrest in G1.
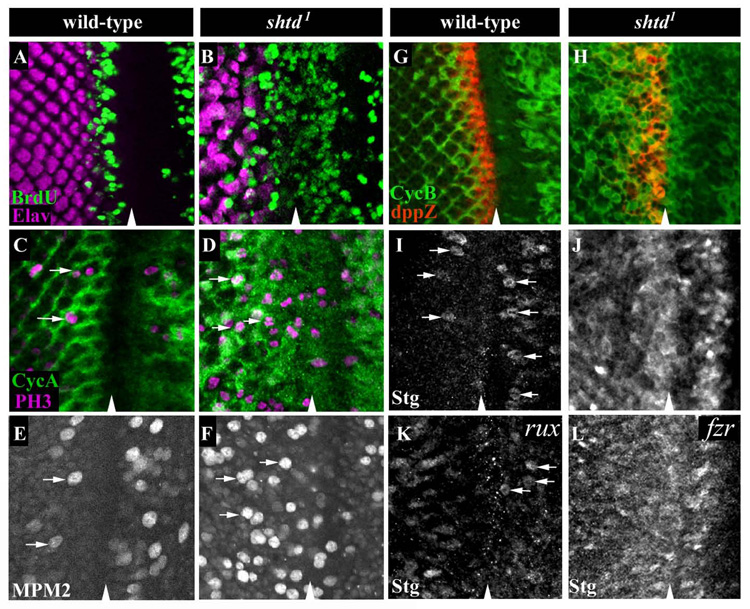
(A, B) S phase cells are labeled with BrdU (green), and differentiating cells are labeled with the neuronal marker Elav (purple). BrdU incorporation in the SMW is seen just behind the MF and anterior to the Elav expressing neuronal cells in wild-type (A). In shtd¹, precocious BrdU incorporation is seen in many cells in the MF (B). For wild-type, basal BrdU and medial Elav optical sections were taken separately and two images merged. For shtd¹, a basal optical section is shown. (C-F) mitotic cells were marked with PH3 (purple) (C, D) or MPM2 (E, F) (arrows). A large increase in PH3 and MPM2-positive cells is seen in and behind the MF in shtd¹ (D, F) compared to wild-type (C, E). CycA is shown in green (C, D). (G, H) CycB (green) prematurely accumulates in the MF (dpp-lacZ, red) in shtd¹ (H), but not in wildtype (G). (I) In wild-type, String protein is expressed transiently in metaphase cells just ahead of and behind the MF (arrows). (J) In shtd¹, String protein is highly expressed in cells in a band which corresponds to those cells expressing high levels of stg mRNA in wild-type. (K) String accumulation is not significantly increased in the rux8 null mutant. A modest accumulation of String protein is seen posterior to the MF. Metaphase cells are identified in the MF (white arrows). (L) fzr mutants show an increased String protein levels. Arrowheads indicate the MF in all panels.
As shtd¹ mutant flies have much smaller eyes than wild-type, we also examined whether there is increased apoptosis in shtd¹ eye discs. Using Acridine Orange staining, there is a dramatic increase in the number of cells undergoing apoptosis in the posterior of the developing eye discs (compare Fig. 2E and 2F). Increased apoptosis is also observed in a pupal lethal allele of shtd, shtd³. Larvae mutant for shtd³ have greatly reduced imaginal tissues. We generated shtd³ mutant clones in imaginal discs using the FLP/FRT method. Increased expression of activated Caspase-3 was found in the shtd³ mutant clones but not in the adjacent wild-type tissues (Fig. S2C). These data indicate that increased apoptosis also contributed to the shtd¹ mutant phenotype.
Accumulation of mitotic Cyclins and String protein in shtd mutants
To further characterize the observed cell cycle defect in shtd¹ mutants, we examined the expression pattern of cell cycle regulators. In the MF of the developing eye, the levels of mitotic cyclins CycA and CycB were low in wild-type eye discs (Fig. 3C and 3G, Fig. S1A). In shtd¹ eye discs, cells showed premature accumulation of CycA and CycB in the G1 arrest domain (Fig. 3D and 3H, Fig. S1B). Double-labeling showed that ectopic accumulation of CycA correlated with the appearance of S phase cells in the mutant eye discs (Fig. S1B). In higher eukaryotes, entry into S phase normally requires CycE. In Drosophila, CycE is sufficient to trigger quiescent cells to enter S phase and CycE activation often led to accumulation of the mitotic cyclins, CycA and CycB (Knoblich et al., 1994). In the eye disc, CycE accumulation begins in a subset of cells in the MF (Fig. S1C) (Richardson et al., 1995). Surprisingly, neither premature CycE enhancer activation nor ectopic CycE protein accumulation was observed in the MF of shtd¹ mutant eye discs (Fig. S1D and data not shown), suggesting that ectopic S phase entry in the mutant is independent of CycE. In Drosophila, high-level expression of CycA triggers the G1/S transition in wild-type embryos and eye discs (Dong et al., 1997; Sprenger et al., 1997) and this effect is also observed in mutant embryos lacking CycE (Sprenger et al., 1997). Thus, ectopic S phase entry of G1 arrested cells in shtd¹ correlated with the accumulation of the mitotic cyclin CycA.
In wild-type eye discs, stg mRNA expression is induced to high levels adjacent and anterior to a band of dpp-lacZ (BS3.0) in the MF and in a lattice pattern posterior to the furrow (Baonza et al., 2002; Blackman et al., 1991; Heberlein et al., 1995; Thomas and Zipursky, 1994). However, Stg protein is only observed transiently in metaphase cells in the proliferating population of cells anterior and posterior to the MF (Fig. 3I). By contrast, shtd¹ mutants accumulate high levels of Stg protein. Anterior to the MF, Stg accumulation corresponds to the band of stg mRNA expression (Fig. 3J and data not shown). The increased levels of Stg protein anterior to the MF are not due to an increase in stg transcription, since stg mRNA expression in this region is not significantly elevated in shtd¹ mutants as compared to wild-type (data not shown). Stg accumulation is also consistently observed in the shtd³ allele. FLP/FRT-mediated mosaic clone analysis revealed that Stg protein accumulated in mutant clones that spanned the MF (Fig. S2A). In addition, increased CycB was also observed in shtd³ mutant clones in the MF (Fig. S2B). These data indicate that Shtd regulates Stg protein level in addition to mitotic cyclins CycA and CycB.
The observation that shtd¹ mutant eye discs exhibit ectopic BrdU incorporation and CycA accumulation in the MF is similar to that of the previously characterized roughex (rux) mutants (Thomas et al., 1994). Rux was shown to be required for the G1 arrest in the MF by preventing the accumulation and/or activity of the mitotic cyclin, CycA (Avedisov et al., 2000; Foley et al., 1999; Thomas et al., 1997). Interestingly, shtd¹ and rux are distinct in their ability to regulate Stg protein. As shown in Figure 3K, there are only a few Stg positive cells observed near the MF in rux null mutants (Fig.3K, white arrows) as compared to the large number of cells accumulate Stg in shtd¹ mutants.
Mitotic delay in the shtd¹ mutants
In wild-type eye discs behind the MF, large populations of cells between each developing cluster accumulate mitotic cyclins, CycA and CycB, indicating that cells are in G2 (Fig. 3C, 3G in green). All of these G2 cells eventually undergo mitosis, which is regulated in part through EGFR signaling (Fig. 3C, 3E white arrows (Baker and Yu, 2001; Baonza et al., 2002). In wild-type eye discs stained with the anti-PH3 antibody, cells displaying anaphase-like chromosomal configurations can readily be observed (Fig. 4B). In the shtd¹ mutants, however, many more PH3-positive cells were observed in and behind the MF and many cells displayed a condensed chromosomal morphology. In contrast, anaphase-like chromosomal configurations were quite rare (Fig. 4D). Double labeling of PH3 and CycA showed many cells have stabilized CycA (Fig. 3D and data not shown). These observations suggest that the shtd¹ mutants have a defect in progression through mitosis, in particular during the transition from metaphase to anaphase. To further characterize the mitotic phenotype of shtd¹, we used the anti-MPM2 antibody to visualize and quantify mitotic cells (Ryo et al., 2001). It is expected that a mitotic delay will significantly increase the number of cells in mitosis. An average of 59±5 mitotic cells was seen in and behind the MF in shtd¹ as compared to 17±5 in wild-type (Fig. 3E and 3F, n=6 for both mutant and wild-type). These observations support the idea that there is a mitotic delay in the shtd¹ mutants. Interestingly, the number of mitotic cells anterior to the MF was also slightly increased in shtd¹ mutant as compared to that in wild-type (18±6 versus 10±2, respectively) while no difference was seen in the number of S phase cells in the mutant as compared to wild-type anterior to the MF (24±9 for shtd¹, n=8, versus 24±12 for wild-type, n=9). These observations suggest that shtd¹ may also cause a delay in progression through mitosis in the anterior asynchronously proliferating cells even though it did not affect the rate of S phase entry there.
Figure 4. Reducing stg dosage suppresses the mitotic delay phenotype of shtd¹.
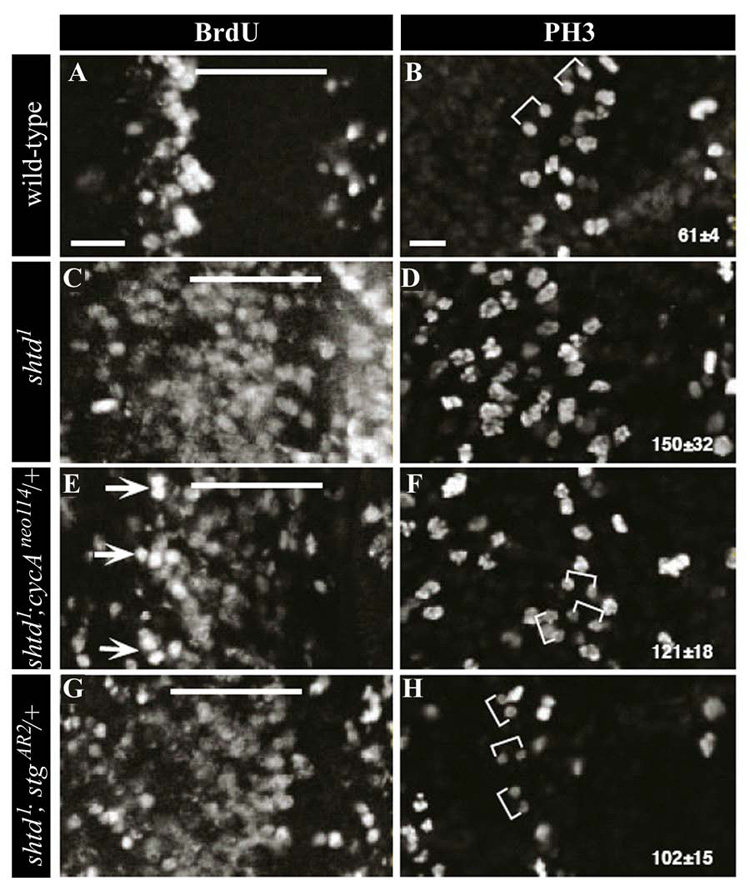
BrdU- (A, C, E, G) and PH3-labeling (B, D, F, H) to show S phase and mitotic cells, respectively, in wild-type (A, B), shtd¹(C, D), shtd¹; CycAneo114/+ (E, F), and shtd¹; stgRXT13/+ (G, H). The MF is indicated by a line and brackets show pairs of anaphase or telophase cells. Arrows indicate clusters of S phase cells with a more wild-type appearance that arise upon reducing CycA gene dosage in the mutant. Mitotic cells were counted in a unit area (wild-type, n=3; shtd¹, n=7; shtd¹; CycAneo114/+, n=4; shtd¹; stgRXT13/+, n=4) and the average number of PH3-positive cells is shown ± s.d. Nuclei with visibly divided chromosome masses were counted as two units. Scale bars, 10µm.
The shtd gene encodes Drosophila Apc1
In order to further characterize the gene corresponding to shtd, the shtd locus was mapped to the 13C–D region by deficiency mapping (Fig. 5A, See Materials and Methods). Imprecise excision of EP1109 (Fig. 5B and Materials and methods) generated one allele, shtdEPΔ, in addition, two gamma ray-induced lethal alleles, shtd² and shtd³ were generated that also failed to complement the shtd¹ eye phenotype. Inverse PCR analysis from shtdEPΔ showed that this allele results from a 33 kb deletion (Fig. 5B). To determine whether the remaining alleles contained chromosomal rearrangements, we performed PCR mapping across a 519Kb region corresponding to cytological bands 13B–E from shtd¹, shtd² and shtd³ (Materials and methods). The shtd² allele also results from a deletion minimally 56 kb in size that completely overlaps the 33 kb deletion in shtdEPΔ; no chromosomal rearrangements were detected in shtd¹ or shtd³ by this analysis. We conclude that the 33 kb region uncovered by shtdEPΔ contains shtd.
Figure 5. shtd encodes the APC-1 homolog.
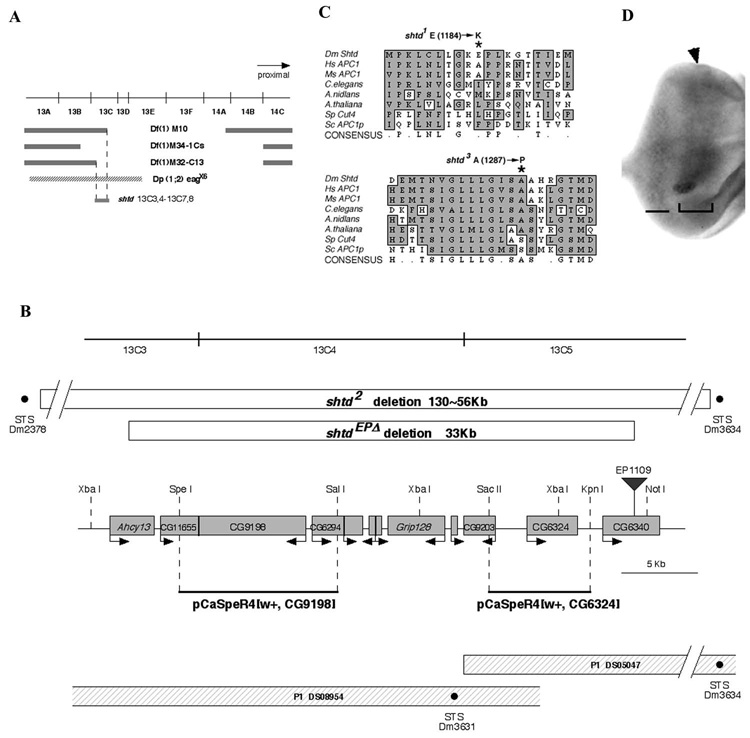
(A) shtd deficiency mapping. Deficiencies uncovering the 13C region and the duplication chromosome Dp(1;2) eagx6 are shown. The shtd gene maps by complementation to 13C3,4-13C7,8. (B) Schematic representation of the shtd locus. An approximately 35 kb region surrounding CG9198 is shown. The shtdEPΔ and shtd² deletions are shown by open boxes; the breakpoints of shtd² fall outside of the range of this map, and the nearest intact STS markers are shown. The insertion site of EP1109, which was used for the imprecise excision, is shown by an inverted triangle. Predicted open reading frames are shown by shaded boxes, with the putative direction of transcription showed by the arrow. P1 clones and STS markers (black dots) are shown at the bottom. For the rescue experiment, genomic fragments were isolated from P1 clones (solid black lines). The genomic fragment that contains the CG9198 ORF rescued shtd mutant phenotype while the fragment that contains the CG6324 did not. The restriction enzyme sites used for subcloning are shown on the map. (C) The shtd¹ allele results from a substitution of lysine for glutamic acid at position 1184 (asterisk). The shtd³ allele results from a substitution of proline for a conserved alanine at position 1287 (asterisk). (D) shtd mRNA expression pattern in third instar eye disc. Expression is higher in the domain surrounding the MF (bracketed region) and posterior portion of eye disc (bar). The arrowhead indicates the MF.
The 33 kb region contains ten annotated genes (Grumbling, 2006). The cell cycle defects of shtd mutants described above led us to focus on CG9198, which encodes the Anaphase-Promoting Complex 1 (Apc1), the largest subunit of the APC/C. The Drosophila Apc1 shows 36% identity and 54% similarity to the human and mouse proteins over its entire length. To determine if shtd corresponds to Apc1, we performed genomic rescue experiments. A genomic fragment containing Apc1 was isolated from a P1 clone (Fig. 5B and Materials and Methods), and multiple transgenic flies were established. Both the shtd¹ eye phenotype and theshtd³ lethality were rescued by the genomic fragment containing Apc1. Sequence analysis showed that both shtd¹ and shtd³ contain point mutations in Apc1 (Fig. 5C). The shtd¹ allele contains a single Glu-to-Lys substitution at position 1184, while shtd³ contains a substitution of Ala-to-Pro at 1287 and a five-base pair deletion in the promoter region of Apc1. Therefore, both shtd¹ and shtd³ are loss of function alleles of Apc1.
Analysis of shtd mRNA expression pattern in embryos by in situ hybridization showed a large component of maternal mRNA during early stages of embryogenesis (Fig. S3A). Later expression is restricted to a particular patterned domain (Fig. S3C and S3D). In third instar eye discs, shtd expression is higher in the region covering the MF, and also in the posterior domain of eye discs (Fig. 5D). This mRNA expression pattern, particularly the region covering the MF, correlates with the region in which we observed the high level accumulation of Stg and mitotic cyclins in the shtd¹ mutants.
Genetic interactions identify stg, CycA, and apoptotic regulators as modifiers of shtd¹
Dosage-sensitive enhancement or suppression of mutant phenotypes in Drosophila is a powerful method to identify genes that act in common genetic pathways. In particular, loci that dominantly suppress the shtd¹ mutant phenotype at reduced gene dosage are potential substrates for APC/C-mediated proteolysis or regulators of APC/C activity. We used a series of overlapping deficiencies of the second and third chromosomes to search for modifiers of the shtd¹ eye phenotype (Fig. 6B). Two deficiencies that uncovered the 99A region of the third chromosome suppressed shtd¹; this region contains stg. Multiple alleles of stg strongly suppressed the shtd1 eye phenotype (Fig. 6E, Table 1).
Figure 6. Suppression of shtd¹ adult eye phenotypes.
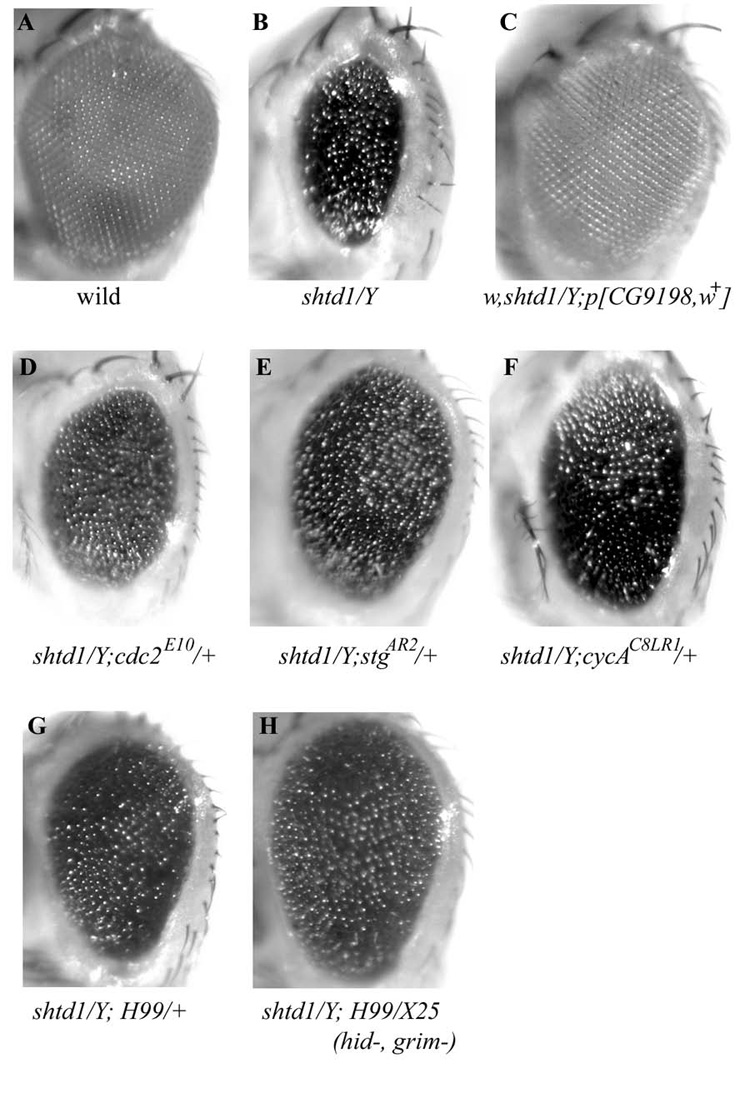
(A, B) A shtd¹ mutant male fly eye (B) displays a rough and reduced eye compared to wild-type (A). (C) The shtd¹ rough eye phenotype is completely rescued by introducing one copy of the CG9198 gene. (D-H) shtd¹ modifiers. A one copy reduction of cdc2 (D), stg (E), or CycA (F) suppresses the shtd¹ eye phenotype. A one copy reduction of the apoptosis regulators rpr, hid and grim (Df(3L)H99/+, G) or removal hid and grim (Df(3L)H99/Df(3L)X25, H) also show suppression. The size of the compound eye is larger and the eye roughness is milder than in shtd¹ alone. Adult male fly eye images were taken under a Leica dissecting microscope.
Table 1.
shtd¹ genetic interactions.
| Gene | Allele/deficiency/transgene | Dosage | Effect1 |
|---|---|---|---|
| cell cycle regulators | |||
| stg/Cdc25 | AR2, RXT13, 01215, 7B, 7M | ↓ | suppress |
| CycA | neo114, C8LR1, 03946 | ↓ | suppress |
| l(3)183, hari | ↓ | none | |
| CycB | Df(2R)59AB | ↓ | none |
| CycB3 | L6540 | ↓ | none |
| CycE | AR95, 05206, P4, JP, | ↓ | none |
| l(2)35Dd | |||
| CycH | EP3658 | ↓ | enhance |
| cdc2 | B47, E10, E1-24, E51Q | ↓ | suppress |
| Df(2L)J27 | |||
| cdc2c | Df(3R)H81 | ↓ | none |
| Cdk4 | k06503, 05428, s4639 | ↓ | none |
| rux | 3, 8 | ↓ | lethal2 |
| pRux+6.0D1 | ↑ | suppress3 | |
| APC subunits and regulators | |||
| mr /Apc2 | 1, 2 | ↓ | suppress |
| lmg /Apc11 | 03424 | ↓ | suppress |
| ida /Apc5 | B4, D14 | ↓ | suppress |
| mks/Cdc27/Apc3 | L7123 | ↓ | none |
| fzr/rap/Cdh1 | x-3, e6 | ↓ | lethal2 |
| Dp(1;2)rb±71g | ↑ | suppress | |
| fzy/Cdc20 | 1, 4, 5, 6, Df(2L)r10 | ↓ | none |
| pFzy8+II.1, pFzy8+III.1 | ↑ | none3 | |
| rca1/Emi1 | IX KS2.30.1 | ↓ | suppress |
| Apoptosis regulators | |||
| rpr, hid, grim | Df(3L)H99 | ↓ | suppress |
| hid, grim | Df(3L)X25 | ↓ | suppress4 |
| rpr | Df(3L)XR38 | ↓ | suppress4 |
| hid | 05014 | ↓ | suppress4 |
| th /diap1 | 4,5 | ↓ | enhance |
Enhance or suppress refers to the shtd¹ eye phenotype. Unless otherwise indicated, genetic interactions were tested by reducing the dosage of the indicated gene in males (i.e., shtd¹/Y; gene/+).
Interactions were assessed as double-mutant combinations.
These crosses assessed an increase in gene dosage using genomic rescue constructs.
Interactions were assessed as double mutant combinations. (shtd¹/Y; Df(3L)X25/Df(3L)H99, shtd¹/Y; Df(3L)XR38/Df(3L)H99 or shtd¹/Y; hid05014 / Df(3L)H99).
We also recovered genetic interactions between shtd¹ and genes previously shown to regulate CycA-dependent kinase activity (Table 1). Reducing the dosage of cdc2, CycA, or rca1 all partially suppressed shtd¹ eye phenotype (Fig. 6D and 6F). CycA was shown to be regulated by rux (Sprenger et al., 1997; Thomas et al., 1997). Increasing the gene dosage of rux or fzr partially reduced shtd¹ eye roughness (Table 1) whereas double mutant combinations of the viable alleles shtd¹ and rux³ or fzrrape6 are synthetic lethal at pupal or early larval stages, respectively. Furthermore, the rux³, shtd¹ double mutant eye discs exhibited more severe defects of aberrant mitotic figures and abnormal ommatidial spacing than that seen in either rux3 or shtd¹ alone (data not shown). These genetic interactions are consistent with previous observations that rux mutants are defective in their ability to prevent the accumulation and/or activity of the mitotic cyclin, CycA (Avedisov et al., 2000; Foley et al., 1999; Foley and Sprenger, 2001; Thomas et al., 1997), and those showing a failure to degrade mitotic cyclins in fzr mutants (Sigrist and Lehner, 1997).
In addition, decreasing the dosage of apoptotic regulators such as hid, rpr or grim also significantly suppressed the shtd¹ eye phenotype (Fig. 6G and 6H). This is consistent with the observation that there is significantly increased apoptosis in shtd¹ mutants (Fig. 2F). Surprisingly, reducing the gene dosage of several APC/C subunits using available mutations suppressed shtd¹ (Table 1, Fig.S5). Examination of ectopic cell proliferation by BrdU incorporation and anti-PH3 staining showed that the observed suppression was correlated with a decreased ectopic BrdU incorporation in the MF and a corresponding increase in the number of anaphase cells (Fig. S6). Interestingly, the level of CycA was slightly reduced in shtd¹; mr² /+ and shtd¹; idaB4/+ eye discs as compared to that of shtd¹ (data not shown). It is possible that the partial APC/C complex without Apc1 may exert a dominant negative effect on the degradation of some key substrates.
The shtd¹ mutant phenotype is not limited to the eye. The wing margin bristles of the adult wing develop from the zone of non-proliferating cells (ZNC), which were shown to be arrested at G1 or G2 (Johnston and Edgar, 1998). The ordered array of wing margin bristles was disrupted in shtd¹ as compared to that in wild-type (Fig. S4A and S4B). This phenotype was suppressed by reduction of either stg or of apoptosis regulators (Fig. S4C–S4F), indicating that the observed genetic interactions between shtd¹ and stg or the apoptosis regulators are not limited to the eye.
Mutations in stg and CycA suppress shtd¹ through distinct mechanisms
Genetic interaction experiments show that reducing the gene dosage of CycA, cdc2 or stg suppresses the shtd¹ rough eye phenotype. In mammalian cells, a G1 form of the phosphatase, Cdc25A, functions in G1 to activate CycE- and CycA-Cdk2 complexes to promote S phase entry (Blomberg and Hoffmann, 1999). One model that would account for the observed genetic interactions is that Stg functions similarly in Drosophila to activate a CycA-dependent kinase complex in G1 cells which drives S phase entry. Alternatively, it is possible that stg suppresses the shtd¹ eye phenotype through a distinct mechanism. To distinguish between these two possibilities, we examined shtd¹ eye discs with reduced gene dosage of either CycA or stg for their ability to undergo G1 arrest and mitosis (Fig. 4).
Reducing the dosage of either CycA or cdc2 in shtd¹ partially rescued the ectopic S phase phenotype seen in the mutants; the numbers of ectopic S phase cells within the MF was reduced relative to shtd¹ alone (Fig. 4E, data not shown), and groups of cells more strongly labeled with BrdU were observed just behind the MF in a position equivalent to the synchronous band of S phase cells seen in wild-type discs (Fig. 4E, arrows). In addition, reducing the gene dosage of CycA in shtd¹ also led to a moderate increase in the number of anaphase or telophase cells and a slight reduction in the total number of PH3-positive cells (from 150±32 to 121±18, Fig. 4D and 4F). It is possible that the increase in anaphase or telophase cells results from the restoration of normal S phase progression in a subset of cells behind the MF. By contrast, reducing the dosage of stg had no significant effect on the ectopic S phase phenotype of shtd¹ (Fig. 4G), but instead showed a significant suppression of the mitotic delay phenotype seen in and behind the MF than that was observed with reduction of CycA (Fig. 4H). These discs exhibited fewer total number of PH3-labelled cells than the shtd¹ and shtd¹; CycA/+ discs and an corresponding increase in telophase cells. These results suggest that reduction of stg suppressed the shtd¹ mutant phenotype by partially restoring mitotic progression. This conclusion is further supported by the observation that we were unable to drive ectopic S phase entry in the MF by overexpression of Stg (data not shown). Taken together, these observations suggest that partial loss of Apc1 function leads to hyper-accumulation of CycA and Stg, which results in ectopic S phase entry in MF, mitotic delay, and increased apoptosis. These phenotypes can be suppressed by reducing the dosage of CycA, stg, and components of the apoptosis pathway, respectively.
Discussion
Our analysis of the Drosophila Apc1 homologue mutant shtd¹ revealed a failure in establishing G1 cell cycle arrest in the MF and a delay in progression through mitosis. The question is: how do the observed cell cycle defects lead to the dramatic developmental defects observed in shtd¹ mutants? As we did not observe BrdU incorporation of Sens positive cells, it is likely that by the time Sens is induced in the future R8 cells, these cells have mostly completed S phase. Since initial Sens expression in the developing eye disc of shtd¹ mutants appears relatively normal, we propose that the initial specification of the first R8 cells proceeds relatively normally even in the absence of normal G1 arrest in the MF. However, these R8 photoreceptor cells that have completed S phase will proceed into mitosis, leading to highly defective arrangement of R8 photoreceptor cells. Since the rest of the photoreceptor cells and accessory cells are recruited after R8 specification, defects in the arrangement of R8 photoreceptors will cause severe defects in eye development. Of course, additional S phase and mitosis of other R cells types will also contribute to the shtd¹ mutant phenotypes. Consistent with this idea, reducing the dosage of CycA suppressed both the ectopic S phase entry in MF and the shtd¹ adult eye phenotype.
In addition to a failure to establish and maintain G1 arrest in MF, shtd¹ mutants also exhibited delayed progression through mitosis. While delayed progression through mitosis in the proliferating cells such as the anterior of the eye discs will not likely cause dramatic defects, it is possible that delayed progression through mitosis in the posterior may result in defects in photoreceptor cell recruitment and contribute to the significantly decreased R3 and R4 cells in shtd¹ mutants. It was shown previously that cell shape changes required for ventral furrow formation during Drosophila embryogenesis was incompatible with mitosis and that precocious mitosis in this region blocked ventral furrow formation (Grosshans and Wieschaus, 2000). Similarly, photoreceptor differentiation is preceded by significant cell shape rearrangements, forming clusters with differential adhesive properties (Brown et al., 2006). Therefore it is possible that the cell shape changes required for the proper differentiation of photoreceptor cells are not compatible with mitosis. As proper differentiation of photoreceptor cells and the other accessory cells involve stepwise recruitment, it is conceivable that cells that remain in mitosis for an extended period of time can contribute to eye development defects. Consistent with this idea, reducing the gene dosage of stg significantly suppressed the mitotic delay posterior to the MF and significantly suppressed the shtd¹ adult eye phenotypes.
One of the obvious phenotypes of shtd¹ mutants is the dramatically increased apoptosis in the larval eye disc and the small size of the adult eye. Although we do not know the exact cause for the extensive apoptosis in the eye disc, it is likely a consequence of the defects in cell cycle control or defects in differentiation. Consistent with this, dosage reduction of either stg or CycA partially restored cell cycle defects of shtd¹ mutant eye discs and partially suppressed adult eye size defect as well.
Although the primary developmental phenotypes observed in shtd¹1 mutants are likely caused by defects in cell cycle control, this does not exclude the possibility that the APC/C may also function directly in regulating the properties of the postmitotic differentiating cells. Recently, a few studies identified crucial roles of APC/C-Cdh1 in controlling axon growth and patterning in developing brain and synapse size/function through proteolysis of key proteins (Juo and Kaplan, 2004; Konishi et al., 2004; Lasorella et al., 2006; van Roessel et al., 2004). Interestingly, the APC/C regulatory subunit Cdh1 but not Cdc20 is required to exert this postmitotic APC/C function.
The shtd¹ mutant phenotype has both similarities and differences with the rux mutant phenotypes (Thomas et al., 1994). Both the shtd¹ and the rux mutants showed accumulation of CycA and ectopic S phase in MF. In contrast, only shtd¹ but not the rux mutants displayed accumulation of Stg and delayed progression through mitosis. These observations are consistent with the observed suppression of shtd¹ and rux mutants by reducing the gene dosage of CycA: while rux mutants were fully suppressed by reducing CycA gene dosage (Avedisov et al., 2000; Foley et al., 1999; Foley and Sprenger, 2001; Thomas et al., 1997), the shtd¹ rough eye phenotype was only partially suppressed (Fig. 6F ). These observations suggest that while the rux mutant phenotype results primarily from ectopic S phase entry due to CycA accumulation, the shtd¹ mutant phenotype results from both ectopic S phase entry and mitotic delay as a consequence of CycA and Stg accumulation, respectively. Currently, it is not clear how accumulation of Stg protein delays progression through mitosis. It is possible that in shtd¹, reduced APC/C activity is unable to quickly inactivate mitotic Cyclin/Cdk activities, leading to delayed mitotic exit. Reducing the dosage of stg may help the inactivation of mitotic Cyclin/Cdk activity during mitotic exit and promote mitotic progression. Alternatively, several APC/C subunits require phosphorylation on multiple sites for activity and dephosphorylation of the APC/C is sufficient for its inactivation (Herzog et al., 2005; King et al., 1995; Peters et al., 1996; Yamada et al., 1997). It is possible that Stg may directly or indirectly regulate APC/C activity to promote progression through mitosis.
In Drosophila, Stg protein levels are precisely controlled to promote the G2-M transition. Previous studies indicated that Stg is rapidly degraded upon exit from mitosis, although the mechanisms controlling its stability were not known (Edgar et al., 1994). More recently, studies in mammalian cells showed that degradation of Cdc25 upon exit from mitosis is dependent on APC/C-Cdh1 and a KEN-box motif at the N-terminus of Cdc25 (Donzelli et al., 2002). We found that the Drosophila Cdc25 homologue Stg accumulates in a region that displays high levels of stg mRNA expression in shtd¹ mutants, suggesting that proteolysis of Drosophila Stg in the developing eye requires the Shtd function. Since we observed strong genetic interactions between shtd and fzr, which encodes the Drosophila Cdh1 subunit of APC/C complex, and since fzr mutants also accumulate Stg protein in the MF, we suggest that Stg degradation is regulated by APC/C-Cdh1.
Supplementary Material
The dpp-lacZ enhancer trap (blue) marks the posterior half of the MF. (A) In wild-type, weak CycA expression (green) is detected in S phase cells (red) at the posterior edge of the MF (arrows). (B) In shtd¹, expression of CycA is seen in cells entering S phase ectopically within the MF. (C) In wild-type eye discs, CycE protein (green) is expressed at low levels in groups of cells (arrows) entering S phase synchronously behind the MF. (D) In shtd¹, CycE protein accumulates to high levels in the nuclei of many cells (arrows) behind the MF. Arrowheads indicate the MF.
shtd³ mutant cells marked by the absence of Mycepitope expression (green) (outlined). (A-A”) In shtd³ mutant clone anterior to the MF accumulate Stg (purple). (B-B”) Mutant clones in the MF accumulate CycB (purple), however accumulation is not observed in Myc expressing wild-type cells (green). (C-C”) A subset of cells in shtd³ mutant clones labeled with anti-active Caspase3 antibody (purple, arrows) indicating that the cells are undergoing apoptosis. Anterior of the eye disc is to the right.
(A,B) Early stage embryos contain large amounts of maternal mRNA. (A) Preblastoderm stage. (B) Cellular blastoderm stage. (C) At gastrulation, high level expression is observed in the cephalic furrow (cf, arrowhead)‥ (D) Expression in stage 11 embryos. (E) By stage14 most epithelial expression is down regulated.
(A) Wild-type wing margin bristles (arrowhead). (B) In shtd¹, the wing shows a severe loss of wing margin bristles. Many bristles are missing in the double bristle row (black arrowhead). No significant difference is observed in the triple bristle row (anterior wing margin; vein1, not shown). The distance between vein3 and vein4 is also narrower at the wing margin than in wild-type (double arrowhead line). (C) Reducing stg gene dosage restores bristle number and increases the width between vein3 and vein4. (D–F) Removal of rpr (D) hid, grim (E) or hid (F) also suppressed the shtd¹ wing phenotype. The degree of suppression with the simultaneous removal of hid and grim was stronger than removing rpr or hid alone. (G) A schematic diagram of the third instar wing pouch (mid to ventral portion only). In the zone of non-proliferating cells (ZNC), cells arrest at G2 or G1 in distinct domains. Defects in the wing margin phenotype correspond to the posterior domain of the ZNC, in which cells arrest in G1.
(A) A shtd¹ mutant male fly eye displays a reduced and rough eye. (B and C) Suppression of shtd¹ by reducing APC/C components. A one copy reduction of mr/Apc2 (B) or ida/Apc5 (C) suppresses the shtd¹ eye phenotype. The sizes of the resulting compound eyes are larger than those of the shtd¹ mutants. Merged frontal adult male fly eye images were obtained using a Zeiss Axio Imager Z1m light microscope. Thirty sequential images (5µm thickness each) were merged using a Zeiss Axio Vision imaging software (Ver. 4.5).
BrdU- (A, C, E, G) and PH3-labeling (B, D, F, H) were carried out to show S and M phase cells in wild-type (A, B), shtd¹(C, D), shtd¹; mr²/+ (E, F), and shtd¹; idaB4/+ (G, H) eye discs. The MF is indicated by a line and red brackets show pairs of anaphase or telophase cells. BrdU labeled cells were dramatically decreased in the MF when the dosages of APC/C components were reduced (C, D) compared to shtd¹ alone (A). More pairs of anaphase or telophase cells were observed anterior and posterior to the MF (F, H) compared to shtd¹ alone (D). Anterior of the eye disc is to the right. Images were obtained using a Zeiss Axio Imager Z1m fluorescence microscope with ApoTome. Five to six sequential optical sections (0.67µm thickness each) were merged using the Zeiss AxioVision software.
Acknowledgements
We gratefully acknowledge B. Edgar, T. Venkatesh, H. Richardson, I. Dawson, S. Frankel, S. Artavanis-Tsakonas, and J. Carlson, C. Desplan, S. Bray, B. Mollereau for gifts of fly strains, antibodies and cDNA, and the DSHB for monoclonal antibodies and the Bloomington Drosophila Stock Center for fly stocks. We thank J. Cho and J. Fewell for generating the shtd gamma-ray alleles, and Dr. Jennifer Searle for reading the manuscript. M.T.-M. gratefully thanks Dr. Hitoshi Matakatsu for encouragement. M.T.-M. was supported by fellowships from the Japan Society for the Promotion of Science (JSPS Research Fellowship for Young Scientists, and JSPS Fellowship for Japanese Biomedical and Behavioral Research at NIH). This research was supported by the Intramural Research Program of the NIH, NCI, CCR and by an NIH grant to WD (GM074197). WD is a Leukemia and Lymphoma Society Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avedisov SN, Krasnoselskaya I, Mortin M, Thomas BJ. Roughex mediates G1 arrest through a physical association with Cyclin A. Mol. Cell. Biol. 2000;20:8220–8229. doi: 10.1128/mcb.20.21.8220-8229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE, Yu S, Han D. Evolution of proneural atonal expression during distinct regulatory phases in the developing Drosophila eye. Curr Biol. 1996;6:1290–1301. doi: 10.1016/s0960-9822(02)70715-x. [DOI] [PubMed] [Google Scholar]
- Baker NE, Yu SY. The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell. 2001;104:699–708. doi: 10.1016/s0092-8674(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Baonza A, Freeman M. Control of cell proliferation in the Drosophila eye by Notch signaling. Dev Cell. 2005;8:529–539. doi: 10.1016/j.devcel.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Baonza A, Murawsky CM, Travers AA, Freeman M. Pointed and Tramtrack69 establish an EGFR-dependent transcriptional switch to regulate mitosis. Nat Cell Biol. 2002;4:976–980. doi: 10.1038/ncb887. [DOI] [PubMed] [Google Scholar]
- Bentley AM, Williams BC, Goldberg ML, Andres AJ. Phenotypic characterization of Drosophila ida mutants: defining the role of APC5 in cell cycle progression. J. Cell Sci. 2002;115:949–961. doi: 10.1242/jcs.115.5.949. [DOI] [PubMed] [Google Scholar]
- Blackman RK, Sanicola M, Raftery LA, Gillevet T, Gelbart WM. An extensive 3′ cis-regulatory region directs the imaginal disk expression of decapentaplegic, a member of the TGF-β family in Drosophila. Devel. 1991;111:657–665. doi: 10.1242/dev.111.3.657. [DOI] [PubMed] [Google Scholar]
- Blomberg I, Hoffmann I. Ectopic expression of Cdc25A accelerates the G1/S transition and leads to premature activation of Cyclin E- and Cyclin A-dependent kinases. Mol. Cell. Biol. 1999;19:6183–6194. doi: 10.1128/mcb.19.9.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan CA, Moses K. Determination of Drosophila photoreceptors: timing is everything. Cell Mol Life Sci. 2000;57:195–214. doi: 10.1007/PL00000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KE, Baonza A, Freeman M. Epithelial cell adhesion in the developing Drosophila retina is regulated by Atonal and the EGF receptor pathway. Dev Biol. 2006;300:710–721. doi: 10.1016/j.ydbio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Cohen-Fix O, Peters JM, Kirschner MW, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- Cooper MT, Bray SJ. Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature. 1999;397:526–530. doi: 10.1038/17395. [DOI] [PubMed] [Google Scholar]
- de Nooij JC, Hariharan IK. Uncoupling cell fate determination from patterned cell division in the Drosophila eye. Science. 1995;270:983–985. doi: 10.1126/science.270.5238.983. [DOI] [PubMed] [Google Scholar]
- Déak P, Donaldson M, Glover DM. Mutations in mákos, a Drosophila gene encoding the Cdc27 subunit of the anaphase-promoting complex, enhance centrosomal defects in polo and are suppressed by mutations in twins/aar, which encodes a regulatory subunit of PP2A. J. Cell Sci. 2003;116:4147–4158. doi: 10.1242/jcs.00722. [DOI] [PubMed] [Google Scholar]
- Dokucu ME, Zipursky SL, Cagan RL. Atonal, rough and the resolution of proneural clusters in the developing Drosophila retina. Devel. 1996;122:4139–4147. doi: 10.1242/dev.122.12.4139. [DOI] [PubMed] [Google Scholar]
- Dong X, Zavitz KH, Thomas BJ, Lin M, Campbell S, Zipursky SL. Control of G1 in the developing Drosophila eye: rca1 regulates Cyclin A. Genes Dev. 1997;11:94–105. doi: 10.1101/gad.11.1.94. [DOI] [PubMed] [Google Scholar]
- Donzelli M, Squatrito M, Ganoth D, Hershko A, Pagano M, Draetta GF. Dual modes of degradation of Cdc25A phosphatase. EMBO J. 2002;21:4875–4884. doi: 10.1093/emboj/cdf491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA, Sprenger F, Duronio RJ, Leopold P, O’Farrell PH. Distinct molecular mechanism regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes Dev. 1994;8:440–452. doi: 10.1101/gad.8.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth LC, Baker NE. Extracellular signals responsible for spatially regulated proliferation in the differentiating Drosophila eye. Dev Cell. 2005;8:541–551. doi: 10.1016/j.devcel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Foley E, O’Farrell PH, Sprenger F. Rux is a cyclin-dependent kinase inhibitor (CKI) specific for mitotic cyclin-Cdk complexes. Curr. Biol. 1999;9:1392–1402. doi: 10.1016/s0960-9822(00)80084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley E, Sprenger F. The cyclin-dependent kinase inhibitor Roughex is involved in mitotic exit in Drosophila. Curr. Biol. 2001;11:151–160. doi: 10.1016/s0960-9822(01)00050-1. [DOI] [PubMed] [Google Scholar]
- Frankfort BJ, Mardon G. R8 development in the Drosophila eye: a paradigm for neural selection and differentiation. Development. 2002;129:1295–1306. doi: 10.1242/dev.129.6.1295. [DOI] [PubMed] [Google Scholar]
- Frankfort BJ, Nolo R, Zhang Z, Bellen H, Mardon G. senseless repression of rough is required for R8 photoreceptor differentiation in the developing Drosophila eye. Neuron. 2001;32:403–414. doi: 10.1016/s0896-6273(01)00480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H, Tomono Y, Ajiro K, Kosako H, Fujita M, Sakurai M, Okawa K, Iwamatsu A, Okigaki T, Takahashi T, Inagaki M. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J. Biol. Chem. 1999;274:25543–25549. doi: 10.1074/jbc.274.36.25543. [DOI] [PubMed] [Google Scholar]
- Grosshans J, Wieschaus E. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell. 2000;101:523–531. doi: 10.1016/s0092-8674(00)80862-4. [DOI] [PubMed] [Google Scholar]
- Grumbling S a. T. F. C. FlyBase: anatomical data, images and queries. Nucleic Acids Research. 2006;34:D484–D488. doi: 10.1093/nar/gkj068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein U, Singh CM, Luk AY, Donohoe TJ. Growth and differentiation in the Drosophila eye coordinated by hedgehog. Nature. 1995;373:709–711. doi: 10.1038/373709a0. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Herzog F, Mechtler K, Peters JM. Identification of cell cycle-dependent phosphorylation sites on the anaphase-promoting complex/cyclosome by mass spectrometry. Methods Enzymol. 2005;398:231–245. doi: 10.1016/S0076-6879(05)98019-1. [DOI] [PubMed] [Google Scholar]
- Horsfield J, Penton A, Secombe J, Hoffman FM, Richardson H. decapentaplegic is required for arrest in G1 phase during Drosophila eye development. Devel. 1998;125:5069–5078. doi: 10.1242/dev.125.24.5069. [DOI] [PubMed] [Google Scholar]
- Irniger S, Nasmyth K. The anaphase-promoting complex is required in G1 arrested yeast cells to inhibit B-type cyclin accumulation and to prevent uncontrolled entry into S-phase. J. Cell Sci. 1997;110:1523–1531. doi: 10.1242/jcs.110.13.1523. [DOI] [PubMed] [Google Scholar]
- Irniger S, Piatti S, Michaelis C, Nasmyth K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell. 1995;81:269–278. doi: 10.1016/0092-8674(95)90337-2. [DOI] [PubMed] [Google Scholar]
- Jacobs H, Richter D, Venkatesh T, Lehner C. Completion of mitosis requires neither fzr/rap nor fzr2, a male germline-specific Drosophila Cdh1 homolog. Curr. Biol. 2002;12:1435–1441. doi: 10.1016/s0960-9822(02)01074-6. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Grell EH, Ackerman L, Jan LY, Jan YN. Atonal is the proneural gene for Drosophila photoreceptors. Nature. 1994;369:398–400. doi: 10.1038/369398a0. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Sun Y, Jan LY, Jan YN. Role of the proneural gene, atonal, in formation of Drosophila chordotonal organs and photoreceptors. Development. 1995;121:2019–2030. doi: 10.1242/dev.121.7.2019. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Edgar BA. Wingless and Notch regulate cell-cycle arrest in the developing Drosophila wing. Nature. 1998;394:82–84. doi: 10.1038/27925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juo P, Kaplan JM. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr Biol. 2004;14:2057–2062. doi: 10.1016/j.cub.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Kashevsky H, Wallace J, Reed B, Lai C, Hayashi-Hagihara A, Orr-Weaver T. The anaphase promoting complex/cyclosome is required during development for modified cell cycles. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11217–11222. doi: 10.1073/pnas.172391099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- Knoblich JA, Sauer K, Jones L, Richardson H, Saint R, Lehner CF. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 1994;77:107–120. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Stegmuller J, Matsuda T, Bonni S, Bonni A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 2004;303:1026–1030. doi: 10.1126/science.1093712. [DOI] [PubMed] [Google Scholar]
- Lasorella A, Stegmuller J, Guardavaccaro D, Liu G, Carro MS, Rothschild G, de la Torre-Ubieta L, Pagano M, Bonni A, Iavarone A. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature. 2006;442:471–474. doi: 10.1038/nature04895. [DOI] [PubMed] [Google Scholar]
- Lisi S, Mazzon I, White K. Diverse domains of THREAD/DIAP1 are required to inhibit apoptosis induced by REAPER and HID in Drosophila. Genetics. 2000;154:669–678. doi: 10.1093/genetics/154.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet. 2001;35:673–745. doi: 10.1146/annurev.genet.35.102401.091334. [DOI] [PubMed] [Google Scholar]
- Page AM, Hieter P. The anaphase-promoting complex: new subunits and regulators. Annu. Rev. Biochem. 1999;68:583–609. doi: 10.1146/annurev.biochem.68.1.583. [DOI] [PubMed] [Google Scholar]
- Peters JM. Subunits and substrates of the anaphase-promoting complex. Exp. Cell Res. 1999;248:339–349. doi: 10.1006/excr.1999.4443. [DOI] [PubMed] [Google Scholar]
- Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- Peters JM, King RW, Hoog C, Kirschner MW. Identification of BIME as a subunit of the anaphase-promoting complex. Science. 1996;274:1199–1201. doi: 10.1126/science.274.5290.1199. [DOI] [PubMed] [Google Scholar]
- Pimentel AC, Venkatesh TR. rap gene encodes Fizzy-related protein (Fzr) and regulates cell proliferation and pattern formation in the developing Drosophila eye-antennal disc. Dev Biol. 2005;285:436–446. doi: 10.1016/j.ydbio.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Reed B, Orr-Weaver T. The Drosophila gene morula inhibits mitotic functions in the endo cycle and the mitotic cell cycle. Devel. 1997;124:3543–3553. doi: 10.1242/dev.124.18.3543. [DOI] [PubMed] [Google Scholar]
- Richardson H, O’Keefe LV, Marty T, Saint R. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Devel. 1995;121:3371–3379. doi: 10.1242/dev.121.10.3371. [DOI] [PubMed] [Google Scholar]
- Ryo A, Nakamura M, Wulf G, Liou YC, Lu KP. Pin1 regulates turnover and subcellular localization of beta-catenin by inhibiting its interaction with APC. Nat. Cell Biol. 2001;3:793–801. doi: 10.1038/ncb0901-793. [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Lehner CF. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell. 1997;90:671–681. doi: 10.1016/s0092-8674(00)80528-0. [DOI] [PubMed] [Google Scholar]
- Sprenger F, Yakubovich N, O’Farrell PH. S-phase function of Drosophila cyclin A and its downregulation in G1 phase. Curr. Biol. 1997;7:488–499. doi: 10.1016/s0960-9822(06)00220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca FC, Ruderman JV, Hershko A. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol. Biol. Cell. 1995;6:185–198. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T, Ota Y, Kotani S, Nakao M, Takami Y, Takeda S, Saya H. Activation of Cdh1-dependent APC is required for G1 cell cycle arrest and DNA damage-induced G2 checkpoint in vertebrate cells. EMBO J. 2001;20:6499–6508. doi: 10.1093/emboj/20.22.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BJ, Gunning DA, Cho J, Zipursky SL. Cell cycle progression in the developing Drosophila eye: roughex encodes a novel protein required for the establishment of G1. Cell. 1994;77:1003–1014. doi: 10.1016/0092-8674(94)90440-5. [DOI] [PubMed] [Google Scholar]
- Thomas BJ, Zavitz KH, Dong X, Lane ME, Weigmann K, Finley RL, Jr, Brent R, Lehner CF, Zipursky SL. Roughex down-regulates G2 cyclins in G1. Genes Dev. 1997;11:1289–1298. doi: 10.1101/gad.11.10.1289. [DOI] [PubMed] [Google Scholar]
- Thomas BJ, Zipursky SL. Early pattern formation in the developing Drosophila eye. Trends Cell Biol. 1994;4:389–394. doi: 10.1016/0962-8924(94)90051-5. [DOI] [PubMed] [Google Scholar]
- van Roessel P, Elliott DA, Robinson IM, Prokop A, Brand AH. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell. 2004;119:707–718. doi: 10.1016/j.cell.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Wolff T. Drosophila Protocols. In: Sullivan W, Ashburner M, Hawley SR, editors. Drosophila Protocols. New York: Cold Spring Harbor laboratory Press; 2000. pp. 201–227. [Google Scholar]
- Wolff T, Ready DF. Pattern formation in the Drosophila retina. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. Vol. II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1277–1325. [Google Scholar]
- Yamada H, Kumada K, Yanagida M. Distinct subunit functions and cell cycle regulated phosphorylation of 20S APC/cyclosome required for anaphase in fission yeast. J Cell Sci. 1997;110(Pt 15):1793–1804. doi: 10.1242/jcs.110.15.1793. [DOI] [PubMed] [Google Scholar]
- Yoon HJ, Feoktistova A, Wolfe BA, Jennings JL, Link AJ, Gould KL. Proteomics analysis identifies new components of the fission and budding yeast anaphase-promoting complexes. Curr. Biol. 2002;12:2048–2054. doi: 10.1016/s0960-9822(02)01331-3. [DOI] [PubMed] [Google Scholar]
- Zachariae W, Nasmyth K. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- Zavitz KH, Zipursky SL. Controlling cell proliferation in differentiating tissues: genetic analysis of negative regulators of G1-->S-phase progression. Curr Opin Cell Biol. 1997;9:773–781. doi: 10.1016/s0955-0674(97)80077-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The dpp-lacZ enhancer trap (blue) marks the posterior half of the MF. (A) In wild-type, weak CycA expression (green) is detected in S phase cells (red) at the posterior edge of the MF (arrows). (B) In shtd¹, expression of CycA is seen in cells entering S phase ectopically within the MF. (C) In wild-type eye discs, CycE protein (green) is expressed at low levels in groups of cells (arrows) entering S phase synchronously behind the MF. (D) In shtd¹, CycE protein accumulates to high levels in the nuclei of many cells (arrows) behind the MF. Arrowheads indicate the MF.
shtd³ mutant cells marked by the absence of Mycepitope expression (green) (outlined). (A-A”) In shtd³ mutant clone anterior to the MF accumulate Stg (purple). (B-B”) Mutant clones in the MF accumulate CycB (purple), however accumulation is not observed in Myc expressing wild-type cells (green). (C-C”) A subset of cells in shtd³ mutant clones labeled with anti-active Caspase3 antibody (purple, arrows) indicating that the cells are undergoing apoptosis. Anterior of the eye disc is to the right.
(A,B) Early stage embryos contain large amounts of maternal mRNA. (A) Preblastoderm stage. (B) Cellular blastoderm stage. (C) At gastrulation, high level expression is observed in the cephalic furrow (cf, arrowhead)‥ (D) Expression in stage 11 embryos. (E) By stage14 most epithelial expression is down regulated.
(A) Wild-type wing margin bristles (arrowhead). (B) In shtd¹, the wing shows a severe loss of wing margin bristles. Many bristles are missing in the double bristle row (black arrowhead). No significant difference is observed in the triple bristle row (anterior wing margin; vein1, not shown). The distance between vein3 and vein4 is also narrower at the wing margin than in wild-type (double arrowhead line). (C) Reducing stg gene dosage restores bristle number and increases the width between vein3 and vein4. (D–F) Removal of rpr (D) hid, grim (E) or hid (F) also suppressed the shtd¹ wing phenotype. The degree of suppression with the simultaneous removal of hid and grim was stronger than removing rpr or hid alone. (G) A schematic diagram of the third instar wing pouch (mid to ventral portion only). In the zone of non-proliferating cells (ZNC), cells arrest at G2 or G1 in distinct domains. Defects in the wing margin phenotype correspond to the posterior domain of the ZNC, in which cells arrest in G1.
(A) A shtd¹ mutant male fly eye displays a reduced and rough eye. (B and C) Suppression of shtd¹ by reducing APC/C components. A one copy reduction of mr/Apc2 (B) or ida/Apc5 (C) suppresses the shtd¹ eye phenotype. The sizes of the resulting compound eyes are larger than those of the shtd¹ mutants. Merged frontal adult male fly eye images were obtained using a Zeiss Axio Imager Z1m light microscope. Thirty sequential images (5µm thickness each) were merged using a Zeiss Axio Vision imaging software (Ver. 4.5).
BrdU- (A, C, E, G) and PH3-labeling (B, D, F, H) were carried out to show S and M phase cells in wild-type (A, B), shtd¹(C, D), shtd¹; mr²/+ (E, F), and shtd¹; idaB4/+ (G, H) eye discs. The MF is indicated by a line and red brackets show pairs of anaphase or telophase cells. BrdU labeled cells were dramatically decreased in the MF when the dosages of APC/C components were reduced (C, D) compared to shtd¹ alone (A). More pairs of anaphase or telophase cells were observed anterior and posterior to the MF (F, H) compared to shtd¹ alone (D). Anterior of the eye disc is to the right. Images were obtained using a Zeiss Axio Imager Z1m fluorescence microscope with ApoTome. Five to six sequential optical sections (0.67µm thickness each) were merged using the Zeiss AxioVision software.


