Abstract
Phthalate dioxygenase (PDO), a hexamer with one Rieske-type [2Fe-2S] and one Fe (II) - mononuclear center per monomer, and its reductase (PDR), which contains flavin mononucleotide and a plant-type ferredoxin [2Fe-2S] center, are expressed by Burkholderia cepacia at ∼30 mg of crude PDO and ∼1 mg of crude PDR per liter of cell culture when grown with phthalate as the main carbon source. A high level expression system in Escherichia coli was developed for PDO and PDR. Optimization relative to Escherichia coli cell line, growth parameters, time of induction, media composition, and iron-sulfur additives resulted in yields of about 1 g/L for PDO and about 0.2 g/L for PDR. Protein expression was correlated to the increase in pH of the cell culture and exhibited a pronounced (variable from 5 to 20 hours) lag after the induction. The specific activity of purified PDO did not depend on the pH of the cell culture when harvested. However, when the pH of the culture reached 8.5-9, a large fraction of the PDR that was expressed lacked its ferredoxin domain, presumably because of proteolysis. Termination of growth while the pH of the cell culture was < 8 decreased the fraction of proteolyzed enzyme, whereas yields of the unclipped PDR were only marginally lower. Overall, changes in pH of the cell culture were found to be an excellent indicator of the overall level of native protein expression. Its monitoring allowed the real time tracking of the protein expression and made it possible to tailor the expression times to achieve a combination of high quality and high yield of protein.
Among the various systems used for the expression of recombinant proteins, Escherichia coli has the advantage of being available in a wide array of mutant host strains, the ability to grow rapidly and to high density, of being better characterized genetically than other microorganisms, of having many compatible expression plasmids, and it gives good yields of target proteins. Considerable attention has been paid to the improvement of E. coli systems for expression of complex eukaryotic proteins (1, 2). However, expression has been especially troublesome for proteins containing Fe-S clusters that are involved in a number of physiological processes including catalysis, electron transfer, biosynthesis, DNA repair and transcriptional regulation, and sensing for regulatory processes (3). In many cases supplementation of the growth media with Fe2+ and S2− was not sufficient for good expression (3). The formation of Fe-S clusters was found to depend on the so-called isc (iron-sulfur cluster) genes (4, 5), all nine individual components of which are important for the expression of some active Fe-S proteins (e.g. (6-10)). In a number of cases it was found that coexpression of the isc cluster was essential for achieving high yields of active recombinant Fe-S proteins (11-13). Cluster inactivation in E. coli resulted in a marked decrease in the production of native and recombinant iron-sulfur proteins, as well as in the overall growth rate of the bacterial cells (9, 10). The Suf operon was also shown to assist in Fe-S cluster assembly (14, 15). Recent findings suggest that the isc operon is responsible for routine Fe-S cluster assembly in E. coli, but the suf operon becomes activated for Fe-S cluster synthesis when iron or sulfur metabolism is disrupted by oxidative stress or iron starvation (16).
In the present study we show that co-expression of the isc gene cluster is not essential for the high yield expression of the iron-sulfur proteins, phthalate dioxygenase (PDO), which contains a [2Fe-2S] Rieske center, and phthalate dioxygenase reductase (PDR), in which FMN and [2Fe-2S] plant-type ferredoxin are present in two covalently linked domains. We optimized the growth conditions to achieve high yields of the recombinant protein. We also present data that shows a correlation between expression of recombinant proteins in E. coli and pH changes of the media, which provide a convenient tool to monitor the protein expression.
Materials and Methods
Bacterial growth media components were from Fisher Scientific and Difco, carbenicillin was from Apollo Scientific, IPTG was from Research Products International, and other reagents were from Fisher Scientific and Sigma. Spectrophotometric measurements were made with a Cary 3 UV-vis spectrophotometer with the concentration of purified and partially purified PDO and PDR determined using Δε575 = 2.38 mM−1cm−1 and Δε466 = 17.5 mM−1cm−1 for the oxidized minus reduced PDO and PDR correspondingly.
Construction of Plasmids
Genomic DNA was isolated from Bulkholderia cepacia using the DNeasy tissue kit from Qiagen. PDO and PDR genes were amplified by PCR using DyNAzyme DNA polymerase from Finnzymes. Primers used to amplify the PDR gene: 5'-GGGAATTCCATATGACTACCCCCCAGGAAG-3' (forward primer, the underlined bases indicate an Nde I site) and 5'-CGCGGATCCAAGCGTAGGTGTTTGCTCCG-3' (reverse primer, the underlined bases indicate a BamH I site). Primers for PDO amplification: 5'-GGGGAATTCCATATGCTGACCCACCAAGAAAACGAATTGC-3' (forward primer, the underlined bases indicate an Nde1 site) and 5'-CGCGGATCCGTTATTGCTTGACTTGATAGTCCGTTGCGAG-3' (reverse primer, the underlined bases indicate a BamH I site) were obtained from IDT. BamH I and Nde I restriction enzymes were obtained from Invitrogen and used according to the manufacturer recommendations. Digested PCR products were further purified by QIAquick Gel extraction (Qiagen) and inserted into the pET11a vector (Novagen) using DNA Ligation kits from Novagen, transformed into competent DH5α cells, and purified using QIAprep Spin Miniprep kits from Qiagen. Ligation was verified by sequencing the entire PDO and PDR genes in their respective plasmids at the University of Michigan Core Facility. Both PDR and PDO were expressed in either E. coli BL21(DE3) or in C41(DE3) cells that were purchased from Avidis.
Protein expression
Plasmids containing PDR or PDO genes were transformed into E. coli BL21(DE3) or C41(DE3) cells and plated on Luria Broth (LB)-Agar, containing 100 mg/L carbenicillin. After an overnight incubation at 37 °C, a single colony was used to inoculate 50 mL of LB media containing 100 ng/L carbenicillin1. These starter cultures were incubated overnight at 37 °C with shaking at 250 rpm. Starter culture (10 mL/L) was added to the growth media, which was supplemented with 100 mg/L carbenicillin. Growth media used in our experiments (Luria Broth (LB), Minimal media (M9) and Terrific Broth (TB)) were prepared as specified previously (17), with the exception of using double (8 mL/L) amounts of glycerol in the preparation of TB medium. Expression was induced by the addition of 0.7 mM IPTG when the cell culture reached the apparent absorbance values at 600 nm specified in the text. At the same time, the cultures were also supplemented with 2 mM cysteine, 0.2 mg/mL ferrous sulfate, 0.2 mg/mL ferric citrate, and/or 0.2 mg/mL ferric ammonium citrate. Cell growth was monitored by the optical density (OD) of the cell culture at 600 nm and by pH. Expression of PDO and PDR was determined in 1 mL aliquots that were lysed on ice by sonication for 1 min. PDR concentration was determined by the rate of reduction of cytochrome c (50 μM) in a catalytic assay in the presence of 200 μM NADH (18). PDR concentrations were calculated assuming that homogeneous PDR specific activity is 200 u/mg (average activity of purified PDR that contains both FMN and ferredoxin domains). Lysates were diluted with 0.1 M HEPES, pH 7.8, as necessary to obtain rates of cytochrome c reduction within the detection limits of the spectrophotometer. PDO concentrations in lysates were estimated using the Easy-Lyse Bacterial Protein Extraction protocol (Epicentre) combined with SDS-PAGE analysis. More precise determination was done spectroscopically using the protein after the first step of purification of PDO (DEAE) performed as described elsewhere (19). Analysis of the impact of the iron and sulfur additives on the yield of the expression used multiple parameter linear regression in Excel.
Results and Discussion
Use of pH to monitor protein expression
In all types of media used (LB, TB and M9), the media undergoes significant acidification after inoculation with bacteria. This effect was concomitant with cell proliferation, as indicated by the increase in the observed absorbance at 600 nm (Figure 1A and 1B). The overall decrease in pH during this stage of bacterial growth was typically less for TB medium, as compared to LB and M9 media, probably due to its higher buffering capacity. Supplementation of cell culture with IPTG (time zero on Figure 1) induces protein expression, even though the measured onset of the expression of both PDR and PDO (not shown) lagged the induction (Figure 1C). Observed lag times varied from 30 minutes to 20 hours, depending on the growth conditions. Conditions that support slower cell proliferation - i.e., low temperature, slow agitation rates - typically exhibited longer lags. Subsequent cell growth can be monitored both by the increase in OD600 and by changes in the pH of the culture. Concurrently with the observed expression of the target protein, the pH of the media starts to rise. The overall increase in the pH correlated to the amount of the PDR (Figure 1B and 1C) and PDO (data not shown) expressed in the culture. If no adjustments were made to the media, the pH approached 8.5-9, at which the extremely low acidity of the media started to adversely affect the ability of the cells to grow. In a typical experiment, the amount of expressed protein was nearly linearly correlated to the increase in pH up to about pH 8.
Figure 1.
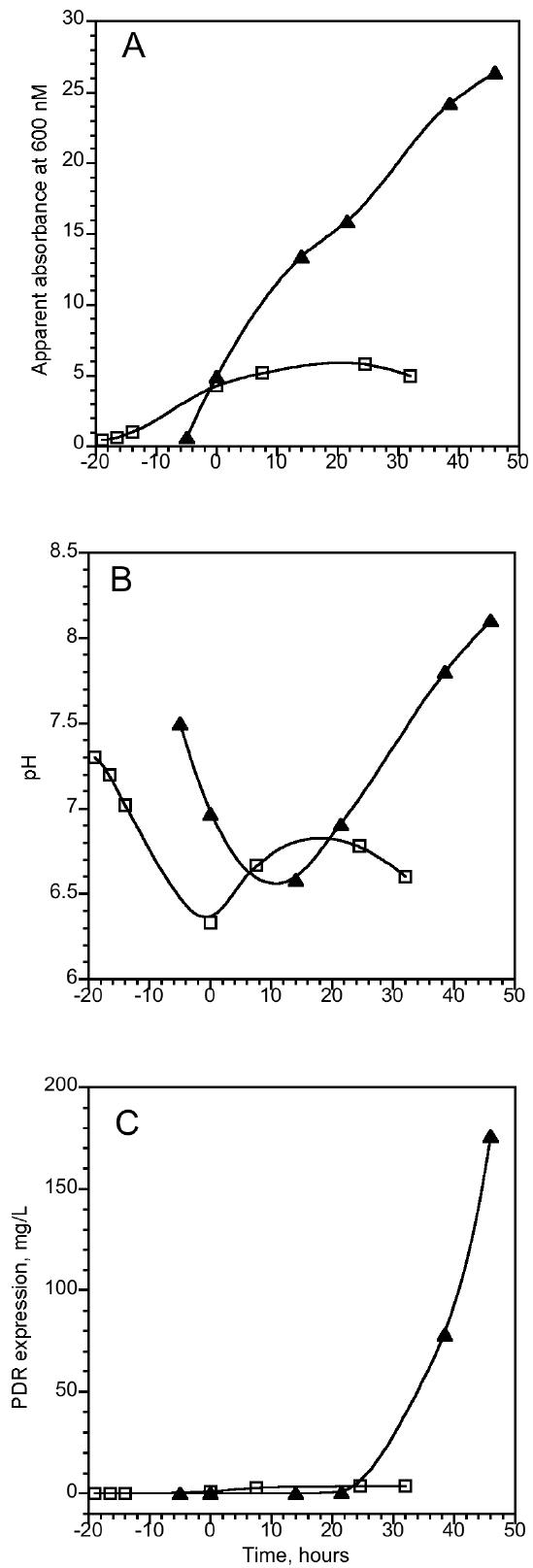
Expression of PDR in E. coli C41 in TB ( ▲ ) and minimal ( □ ) media in the presence of 0.2 mg/mL Ferric citrate, 2 mM cysteine, 0.2 mg/ml FeSO4 and 100 mg/L carbenicillin. Starter cultures were grown in LB overnight at 37 °C, 250 rpm in the presence of carbenicillin, but without iron-sulfur additives. Starter cultures (0.5 mL) were used to inoculate 50 ml of media in 0.25 L conical flasks for both TB and minimal media flasks. Cultures were grown at 26 °C, 200 rpm. PDR expression was induced by adding 0.5 mM IPTG at time zero. Changes in the apparent absorbance at 600 nm (A), pH (B) and enzyme expression in cell culture (C) were evaluated by enzymatic assays as described in Material and Methods.
Prolonged growth of cell cultures that lack expression vector can also result in an increase in pH (after initial acidification), an effect, probably related to depletion of the original carbon sources. However, such an increase, which is unrelated to the addition of IPTG to the media, typically comes about much later in the course of cell proliferation. Although not an unqualified indicator, changes in the pH of the cell culture can be used to monitor the yields of protein expression. We were thus able to use pH to select the appropriate harvesting time to maximize the yields of the expressed proteins. This provides a convenient tool to monitor expression. In the case of PDR, as described below, it also made it possible to significantly improve the quality of the expressed protein.
Effect of E. coli cell line
We evaluated PDO and PDR expression in two E. coli hosts BL21(DE3) and C41(DE3). Both cell lines were grown simultaneously, under the same conditions. Results were compared when using the same concentrations of additives with induction at similar cell densities. In all cases expression of PDO or PDR in BL21 only constituted 10 ± 5 percent of that in the E. coli C41 strain. Thus, even though the two strains used in our experiments are very similar, the choice of the bacterial host significantly affects the yield of recombinant protein.
Effect of Iron and Sulfur additives
A number of studies have stressed the importance of enhancing the supply of iron and sulfur when overexpressing iron-sulfur proteins in E. coli. In particular, cysteine was previously identified as a sulfur source for Fe-S cluster formation (20). Addition of cysteine was also shown to increase the yield of holo ferredoxins under different growth conditions (11). Ferric ammonium citrate (10, 11, 21) or ferrous sulfate (22-24) was also shown to be beneficial for high expression of ferredoxins. We evaluated the effects of the following additives on the expression of PDR: cysteine; ferrous sulfate (FeSO4), ferric citrate (FC, FeC6H5O7), and ferric ammonium citrate (FAC, C6H8O7xFexNH3). E. coli C41 cells were transformed with an expression plasmid containing the PDR gene, and starter cultures were prepared as described in Materials and Methods. Starter cultures were used to inoculate 50 mL of TB media in a 250 mL flask. Supplements were added as specified in Material and Methods. Cultures were grown at 37 °C and 250 rpm until induction with 0.7 mM IPTG at OD600 = 4-5, when the temperature was decreased to 30 °C and the agitation rate to 200 rpm. Cells were harvested when the pH of the culture reached 8. Yields of expression were determined by the enzymatic assay described in Material and Methods.
Analysis of 94 independent growth conditions was performed using the multiparameter linear regression function in Excel. Evaluation of the overall effects of iron or sulfur supplementation on the expression yield of PDR is presented in Table 1A. (Because quantification of the yields of PDO expression by enzymatic activity in crude cell lysates was not convenient, and the PDO concentration evaluated using SDS-PAGE as described in Materials and Methods for a large number of samples was cumbersome, the sample size collected was not statistically significant for analysis). Addition of Fe-containing compounds to the growth media significantly enhanced the yield of PDR and was responsible for about 42% of the variation in the observed yield. Addition of sulfur containing compounds did not have a statistically significant effect on the expression, as evidenced by the high p-value (∼0.7, compared to the low p-value of 3.2 10−5 for Fe addition). Of the four additives tested in this work, only two appear to have statistically significant effects on PDR expression yield (ferric citrate and ferric ammonium citrate with p-values ≪ 0.1), while the contribution of neither cysteine nor FeSO4 was statistically significant (Table 1B). If FC or FAC were present in the media, addition of cysteine did not result in a statistically significant change in the PDR expression (Table 1C). However, addition of FeSO4 to cultures containing either one or both of the citrates was positively correlated with the protein yield (Table 1D), with the additions accounting for up to 50% of the yield variations. These results indicate that i) Both FC and FAC are efficient donors of iron for PDR expression, while FeSO4 is not; ii) In the absence of additional iron, sulfur provided by either cysteine or FeSO4 is not essential for the expression; iii) In the presence of additional iron from either FC or FAC, additional sulfur can be taken up from the FeSO4 (but not from cysteine), and this process is positively correlated with high PDR yields. The ineffectiveness of additional cysteine as a sulfur source for PDR expression can possibly be explained by the background presence of the cysteine in the sample. However, cysteine has been found to elicit higher yields for some ferredoxins in both 2XYT and TB media (11). The effect of the additives on the expression yield appeared to be similar for both PDO and PDR. We did not observe a statistically significant increase in the expression yields for either PDR or PDO when the amounts of the Fe-S supplements were doubled.
Table 1.
| Additions affecting PDR yield1 | P-value | Increase in PDR expression yield , % ± SE |
||
|---|---|---|---|---|
| A | 1 | Any Fe containing compound2 | 3.2 10−5 | 83 ± 19 |
| 2 | Any Sulfur containing compound3 | 0.79 | − 6 ± 23 | |
| B | 1 | Cysteine | 0.63 | 16 ± 33 |
| 2 | Ferrous Sulfate | 0.17 | 44 ± 37 | |
| 3 | Ferric Citrate | 9.6 10−5 | 130 ± 32 | |
| 4 | Ferric Ammonium Citrate | 1.6 10−2 | 85 ± 35 | |
| C | 1 | Ferric Citrate | 1.3 10−3 | 92 ± 28 |
| 2 | Ferric Ammonium Citrate | 2.7 10−2 | 57 ± 25 | |
| 3 | Cysteine (if FC or FAC is also present ) | 0.71 | 11 ± 28 | |
| D | 1 | Ferric Citrate | 3.6 10−3 | 78 ± 26 |
| 2 | Ferric Ammonium Citrate | 8.2 10−2 | 47 ± 26 | |
| 3 | Ferrous Sulfate ( if FC or FAC is also present ) | 3.2 10−2 | 60 ± 28 |
See Materials and Methods for concentrations.
Including Ferrous Sulfate, FC and FAC
Including Cysteine and Ferrous Sulfate
Effect of cell medium on protein expression
The highest yields of expression of both PDO and PDR were achieved using TB as a growth medium. When LB or M9 media were used, the resultant yields were less than 25 and 2 percent of that in TB, respectively (see Figure 1C; results for growth on LB are not shown).
Effect of the pre-induction temperature conditions on protein expression
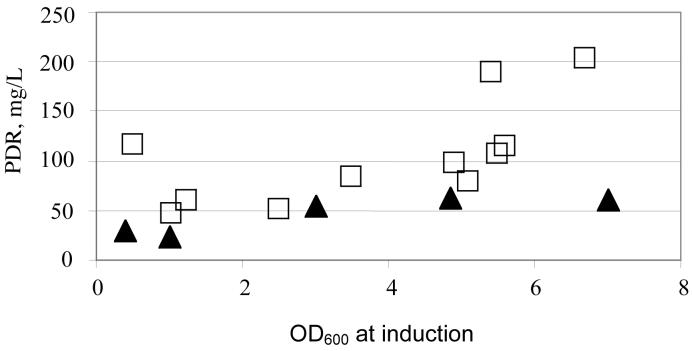
Preinduction growth conditions were shown to have a significant impact on the yields of the recombinant protein expression (25, 26). For both PDO and PDR expression in C41 E. coli, changes in growth rates were achieved by changing the temperature during the pre-induction incubation, with the concomitant changes in the agitation rates. As shown in Figure 2, a decrease to 30 °C with 200 rpm agitation from 37 °C and 250 rpm, resulted in a significantly lower recombinant protein yields.
Figure 2.
Dependence of PDR expression in C41 E. coli on the OD of cell culture at 600 nm at the time of induction with IPTG. Temperature before induction 37 °C ( ■ ) and 30 °C ( ▲ ). Concentration of PDR in cell culture was measured as described in Materials and Methods. Test growths in 50 mL TB media in 0.25 L conical flasks in the presence of 0.2 mg/mL Fe citrate, 2 mM cysteine, 0.2 mg/mL FeSO4 and 100 mg/L carbenicillin at 30 °C, 200 rpm for 40 hours after induction.
Effect of the cell density at the moment of induction
Induction of the main culture with IPTG is typically done when the OD600 reaches 0.4-1, with the optimal OD depending on the culture method and the medium. In our experiments, for both PDR and PDO, an induction in the late logarithmic phase of cell growth resulted in significantly higher yields of the recombinant protein compared to induction at early logarithmic stages (Figure 2). On average, about 4-fold increases in the yields were achieved by the inoculation of the cell culture at OD600 > 4.5.
Effect of growth conditions after the induction
Three sets of conditions for cell growth after induction were tested in our experiments. The best results were obtained when the growth was performed at 30 °C with an agitation rate of 200 rpm. Yields of both PDO and PDR were decreased by as much as 70 percent when the cultures were incubated at 27 °C, 200 rpm. A decrease in the protein yields of about 20 percent was observed when the cultures were incubated at 37 °C, 250 rpm.
Optimization of the protein expression
A recent publication by Gennaro et.al (27) indicates that pH, oxygen delivery to the media (as expressed by air flow rate, stirring or a combination of both), and extra Fe(II) are parameters that can explain up to 80 percent of the variability in the expression of naphthalene dioxygenase, which is an enzyme similar to PDO. Higher Fe(II) concentrations and higher stirring speeds appeared to be beneficial for higher protein expression, while the best results were obtained at an intermediate pH levels (at about pH 7). Other parameters, such as reaction volume, temperature, etc. also affected the yields of the expression, although these effects were found to be much less significant than those due to the presence of iron and high stirring speed.
In contrast to the procedures used for naphthalene dioxygenase, in our expression of PDR and PDO the pH value of the medium was not kept constant. Even though the growth media (typically TB) was well buffered, the pH changed during the expression, and these changes were used to conveniently monitor the progress of protein expression. The optimized protocols for both PDO and PDR expression were as described in Materials and Methods with the following additions:
The C41 cell line was used for the expression
After inoculation, cultures in TB growth medium were grown at 37 °C, 250 rpm
The cultures were induced by 0.7 mM IPTG at OD600 = 4.5-5.5
At the moment of induction, the cultures were supplemented with iron and sulfur (0.1 mg/mL ferric citrate, 1 mM cysteine, 0.1 mg/mL FeSO4 and 0.1 mg/mL ferric ammonium citrate)
After induction, the temperature was decreased to 30 °C and the agitation rate reduced to 200 rpm.
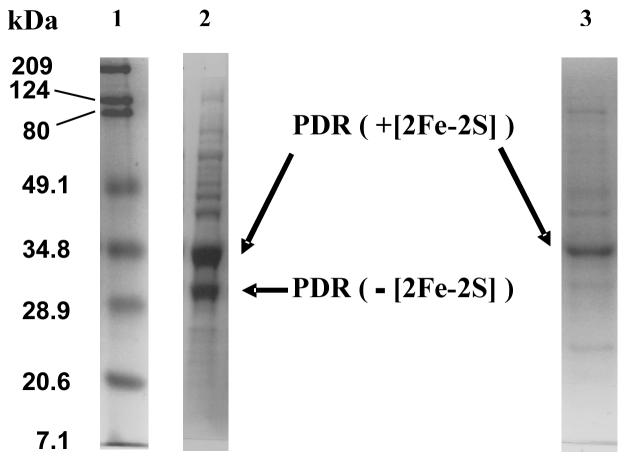
Under these conditions the total yield of crude protein, as estimated by the recombinant protein concentration in the cell lysate, was up to 250 mg/L for PDR and up to 1 g/L for PDO. Spectrophotometric and SDS-PAGE analysis of the partially purified proteins confirmed the yields of up to 200 mg/L for PDR and up to 850 mg/L for PDO. In both cases maximum protein yields were achieved when the cultures were allowed to grow until the pH values stabilized at pH > 8.5. For PDR, however, growth to such a high pH of the media resulted in protein with low apparent enzymatic activity. The SDS-PAGE gel of the lysates of cell cultures grown until the pH exceeded 8, shows two bands related to recombinant PDR (at about 34 kDa and at about 25 kDa, Figure 3). These bands correspond to PDR with and without the ferredoxin domain (28). The enzyme that lacks the [2Fe-2S] domain is catalitically inactive with PDO and its presence in the culture is undesirable because of the complexity of its separation from fully active PDR. Harvesting the cells at pH < 7.5, while decreasing the total yield of PDR, resulted in more homogeneous protein with the fraction of the enzyme lacking the ferredoxin domain significantly decreased (Figure 3). For PDO, we observed no differences in catalytic activity between proteins purified from the cultures grown to pH ≥ 8.5 and the cultures harvested at or below 7.5.
Figure 3.
SDS PAGE of lysed cultures of C41 E. coli expressing PDR. 1. Protein ladder (5 μL, BioRad Broadband); 2. Cells harvested from the media at pH 8.5-8.8; 3. Cells harvested when the pH of the media was less that 7.7. For lanes 2 and 3, 0.2 μL of lysed cell culture (after 1 minute sonication of freeze-thawed aliquote) were applied. Recombinant protein expression was performed using the optimized protocol as described in the text.
The optimization of the expression conditions performed was by no means exhaustive. Although the expression at 30 °C, 200 rpm, appears favorable, a more precise selection of temperature and agitation rate combination might have improved the yield even further. Similarly, the concentration of iron-sulfur additives adds a dimension to optimization that remains largely unexplored. Increased glycerol concentration, either from the start, or supplemented to the growth during the expression might have resulted in even higher protein yields as described by Kwon et.al. (29). However, in practical terms the expression yields achieved even with the limited optimization described above were more than sufficient to cover the needs for recombinant PDO and PDR in our laboratory.
In conclusion, even in the absence of co-expressed suf and isc operons, optimization of the expression conditions allowed for high yield expression of recombinant iron-sulfur proteins. During protein expression, changes in pH were correlated with the amount of protein in the cell culture. The use of pH to monitor protein expression readily allowed for selection of the optimal harvest time for maximizing protein yield and/or high quality of recombinant proteins.
Acknowledgments
Supported by NIH grant (GM20877 to D.P.B.)
Abbreviations
- PDO
phthalate dioxygenase
- PDR
phthalate dioxygenase reductase
- IPTG
isopropyl-beta-D-thiogalactopyranoside
- FC
ferric citrate
- FAC
ferric ammonium citrate
- LB
Luria Broth
- TB
Terrific Broth
- OD
optical density
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We used the more stable carbenicillin instead of ampicillin to decrease the degradation of the antibiotic due to pH changes and β-lactamase secretion into the medium by E. coli.
References
- 1.Baneyx F. Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol. 1999;10:411–421. doi: 10.1016/s0958-1669(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 2.Makrides SC. Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev. 1996;60:512–538. doi: 10.1128/mr.60.3.512-538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beinert H, Holm RH, Munck E. Iron-sulfur clusters: nature's modular, multipurpose structures. Science. 1997;277:653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- 4.Zheng L, Cash VL, Flint DH, Dean DR. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J Biol Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]
- 5.Agar JN, Zheng L, Cash VL, Dean DR, Johnson MK. Role of the IscU Protein in Iron-Sulfur Cluster Biosynthesis: IscS-mediated Assembly of a [Fe2S2] Cluster in IscU. J Am Chem Soc. 2000;122:2136–2137. [Google Scholar]
- 6.Ollagnier-de-Choudens S, Mattioli T, Takahashi Y, Fontecave M. Iron-sulfur cluster assembly: characterization of IscA and evidence for a specific and functional complex with ferredoxin. J Biol Chem. 2001;276:22604–22607. doi: 10.1074/jbc.M102902200. [DOI] [PubMed] [Google Scholar]
- 7.Hoff KG, Silberg JJ, Vickery LE. Interaction of the iron-sulfur cluster assembly protein IscU with the Hsc66/Hsc20 molecular chaperone system of Escherichia coli. Proc Natl Acad Sci U S A. 2000;97:7790–7795. doi: 10.1073/pnas.130201997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silberg JJ, Hoff KG, Tapley TL, Vickery LE. The Fe/S assembly protein IscU behaves as a substrate for the molecular chaperone Hsc66 from Escherichia coli. J Biol Chem. 2001;276:1696–1700. doi: 10.1074/jbc.M009542200. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz CJ, Djaman O, Imlay JA, Kiley PJ. The cysteine desulfurase, IscS, has a major role in in vivo Fe-S cluster formation in Escherichia coli. Proc Natl Acad Sci U S A. 2000;97:9009–9014. doi: 10.1073/pnas.160261497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi Y, Nakamura M. Functional assignment of the ORF2-iscS-iscU-iscA-hscB-hscA-fdx-ORF3 gene cluster involved in the assembly of Fe-S clusters in Escherichia coli. J Biochem (Tokyo) 1999;126:917–926. doi: 10.1093/oxfordjournals.jbchem.a022535. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M, Saeki K, Takahashi Y. Hyperproduction of recombinant ferredoxins in escherichia coli by coexpression of the ORF1-ORF2-iscS-iscU-iscA-hscB-hs cA-fdx-ORF3 gene cluster. J Biochem (Tokyo) 1999;126:10–18. doi: 10.1093/oxfordjournals.jbchem.a022409. [DOI] [PubMed] [Google Scholar]
- 12.Kriek M, Peters L, Takahashi Y, Roach PL. Effect of iron-sulfur cluster assembly proteins on the expression of Escherichia coli lipoic acid synthase. Protein Expr Purif. 2003;28:241–245. doi: 10.1016/s1046-5928(02)00680-0. [DOI] [PubMed] [Google Scholar]
- 13.Grawert T, Kaiser J, Zepeck F, Laupitz R, Hecht S, Amslinger S, Schramek N, Schleicher E, Weber S, Haslbeck M, Buchner J, Rieder C, Arigoni D, Bacher A, Eisenreich W, Rohdich F. IspH protein of Escherichia coli: studies on iron-sulfur cluster implementation and catalysis. J Am Chem Soc. 2004;126:12847–12855. doi: 10.1021/ja0471727. [DOI] [PubMed] [Google Scholar]
- 14.Ollagnier-de Choudens S, Nachin L, Sanakis Y, Loiseau L, Barras F, Fontecave M. SufA from Erwinia chrysanthemi. Characterization of a scaffold protein required for iron-sulfur cluster assembly. J Biol Chem. 2003;278:17993–18001. doi: 10.1074/jbc.M300285200. [DOI] [PubMed] [Google Scholar]
- 15.Loiseau L, Ollagnier-de-Choudens S, Nachin L, Fontecave M, Barras F. Biogenesis of Fe-S cluster by the bacterial Suf system: SufS and SufE form a new type of cysteine desulfurase. J Biol Chem. 2003;278:38352–38359. doi: 10.1074/jbc.M305953200. [DOI] [PubMed] [Google Scholar]
- 16.Outten FW, Djaman O, Storz G. A suf operon requirement for FeS cluster assembly during iron starvation in Escherichia coli. Mol Microbiol. 2004;52:861–872. doi: 10.1111/j.1365-2958.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- 17.Ausubel FM, Brent R, Kingston RE, Moore DD, Siedman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. John Wiley & Sons, Inc.; New York: 2001. Escherichia Coli, Plasmids and Bacteriophages; pp. 1.1.1–1.1.4. [Google Scholar]
- 18.Batie CJ, Ballou DP. Phthalate dioxygenase. Methods Enzymol. 1990;188:61–70. doi: 10.1016/0076-6879(90)88013-z. [DOI] [PubMed] [Google Scholar]
- 19.Tarasev M, Rhames F, Ballou DP. Rates of the Phthalate Dioxygenase Reaction with Oxygen Are Dramatically Increased by Interactions with Phthalate and Phthalate Oxygenase Reductase. Biochemistry. 2004;43:12799–12808. doi: 10.1021/bi0490587. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy MB, White RE. Functional differences between peroxidase compound I and the cytochrome P-450 reactive oxygen intermediate. J Biol Chem. 1983 Aug 10;258:9153–9158. [PubMed] [Google Scholar]
- 21.Metzler DE, Metzler CM, Scott RD, Mollova ET, Kagamiyama H, Yano T, Kuramitsu S, Hayashi H, Hirotsu K, Miyahra I. NMR Studies of 1H Resonances in the 10-18-ppm Range for Aspartate Aminotransferase from Escherichia coli. J Biol Chem. 1994;269:28027–28033. [PubMed] [Google Scholar]
- 22.Schrautemeier B, Cassing A, Bohme H. Characterization of the genome region encoding an fdxH-type ferredoxin and a new 2[4Fe-4S] ferredoxin from the nonheterocystous, nitrogen-fixing cyanobacterium Plectonema boryanum PCC 73110. J Bacteriol. 1994;176:1037–1046. doi: 10.1128/jb.176.4.1037-1046.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen B, Menon NK, Dervertarnian L, Moura JJ, Przybyla AE. Cloning, sequencing and overexpression of the Desulfovibrio gigas ferredoxin gene in E. coli. FEBS Lett. 1994;351:401–404. doi: 10.1016/0014-5793(94)00891-4. [DOI] [PubMed] [Google Scholar]
- 24.Grabau C, Schatt E, Jouanneau Y, Vignais PM. A new [2Fe-2S] ferredoxin from Rhodobacter capsulatus. Coexpression with a 2[4Fe-4S] ferredoxin in Escherichia coli. J Biol Chem. 1991;266:3294–3299. [PubMed] [Google Scholar]
- 25.Curless C, Pope J, Tsai L. Effect of preinduction specific growth rate on recombinant alpha consensus interferon synthesis in Escherichia coli. Biotechnol Prog. 1990;6:149–152. doi: 10.1021/bp00002a009. [DOI] [PubMed] [Google Scholar]
- 26.Kim SS, Kim EK, Rhee JS. Effects of growth rate on the production of Pseudomonas fluorescens lipase during the fed-batch cultivation of Escherichia coli. Biotechnol Prog. 1996;12:718–722. doi: 10.1021/bp960047h. [DOI] [PubMed] [Google Scholar]
- 27.Di Gennaro P, Conforti P, Lasagni M, Bestetti G, Bernasconi S, Orsini F, Sello G. Dioxygenation of naphthalene by Pseudomonas fluorescens N3 dioxygenase: optimization of the process parameters. Biotechnol Bioeng. 2006;93:511–518. doi: 10.1002/bit.20736. [DOI] [PubMed] [Google Scholar]
- 28.Gassner GT, Ballou DP. Preparation and characterization of a truncated form of phthalate dioxygenase reductase that lacks an iron-sulfur domain. Biochemistry. 1995;34:13460–13471. doi: 10.1021/bi00041a025. [DOI] [PubMed] [Google Scholar]
- 29.Kwon S, Kim S, Kim E. Effects of glycerol of beta-lactamase production during high cell density cultivation of recombinant Escherichia coli. Biotechnol Prog. 1996;12:205–208. doi: 10.1021/bp9500728. [DOI] [PubMed] [Google Scholar]